Abstract
The number of devices for electrical stimulation of nerve fibres implanted worldwide for medical applications is constantly increasing. Stimulation charge is one of the most important parameters of stimulation. High stimulation charge may cause tissue and electrode damage and also compromise the battery life of the electrical stimulators. Therefore, the objective of minimizing stimulation charge is an important issue. Delaying the second phase of biphasic stimulation waveform may decrease the charge required for fibre activation, but its impact on stimulation selectivity is not known. This information is particularly relevant when transverse intrafascicular multichannel electrode (TIME) is used, since it has been designed to provide for high selectivity. In this in vivo study, the rat sciatic nerve was electrically stimulated using monopolar and bipolar configurations with TIME. The results demonstrated that the inclusion of a 100-μs delay between the cathodic and the anodic phase of the stimulus allows to reduce charge requirements by around 30 %, while only slightly affecting stimulation selectivity. This study shows that adding a delay to the typical stimulation waveform significantly (\(P < 0.001\)) reduces the charge required for nerve fibres activation. Therefore, waveforms with the delayed discharge phase are more suitable for electrical stimulation of nerve fibres.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The number of electrical stimulators implanted around the world for various medical applications is constantly increasing [2]. However, many challenges related to electrical stimulation of nerve fibres remain unsolved. Among the main issues are safety, power consumption and selectivity of stimulation, these issues depend among others on the interface (i.e. electrode) shape and material, stimulation parameters and electrode to nerve distance [18]. A minimal charge injection is desired to maintain safety and reduce power consumption [16]. The selectivity of stimulation indicates how much the target population of nerve fibres is activated, without activating other neighbouring fibres. Ideally, only the specified cluster of fibres within the nerve should be activated using very small charge [25]. For that purpose, various interfaces between the stimulator and the nerve [7, 21] and various stimulation techniques have been proposed [9].
In order to avoid tissue and electrode damage, biphasic charge balanced stimuli, with either passive or active discharge, are commonly used [16]. In the vast majority of cases, the cathodic phase—responsible for fibre activation—is generated first, and the anodic one—responsible for charge balance—comes later. However, if the anodic phase is generated immediately after the short cathodic phase, it may abolish the activation of some of the targeted fibres. Van den Honert and Mortimer [24] found that such abolition could be eliminated by introducing a delay after the first, cathodic, phase of a biphasic stimulus waveform. This strategy significantly decreased the charge required for activation of the same population of nerve fibres, and also it produced a slightly steeper recruitment curve [8]. This decreases the number of functional levels of responses for a given device. This may be a reason why delaying the second phase of a biphasic waveform has not become popular, although it was used in some research works [10]. There were a few previous studies regarding application of these techniques for various applications, including auditory nerve [17, 22] and retina [26] stimulation, but without analysing the effect on selectivity.
The transverse intrafascicular multichannel electrode (TIME) [23], Fig. 1, belongs to the category of intraneural interfaces designed to achieve relatively high stimulation selectivity. It consists of a polyimide thin film electrode, inserted transversally into the nerve and has a number of stimulation sites along the two sides of the transversal part. These sites are used to independently address various populations of nerve fibres within the same nerve. It has been previously shown that TIME enables higher stimulation selectivity than multipolar cuff and longitudinal intra-fascicular (LIFE) electrodes [5]. The purpose of the current study is to assess the effect of delaying the second phase of the stimulation waveform when using the TIME on the minimal charge required to achieve desired activation of nerve fibres and on the selectivity of stimulation. In addition, it is to investigate whether the effect of delayed discharge is the same for the monopolar and bipolar electrode configurations. It is important, since for TIME, differently from most of the other neural interfaces, in the bipolar configuration current flows mainly transversely to the nerve fibres.
2 Methods
2.1 Experimental procedure
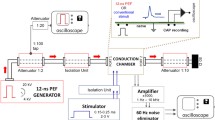
The experiment was performed in four adult Sprague–Dawley rats following protocols approved by the Ethical Committee of the Universitat Autónoma de Barcelona. Under anaesthesia with pentobarbital (40 mg/kg i.p.), the sciatic nerve was surgically exposed at the mid-thigh and carefully freed from adherences to surrounding tissues. A TIME device with four active sites, coated with iridium oxide (80 μm diameter, spread by 200 μm), on the left and four on the right side was carefully implanted into the sciatic nerve perpendicularly to the direction of the nerve fibres. Care was taken to handle the nerve and the thin film electrode delicately with fine surgical tools (for details, see [6]). The insertion was monitored under a dissection microscope to ensure correct placement and that the electrode sites were located adequately in the nerve. The TIME was inserted across the peroneal and the tibial fascicles of the sciatic nerve in this order, although the exact location of the active sites within each fascicle cannot be determined. In order to assess possible variations depending on the exact insertion of the TIME, in two rats the stimulation protocol has been performed twice in different locations. In those rats, first the electrode was implanted in the right sciatic nerve and the whole stimulation protocol (see below) was performed, and then the electrode was retrieved and implanted again in a different location (more proximal) into the same nerve and the same stimulation protocol was repeated. Therefore, the stimulation protocol has been repeated six times, once in two rats and twice in another two rats.
After implantation of the electrode, the same stimulation protocol was applied with several monopolar and bipolar combinations of the electrode sites. Each site of the TIME was used as the cathode while varying the anode. For each active site being a cathode, a monopolar configuration was first tested, in which stimulation was delivered against a small needle electrode placed in the proximity of the nerve. Then, the stimulation was delivered using each of the remaining seven sites of the TIME consecutively as an anode, i.e. in 7 bipolar configurations. Since for each of the 8 TIME sites 1 monopolar and 7 bipolar configurations were tested, during each experiment a total of 8 monopolar and 56 bipolar configurations of electrode sites were tested.
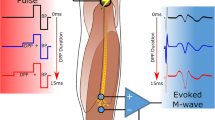
For each configuration tested, the same series of stimuli was generated, which consisted of rectangular stimuli of 20 μs duration and amplitudes ranging from 20 to 300 μA, in steps of 20 μA. The stimuli were delivered using Stim’nD stimulator (DEMAR/MXM-Obelia) [3]. The amplitude was limited to 300 μA because, for some bipolar configurations, relatively high impedance (of the order of few tens k\({\mathrm {\Omega }}\)) between the two sites used for stimulation was observed. Using higher stimulation amplitudes could, in such situation, lead to stimulator saturation. The maximal stimulus charge, 6 nC (20 μs \(\times \) 300 μA), was well below the safe charge injection limit, which for the electrode sites with a diameter of 80 μm was 2.3 mC/cm2, i.e. approximately 120 nC. Each stimulation amplitude was delivered in two conditions (modes), Fig. 2. In the first condition, immediately after the stimulus a passive discharge (1 μF capacitor coupling) was performed. In the second condition, a 100-μs delay was introduced between the stimulus and the passive discharge. During that delay, the outputs of the stimulator were in high impedance state, so that no current flowed. The duration of the delay was set at 100 μs because it was shown by van den Honert and Mortimer to be the most efficient value [24]. For each configuration of electrode sites and stimulation amplitude, the stimulus with immediate discharge was generated 1 s after the corresponding stimulus with delayed discharge. The protocol including all the stimuli for all 64 configurations of electrode sites took approximately 40 minutes per case. During the experiment, the animal body temperature was maintained by means of a thermostated heating pad.
During the stimulation electromyographic (EMG) signals were simultaneously recorded using monopolar needle electrodes from the plantar interossei (PL), gastrocnemius medialis (GM) and tibialis anterior (TA) muscles, which are innervated by different fascicles or subfascicles of the sciatic nerve. Those muscles were chosen upon the knowledge of the topographical distribution of the muscular fascicles in the rat’s sciatic nerve [4] and based also on previous studies [5, 14]. The TA muscle is innervated by the peroneal fascicle, whereas GM and PL muscles are innervated by fibres with distinct locations in the tibial fascicle. The EMG data were collected at 20 kHz, well above the expected frequency of the evoked compound muscle action potentials (CMAP) which was approximately 200–500 Hz (compare with Fig. 3).
Sample recording of the CMAP of PL, GM and TA muscles elicited by the same stimulus delivered to the sciatic nerve. The signals were amplified by \(\times \)1,000 for the PL muscle and by \(\times \)200 for GM and TA muscles, and displayed at scales appropriate for full recording of the maximal CMAPs. From each EMG recording, the amplitude of the CMAP was measured from the onset to the maximal peak, as shown in the inset
For the sake of clarity, the names of the variables used in this study and their values are listed in Table 1.
2.2 Data processing
In order to compare the charge required to activate the muscles in each stimulation mode, the minimal amplitude of stimulation (\(I_{\mathrm{min}}\)) that elicited 10, 30, 50, 70 and 90 % of the maximum CMAP amplitude of each of the three muscles was determined for each electrode configuration tested. The five selected levels of muscle activation were equally spread between the 10 and 90 % of muscle activation to ensure that the observed changes are not specific of a particular segment of the recruitment curve.
In the EMG recordings, the amplitude of the CMAP of each muscle was measured from the baseline to the maximal peak of the M wave and normalized to the maximum CMAP amplitude obtained for each muscle in the experiment [5, 19] in order to evaluate the relative efficacy of each stimulating condition in the selective activation of the three muscles. CMAP amplitudes lower than 5 % of the maximum CMAP were considered as 0. An example of the recorded CMAPs is presented in Fig. 3.
For each electrode configuration and stimulation mode, up to 15 sets (three muscles \(\times \) 5 activation levels) of \(I_{\mathrm{min}}\) to elicit the specified muscle activation level were determined. However, when intraneural electrodes with active sites of small surface area are used, the region of nerve activation is limited to fibres in the vicinity of the cathode [19]. Therefore, with the current amplitude limited to 300 μA, for some configurations of electrode sites, only partial activation of the muscles was achieved. Thus, for those configurations, some sets of \(I_{\mathrm{min}}\) could not be determined. Therefore, the statistical analysis was made only for cases in which the \(I_{\mathrm{min}}\) was determined in both stimulation modes.
In order to assess how the delayed discharge after stimulus influences the stimulus amplitude required to achieve a specified level of muscle activation, \(I_{\mathrm{min}}\) values obtained for various monopolar and bipolar configurations in the two stimulation modes have been averaged for each experiment. Then, a two-way ANOVA was applied with two variables: “configuration type” and “stimulation mode” with repeated measures for “stimulation mode”. When a significant interaction between explanatory variables was detected by ANOVA, multiple comparisons using Holm–Šídák method [1, 12] were performed afterwards for comparing pairs of situations. The \(P\) values provided in the results of the multiple comparisons analysis are the multiplicity-adjusted \(P\) values [27].
Each time \(I_{\mathrm{min}}\) was found, and the corresponding selectivity index (SI) was determined. SI was calculated for each stimulation mode, electrode site configuration, muscle and level of muscle activation, as the ratio between the normalized CMAP of the muscle for which \(I_{\mathrm{min}}\) was found, \(\mu _i\), and the sum of the normalized CMAPs obtained in the same stimulation conditions for all three muscles:
This equation was initially proposed by Veraart et al. [25]. However, in the original version, the normalized contraction forces of the isolated muscles, and not CMAPs, were compared in the equation. Similarly as it has been done in some other studies [5, 13, 19], we have chosen a simplified version, using CMAPs instead.
In order to assess whether the delayed discharge affects the selectivity of muscle activation, ANOVA was performed as above, but instead of \(I_{\mathrm{min}}\), the corresponding SI values were considered.
3 Results
An example of the EMG responses recorded from the three muscles during stimulation in one bipolar configuration is presented in Fig. 4. The differences in the amplitude of the consecutive responses, caused by interleaved generation of stimuli with delayed (stronger responses) and immediate discharge, can be clearly observed.
Sample EMG responses recorded from the three muscles (PL, GM and TA) when stimulating using one of the bipolar configurations. The amplification ratios were same as in Fig. 3. The stimuli with delayed and immediate discharge were interleaved, while the amplitude was increased from 20 to 300 μA in steps of 20 μA. At the bottom of the figure, “X” and “I” signs indicate the times of delivery of stimuli for delayed and immediate discharge modes, respectively
The box-and-whisker plots of the averaged \(I_{\mathrm{min}}\) and SI values, depending on the stimulation mode and configuration type obtained in the six experiments, are presented in Fig. 5. Additional plots presenting mean values and 95 % standard deviations of the \(I_{\mathrm{min}}\) depending on the different variables assessed are presented in the online resources.
When testing charge requirements, ANOVA yielded significant differences for both the configuration type (\(F(1,10) = 28.2, P < 0.001\)) and the stimulation mode (\(F(1,10) = 1{,}277, P<0.001\)), as well as for the interaction between them (\(F(1,10) = 93.9, P < 0.001\)). The multiple comparisons using the Holm–Šídák method indicated significant differences between monopolar stimulation with immediate and with delayed discharge (\(P < 0.001\)) and bipolar stimulation with immediate and with delayed discharge (\(P < 0.001\)).
The ratio between the amplitudes required to achieve the same level of muscle activation using delayed and immediate discharge was calculated. The ratios averaged 0.73 and 0.65 for the monopolar and bipolar configurations, respectively. These ratios indicate that the delayed discharge allowed to decrease by 30 % on average the required amplitude to achieve a specified level of muscle activation and that this effect was slightly stronger for bipolar configurations of electrode sites.
The SIs corresponding to the \(I_{\mathrm{min}}\) were analysed similarly. The ANOVA showed significant influence on selectivity of configuration type (\(F(1,10) = 9.69, P = 0.011\)) and the stimulation mode (\(F(1,10) = 11.28, P = 0.007\)), as well as for the interaction between them (\(F(1,10) = 9.03, P = 0.013\)). The multiple comparisons using Holm–Šídák method indicated significant differences between monopolar stimulation with immediate and with delayed discharge (\(P < 0.01\)), but not between bipolar stimulation with immediate and with delayed discharge (\(P = 0.81\)).
The ratio between the averaged SIs obtained in the two stimulation modes was calculated in a similar way as for the \(I_{\mathrm{min}}\) values. Its amount reached 0.98 and 0.999 for monopolar and bipolar configurations, respectively. These ratios indicate that the use of delayed discharge slightly (\(\approx \)2 %) decreased the selectivity of the monopolar configurations. This decrease was not significant for the bipolar configurations.
As already explained above, it was not always possible to determine \(I_{\mathrm{min}}\). From a total of 5,760 potential \(I_{\mathrm{min}}\) values (6 experiments per 64 electrode sites configurations with five levels for each of three muscles), in 1,820 cases (31.6 %) \(I_{\mathrm{min}}\) was determined in both stimulation modes, and only these values have been taken into account in the statistical comparisons. It is worth to note that in 857 cases (14.9 %) \(I_{\mathrm{min}}\) was measurable only in the stimulation mode with delayed discharge but not with immediate discharge, whereas in only 1 case (0.02 %) \(I_{\mathrm{min}}\) was determined only in the stimulation mode with immediate discharge and not with delayed discharge. Detailed information about the number and proportion of the \(I_{\mathrm{min}}\) determined for the two stimulation modes, with immediate and with delayed discharge, depending on the electrode configuration type, is presented in Table 2.
4 Discussion
The results of this study show that adding a short delay between the first, cathodic, phase of the biphasic stimulus waveform and the anodic discharge phase significantly decreases the charge required to activate nerve fibres. This is also directly observed in the EMG recordings (Fig. 4) and confirmed by the number of configurations for which the specified level of muscle activation was obtained only when using delayed discharge (Table 2). Whenever \(I_{\mathrm{min}}\) could not be determined, it indicates that the required charge was higher than the maximal charge tested (6 nC). When the activation level was achieved in the mode with immediate discharge, it was also (except one case) achieved in the mode with delayed discharge. On the contrary, an important part of the activation levels was achieved only in the mode with delayed discharge (14.9 % of all cases).
Our observations are consistent with the findings reported by van den Honert and Mortimer [24]. They explained that when short stimuli are applied, the first cathodic phase initializes processes in the fibre membrane leading to initialization of the action potential; however, due to the relatively long time constants of these processes, they may be abolished by the anodic phase generated shortly after the first one. They also showed that inclusion of a delay as short as 100 μs between the two phases of the stimulus is sufficient to eliminate this blocking phenomenon.
In addition, our study provides novel contributions, as we show that: i) the effect of the delay between stimulus phases is as relevant using intrafascicular transverse electrodes (in which current flows mainly transversely) as with cuff electrodes (in which current flows mainly longitudinally); ii) the inclusion of the delay between the two phases of the waveform only slightly affects stimulation selectivity (Fig. 5). This last point is important because stimulation selectivity is crucial for many practical applications of electrical nerve stimulation, and it was also the initial aim for designing the TIME concept.
The effect of delayed discharge was more marked for bipolar than for monopolar stimulation configurations. First, it may be due to monopolar stimulation generating more longitudinal current in comparison with the transversal placed bipolar stimulation [20]. Second, the difference may be linked to the more limited region of activation in the bipolar configuration. It is possible that the ratio of the fibres being close to the threshold for activation in that limited region is higher than when monopolar stimulation is performed. Van den Honert and Mortimer [24] showed that the fibres close to activation threshold are those more prone to suffer activation block by the second phase of the stimulus waveform. In such a case, the bipolar configuration would be more sensitive to activation abolition caused by the immediate discharge, and, in consequence, delaying the discharge would be more beneficial than in the monopolar configuration. Further studies are, however, necessary to verify this hypothesis. It should be also investigated why selectivity loss was significant only in the monopolar configurations.
Despite the fact that the recruitment curves are steeper when the second phase of the stimulation waveform is delayed, as it was shown by Gorman and Mortimer [8], it affects in the same manner all muscles; thus, it does not affect much the selectivity of targeted activation. Our study also confirms that the selectivity of stimulation using TIME is higher for bipolar than for monopolar configurations, as already reported in [14]. However, bipolar stimulation requires higher charge to achieve the same level of muscle activation as compared with the monopolar stimulation (Fig. 5). This is why the percentage of combinations for which the specified level of muscle activation was not achieved was much higher for bipolar configurations than for monopolar configurations (Table 2).
Another relevant finding in our study is that the amplitude of stimulation may be decreased by using the delayed discharge mode to around 30 % compared with the amplitude of the stimulation with the immediate discharge mode. This ratio is however dependent on the delay duration and the time constant of the discharge [24]. It is also possible that the reduction in amplitude of the stimulus needed may vary with the type of neural interface used. Therefore, further studies are necessary to determine the extent to which charge requirements may be decreased by modulating the delay to the discharge phase. Simulations may help to provide answer to this question. For example, results of simulation performed by Hofmann et al. [11] suggest that delaying discharge may decrease the required charge by as much as 50 %, but in their study 30 % reduction of required charge was obtained only for delays longer than 1 ms. This discrepancy may be caused by various stimulation conditions in their and our study. Therefore, using more realistic models, for example, in [19], should be considered.
Although delaying the negative phase of stimulation reduces required charge, it also causes the electrode potential to remain relatively negative during a long period. Therefore, long delaying of the reversal phase should be avoided. In our study, the duration of this delay was set to 100 μs, which is sufficient to prevent the suppressing effect of the discharge phase [24] and may be short enough that deleterious Faradaic reaction products do not accumulate to an unacceptable level [16].
There are a few cases in which using a discharge immediately after the first stimulus phase may be desired, for example, when precise control of the muscle activation level is required. In such a case, generating the second phase of stimulus immediately after the first one will produce less steep recruitment curves, so the same changes in the amplitude will produce smaller changes in the amplitude of muscle response [8]. Another situation in which a delayed discharge is not desired refers to neural recordings performed in the proximity of the stimulating electrode. In this case, the immediate discharge will allow for better compensation of the ionic displacement in the volume conductor caused by the first phase of stimulation and will reduce the stimulus artefact duration. Both effects contribute to decrease of the observed stimulus artefact, and thus, it will decrease the stimulation artefact observed in near recording electrodes [15].
5 Conclusion
We confirmed that the inclusion of a delay between the cathodic and the anodic phases of a biphasic stimulus waveform allows to decrease the charge required to achieve the same level of muscle activation as compared to an immediate discharge. This phenomenon appears to be stronger for biphasic than for monophasic stimulation configurations. We have also shown that the proposed modification of the stimulus waveform only slightly affects the stimulation selectivity achieved by using transverse intrafascicular stimulation. Further studies are necessary to confirm to which degree charge requirements may be reduced by delaying the second phase of the stimulus. Since there are no technical limitations for applying such a delay in the stimulus waveform generation, this technique may be of interest for applications involving electrical stimulation of nerve fibres.
References
Abdi H (2007) The Bonferonni and Šídák corrections for multiple comparisons. In: Salkind NJ (ed) Encyclopedia of measurement and statistics. Sage, Thousand Oaks, pp 103–107
Arle JE (2011) The neuromodulation approach. In: Arle JE, Shils JL (eds) Essential neuromodulation. Academic Press, Waltham, pp 1–16
Andreu D, Guiraud D, Souquet G (2009) A distributed architecture for activating the peripheral nervous system. J Neural Eng 6:026001
Badia J, Pascual-Font A, Vivó M, Udina E, Navarro X (2010) Topographical distribution of motor fascicles in the sciatic–tibial nerve of the rat. Muscle Nerve 42:192–201
Badia J, Boretius T, Andreu D, Azevedo-Coste C, Stieglitz T, Navarro X (2011) Comparative analysis of transverse intrafascicular multichannel, longitudinal intrafascicular and multipolar cuff electrodes for the selective stimulation of nerve fascicle. J Neural Eng 8:036023
Badia J, Boretius T, Pascual-Font A, Udina E, Stieglitz T, Navarro X (2011) Biocompatibility of chronically implanted transverse intrafascicular multichannel electrode (TIME) in the rat sciatic nerve. IEEE Trans Biomed Eng 58:2324–2332
Del Valle J, Navarro X (2013) Interfaces with the peripheral nerve for the control of neuroprostheses. Int Rev Neurobiol 109:63–83
Gorman PH, Mortimer JT (1983) The effect of stimulus parameters on the recruitment characteristics of direct nerve stimulation. IEEE Trans Biomed Eng 30:407–14
Grill WM, Mortimer JT (1995) Stimulus waveforms for selective neural stimulation. IEEE Trans Biomed Eng 14:375–85
Guiraud D, Stieglitz T, Taroni G, Divoux JL (2006) Original electronic design to perform epimysial and neural stimulation in paraplegia. J Neural Eng 3:276–86
Hofmann L, Ebert M, Tass PA, Hauptmann C (2011) Modified pulse shapes for effective neural stimulation. Front Neuroeng 4:9
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:6570
Kundu A, Harreby K, Yoshida K, Boretius T, Stieglitz T, Jensen W (2014) Stimulation selectivity of the thin-film longitudinal intrafascicular electrode (tfLIFE) and the transverse intrafascicular multi-channel electrode (TIME) in the large nerve animal model. IEEE Trans Neural Syst Rehabil Eng 22:400–410
Maciejasz P, Badia J, Boretius T, Harreby K, Jensen W, Stieglitz T, Navarro X, Guiraud D (2013) Comparison of stimulation selectivity in monopolar and bipolar configuration using the transversal intrafascicular multichannel electrode (TIME)—preliminary results. In: Pons JL, Torricelli D, Pajaro M (eds) Converging clinical and engineering research on neurorehabilitation, vol 1., Biosystems and BioroboticsSpringer, Berlin, pp 79–83
Marin J, De Lannoy G, Delbeke J (2009) When can we recover charges with a biphasic charge balanced stimulation pulse? In: Proceedings of the international functional electrical stimulation society conference, Seoul, Korea, pp 55–57
Merrill DR, Bikson M, Jefferys JGR (2005) Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J Neurosci Methods 141:171–98
Prado-Guitierrez P, Fewster LM, Heasman JM, McKay CM, Shepherd RK (2006) Effect of interphase gap and pulse duration on electrically evoked potentials is correlated with auditory nerve survival. Hear Res 215:4755
Prodanov D, Marani E, Holsheimer J (2003) Functional electric stimulation for sensory and motor functions: progress and problems. Biomed Rev 14:23–50
Raspopovic S, Capogrosso M, Badia J, Navarro X, Micera S (2012) Experimental validation of a hybrid computational model for selective stimulation using transverse intrafascicular multichannel electrodes. IEEE Trans Neural Syst Rehabil Eng 20:395–404
Rattay F (1986) Analysis of models for external stimulation of axons. IEEE Trans Biomed Eng 33:974–77
Schultz AE, Kuiken TA (2011) Neural interfaces for control of upper limb prostheses: the state of the art and future possibilities. PMR 3:55–67
Shepherd RK, Javel E (1999) Electrical stimulation of the auditory nerve: II. Effect of stimulus waveshape on single fibre response properties. Hear Res 130:17188
Stieglitz T, Boretius T, Navarro X, Badia J, Guiraud D, Divoux JL, Micera S, Rossini PM, Yoshida K, Harreby KR, Kundu A, Jensen W (2012) Development of a neurotechnological system for relieving phantom limb pain using transverse intrafascicular electrodes (TIME). Biomed Tech (Berl) 57:457–465
van den Honert C, Mortimer JT (1979) The response of the myelinated nerve fiber to short duration biphasic stimulating currents. Ann Biomed Eng 7:117–25
Veraart C, Grill WM, Mortimer JT (1993) Selective control of muscle activation with a multipolar nerve cuff electrode. IEEE Trans Biomed Eng 40:640–53
Weitz AC, Behrend MR, Ahuja AK, Christopher P, Wei J, Wuyyuru V, Patel U, Greenberg RJ, Humayun MS, Chow RH, Weiland JD (2014) Interphase gap as a means to reduce electrical stimulation thresholds for epiretinal prostheses. J Neural Eng 11:016007
Wright SP (1992) Adjusted P-values for simultaneous inference. Biometrics 48:1005–1013
Acknowledgments
We would like to thank Mr. Guillaume Souquet from MXM Axonic for developing low-level control software of the Stim’nD stimulator, Mr. François Bonnetblanc from the DEMAR team, INRIA, for help with statistical analysis and Ms. Chloé Picq from MXM Axonic for English proof reading.
Author information
Authors and Affiliations
Corresponding author
Additional information
This research was supported by the European Union through the FP7 Projects: EPIONE—“Natural sensory feedback for phantom limb pain modulation and therapy” (Project Number: 602547), and TIME—“Transverse, Intrafascicular Multichannel Electrode system for induction of sensation and treatment of phantom limb pain in amputees” (Project Number: 224012).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maciejasz, P., Badia, J., Boretius, T. et al. Delaying discharge after the stimulus significantly decreases muscle activation thresholds with small impact on the selectivity: an in vivo study using TIME. Med Biol Eng Comput 53, 371–379 (2015). https://doi.org/10.1007/s11517-015-1244-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-015-1244-4