Abstract
Although feasibility of accurate 3D reconstruction of the proximal epiphysis of the femur from biplanar X-rays (frontal and lateral) has been assessed, in vivo application is limited due to bone superposition. The aim of this study was to propose a specific algorithm to get accurate and reproducible, low dose in vivo 3D reconstruction. To achieve this goal, a parametric subject-specific model was introduced as a priori knowledge. This geometric model was based on a database based on proximal epiphysis of 60 femurs. The accuracy was estimated using comparisons to CT scans on 13 cadaveric femurs, then in vivo intra- and inter- observer reproducibility was assessed using a set of 23 femurs. The mean for the relative difference was 0.2 mm for the in vitro 3D accuracy. The mean error was 1.0 mm with maximum value of 5.1 mm in ideal conditions (in vitro). The confidence interval for the inter-observer reproducibility was within ±2.2 mm. This method gave us a reproducible tool in order to get in vivo 3D reconstructions of the femur proximal epiphysis from biplanar X-rays.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Accurate patient-specific three dimensional (3D) reconstructions of bone structures are needed for clinical and biomechanical applications. For example, in post-operative evaluation of joint arthroplasty, the position of implants is evaluated using accurate 3D subject-specific reconstructions of the knee [1, 2] or the hip [3, 4]. In another kind of application, evaluation of 3D parameters on the proximal epiphysis of the femur could lead to a quantification of the femoral neck mechanical properties [5]. Even if recent method using low-dose computed tomography [6] were developed, these 3D models of bone resulted from methods based on the CT-scan [7, 8] leading to a high radiation dose for the patient. Consequently, obtaining an accurate, low dose and in vivo 3D reconstruction of the proximal epiphysis of the femur is a challenging goal.
The use of low dose bi-planar digital radiographs in standing position [EOS™ low irradiation 2D–3D X-ray scanner (Biospace Med, Paris, France)] to obtain a subject-specific 3D model was proposed [9, 10]. An anatomical atlas model is needed as a priori knowledge to obtain the 3D reconstruction [11, 12]. According to previous methods, this knowledge could be improved with a parametric model and a database [13], or from statistical analysis such as Principal Component Analysis [14, 15]. Regarding the hip, accurate methods were developed for the pelvis [16] and the proximal epiphysis of the femur [17]. However, they were applied and evaluated on excised bone specimens. Moreover, the lack of visibility on the lateral X-ray makes it difficult to use in vivo due to the femurs’ superimposition.
Therefore, the aim of this study was to propose a specific algorithm for the 3D reconstruction of the femur from biplanar X-rays in vivo. To achieve this goal, a parametric subject-specific model was introduced. The accuracy and reproducibility of the technique was then evaluated.
2 Materials and methods
2.1 Subjects
For the accuracy evaluation, proximal epiphysis of 13 in vitro femurs as part of a previous study [17] was available. For the in vivo study, 13 subjects (six males and seven females, 27.9 ± 9.1 years old) participated. Subjects did not have hip or pelvis pathologies, and had bi-planar X-rays using the EOS device (Biospace Med, Paris, France). The EOS™ imaging device takes two perpendicular radiographs simultaneously using low dose X-Ray detectors. The dose received by the patients is eight to ten times lower than for conventional X-ray exams [9, 10]. Informed consent was obtained from each subject (ethical committee of Paris Saint-Antoine approval No. 02547).
Both femurs of each subject were reconstructed by three different observers (except for three subjects where only one femur was reconstructed due to a bad positioning of these patients). One of the observer reconstructed all the subjects three times (intra-observer reproducibility). Thus, proximal epiphyses of 23 femurs were available for the in vivo evaluation.
2.2 3D reconstruction
The 3D reconstruction method (non stereo corresponding contours (NSCC) method [12]) was previously described elsewhere for the proximal epiphysis of the femur [17]. It can be summarized in three steps:
-
1.
Identification of stereo-corresponding landmarks (seen in both radiographs; black crosses, Fig. 1).
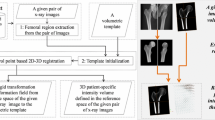
Fig. 2 3D reconstruction process. From the X-ray exploitation and the use of a database, a simplified parametric subject specific model (Fig. 3) was evaluated. Morphorealism was added from CT scan 3D reconstruction to obtain a morphorealistic parametric subject specific model (Fig. 4). This model was used as generic model for the NSCC algorithm in order to have the subject-specific model
-
2.
Semi-automatic extraction of 2D contours in the radiographs (Fig. 1a).
-
3.
Deformation of an anatomical atlas model of femur according to the identified contours shown in Fig. 1a using the NSCC algorithm [12]. This algorithm matched the identified contours with those derived from the anatomical atlas model. Then, the optimization of the initial solution and the optimized object deformation was done to minimize the distances between X-rays contours and projected 3D contours.
The main modification of this algorithm was the definition of a simplified parametric subject specific model (SPSSM, Fig. 3) and a morphorealistic parametric subject specfic model (MPSSM, Fig. 4) instead of the anatomical atlas model. The whole process is described in Fig. 2.
The contours identification was assessed for the in vivo situation. Visibility in X-rays (Fig. 1c) was reduced due to the bone superimposition in the lateral view. Therefore, it was not possible to differentiate contours such as the trochanters (lesser and greater) and the femoral neck in the lateral view (Fig. 1a).
A 3D sphere model was adjusted, in terms of position and radius, on both frontal and sagittal X-rays. The sphere models provided the radius (femoral head radius, FHR) and center of the femoral head.
The anatomical atlas model was replaced by a MPSSM. At first, the 2D contours were used to approximate the shaft and neck axis. Then the 3D neck-shaft angle (NSA_3D) was calculated. Thus both the NSA_3D and the FHR were obtained. A local frame linked to the studied proximal epiphysis of the femur was also assessed.
These two parameters were used as predictors for the construction of an initial parametric subject-specific model from a database (International Patent Number PCT/FR2004/001394 [18]). Proximal epiphyses of 60 excised femurs were used to build this database. For each bone, its 3D CT scan reconstruction made with millimetric slices was available. Then 33 parameters were evaluated, including NSA_3D, neck length, FHR and neck minimal section [17]. Parameters correlation was evaluated using the Pearson’s correlation matrix. Then, a multivariate linear regression was employed to determine all of the parameters with the explicative ones (NSA_3D and FHR). A SPSSM (Fig. 3) was then calculated from the explicative parameters and the database. This 3D model was made of simple geometric object such as sphere or truncated cone.
The anatomical atlas model was then replaced by a MPSSM. This model was evaluated from both the SPSSM and a 3D model extracted from CT scan slices. The resulting 3D model was used as generic model for the NSCC algorithm to obtain a subject specific model.
All of the 3D reconstructions were performed with a custom software package developed in collaboration between the Laboratoire de recherche en Imagerie et Orthopédie, (ETS-CRCHUM, Montréal, Canada) and the Laboratoire de Biomécanique (ENSAM-CNRS, Paris, France).
The reconstruction time for the whole process was approximately 4 min.
2.3 Comparison protocol
2.3.1 In vitro evaluation
For the in vitro evaluation, 13 femurs were selected from the database. Consequently, we had to re-calculate the linear regressions using the database without the selected femur (a ‘leave-one-out’ procedure). For each femur, the two reconstructions were performed, using two methods (the current one and the one proposed by Le Bras et al. [17]). These reconstructions were made using the reduced set of contours visible in vivo (Fig. 1b). This accuracy evaluation was therefore done in the most favorable conditions.
The evaluation was divided into two steps. In the first step, a qualitative comparison between the reconstructed shape and the reference femoral geometry (CT-scan reconstruction) was performed. In addition, visualization of the superimposed reconstruction of the same femur made visible the femoral regions where maximum deviations may occur.
Quantitative accuracy was then expressed as point to surface distances: each point of the model (obtained from stereo-radiography) was projected onto the surface defined by the reference (obtained from CT-scan) [19, 20]. A global comparison consisting of computing the mean point to surface distance, the 2SD (2 × Standard Deviation) and the maximum distance values was made. The 2SD distances represent the maxima for 95% of all points, while the maximum distance values represent the isolated extreme values obtained for the entire sample. This comparison was processed on the entire set of 1,706 points per femur. Then, a local comparison was evaluated. For the femur, the areas of interest were the head, the neck, the trochanters (lesser and greater), and the shaft.
Finally, the inverse operation was made by evaluating the point to surface distances with the EOS™ 3D reconstruction used as reference. The Hausdorff distances (bilateral point to surface distances) were therefore available using the same evaluation as mentioned above.
2.3.2 In vivo evaluation
The intra- and inter-observer reproducibility was evaluated for the in vivo evaluation. In order to make comparisons, a reference object was needed. For each subject, the reference model was calculated from the mean position (X, Y, Z) of each of the nodes obtained from the three observer’s reconstructions.
The shape of each reconstructed object was evaluated by superimposing it with the reference model. The distances between the points of the reconstructed models and the surface of the reference model were computed [19, 20]. Then, the global and local evaluations were done in the same way as in vitro.
A set of 3D parameters was also computed. Each 3D parameter of the stereo-radiographic model was compared to the same parameter given by the reference model. These parameters (3D neck shaft angle, neck length, head diameter, and neck minimal section) were calculated in the same way as Le Bras et al. [17].
For each pair of radiographs of a subject, three reconstructions and a reference model were available. The statistical tests were done as follows. First, a Shapiro-Wilk normality test was applied to the measures defined in the comparison protocol. The normality of the values was tested for each observer. Then, an ANOVA for repeated measures was applied to the normally distributed values. In the case of non-normal values, a Friedman test was used. For normally distributed samples, the 95% limit is equal to 2SD. For non-gaussian measures, it was defined as the 95th percentile of all the values.
3 Results
3.1 In vitro evaluation
The results for the in vitro evaluation are given in Table 1. These results were obtained with the reduced set of contours (Fig. 1b) for both the parametric subject-specific method and the NSCC methods. The mean relative 3D reconstruction error for the parametric subject-specific method was 0.2 mm.
When using the EOS™ 3D model as reference, the mean absolute error and the RMS were very similar to those mentioned in Table 1. The maximum were higher reaching 8.5 mm for the parametric subject-specific method with a reduced set of contours.
3.2 In vivo evaluation
No statistical differences were outlined between the samples (min(p) > 0.05) for both intra- and inter-reproducibility. The results for the inter-observer reproducibility are summarized in Fig. 5. The results were given for both methods as a global evaluation and per area of interest. The localization of the maximal differences for the entire set of femurs is shown in Fig. 6. Summarized for the 3D parameters, the mean values and the coefficient of variation (CV) in absolute value, and percentage are shown in Table 2.
Concerning the intra-observer reproducibility, the mean difference was 0.4 mm (2SD 1.3 mm, max 5.8). For the local comparison, in the head, shaft and neck areas, the mean differences were 0.4 mm for all values, the 2SD were 1.3 mm, 1.0 mm, and 1.2 mm, respectively, and finally the maxima were 4.5, 4.3, and 2.9 mm, respectively. The Intra CV in percentages and absolute values were 1.3% (0.6 mm), 0.7% (0.6 mm), 1.0% (1.3°) and 3.5% (20.6 mm²) for the femoral head diameter, the neck length, the neck shaft angle and the minimal cross section of the neck, respectively.
4 Discussion
The use of a strict lateral X-ray in vivo was challenging because some contours were not visible. However, the study was conducted using the minimal contours available on all the X-rays (Fig. 1c).
Regarding the femoral head area, the use of a sphere model allowed precise and reproducible values for both the center position and the radius. Under no circumstance was the femoral head area imposed as spherical. The contours selected by the observer led the reconstruction of the femoral head.
Moreover, these 3D models were obtained with the EOS™ device leading to a radiation reduction of eight to ten times for the patient [9, 10].
A particular and innovative method was therefore, created to overcome the mentioned difficulties. The use of a parametric subject specific model was done. It was the result of the statistical analysis of a database composed of 60 femurs.
4.1 In vitro evaluation
This in vitro study was designed to validate the parametric subject-specific method. There was no introduction of biases with the use of this method. Indeed, regarding the relative differences, there were no systematic differences (mean relative difference 0.2 mm). Therefore, the uncertainty of measurement for the reconstruction method was given by the inter-observer reproducibility evaluated in vivo.
The results found in the current study are almost the same as those reported by Le Bras et al. [17]. The main differences between the two studies occurred in the in vivo evaluation, where fewer contours were selected by the observer in the lateral X-ray. With the use of all of the contours, both methods are similar in terms of accuracy (mean absolute difference 0.8 mm, 2SD 2.2 mm, max 5.4 mm). The accuracy slightly decreased using the reduced set of contours. Nevertheless, the maximum for the parametric subject-specific method was equivalent to the NSCC method (5.1 mm compared to 7.8 mm), indicating that this method was more precise than the previous one [17]. When using a reverse comparison using the EOS™ model as reference (Hausdorff distances), the results were consistent with those described above except for the maximum growing to 8.5 mm. As the mean and RMS value stayed equal, this only reflected local imperfections on the 3D models.
The accuracy was assessed in ideal conditions (isolated femur). For in vivo applications, a higher difference should be expected.
4.2 In vivo evaluation
4.2.1 Shape evaluation
The mean, 2SD and maximal values were smaller for the anatomical subject-specific method due to the use of the statistical database. The maximum value decreased from 13.9 to 7.3 mm.
The mean reproducibility value is 0.8 mm. Ninety five percent of the differences, representing the variability of measurement, were within ±2.2 mm for the whole femur. It decreased to ±1.6 and ±1.9 mm for the head and neck areas, respectively. Subsequently, a higher quality of reconstruction was available in the most important areas used for the study of hip pathologies.
The maximum values were located on either side of the proximal epiphysis of the femur along an antero-posterior axis. These results were certainly due to the lack of information extracted from the sagittal view.
4.2.2 3D parameters
The 3D parameters evaluated in this study were the same as those measured by Le Bras et al. [17]. In this study [17] the researchers made the measurement on proximal epiphyses of 15 excised femurs. In the current study, 13 subjects (i.e. proximal epiphyses of 23 femurs) with in vivo X-rays were selected. Using the parametric subject-specific method, the reproducibility decreased for the measurement of the 3D parameters. Introducing a parametric subject-specific model improved the reproducibility without modifying the accuracy.
For the NSA_3D, Inter CV was 1.0% (0.9°). For the FHD, it was 2.2% (0.7 mm). These results showed that this method gave us a reproducible tool to assess the proximal epiphysis of the femur geometry. This method could also lead to a quantification of the femoral neck mechanical properties [5]. Therefore, with this application, from just two perpendicular X-rays, surgeons will be able to evaluate both the subject-specific geometry and bone strength.
5 Conclusion
This paper presents the in vitro accuracy and the in vivo inter- and intra-observer reproducibility for the reconstruction of the proximal epiphyses of the femur using bi-planar X-rays. The described method used a database composed of 60 excised femurs. A MPSSM was then calculated using multiple linear regressions. This model was finally adjusted according to the X-ray information to get a 3D reconstruction.
This method gave us a reproducible tool in order to get 3D reconstructions of the proximal epiphysis of the femur from biplanar X-rays, in vivo. The shape evaluation gave measurement variability within ±2.2 mm for the whole femur.
This algorithm was developed with frontal and sagittal views, but could easily be extended to other applications involving different biplanar projections. Similar methods could now be developed for other bone structures such as the pelvis or the whole lower limb.
The current evaluation was made on asymptomatic subjects. The next step for the validation process will be to use the parametric subject-specific method for pathological cases.
References
Mahfouz MR et al (2003) A robust method for registration of three-dimensional knee implant models to two-dimensional fluoroscopy images. IEEE Trans Med Imaging 22(12):1561–1574
Sato T, Koga Y, Omori G. (2004) Three-dimensional lower extremity alignment assessment system: application to evaluation of component position after total knee arthroplasty. J Arthroplasty 19(5):620–628
Hagio K et al (2004) A novel system of four-dimensional motion analysis after total hip arthroplasty. J Orthop Res 22(3):665–670
Otake Y et al (2005) Four-dimensional model of the lower extremity after total hip arthroplasty. J Biomech 38(12):2397–2405
Bousson V et al (2006) Volumetric quantitative computed tomography of the proximal femur: relationships linking geometric and densitometric variables to bone strength. Role for compact bone. Osteoporos Int 17(6):855–864
Van Sint Jan S et al (2006) Low-dose computed tomography: a solution for in vivo medical imaging and accurate patient-specific 3D bone modeling? Clin Biomech (Bristol, Avon) 21(9):992–998
Gautsch TL, Johnson EE, Seeger LL (1994) True three dimensional stereographic display of 3D reconstructed CT scans of the pelvis and acetabulum. Clin Orthop Relat Res 305:138–151
Mortele KJ, McTavish J, Ros PR (2002) Current techniques of computed tomography. Helical CT, multidetector CT, and 3D reconstruction. Clin Liver Dis 6(1):29–52
Kalifa G et al (1998) Evaluation of a new low-dose digital x-ray device: first dosimetric and clinical results in children. Pediatr Radiol 28(7):557–561
Dubousset J et al (2005) A new 2D and 3D imaging approach to musculoskeletal physiology and pathology with low-dose radiation and the standing position: the EOS system. Bull Acad Natl Med 189(2):287–297 (discussion 297–300)
Fleute M, Lavallee S, Julliard R (1999) Incorporating a statistically based shape model into a system for computer-assisted anterior cruciate ligament surgery. Med Image Anal 3(3):209–222
Laporte S et al (2003) A biplanar reconstruction method based on 2D and 3D contours: application to the distal femur. Comput Methods Biomech Biomed Eng 6(1):1–6
Pomero V et al (2004) Fast accurate stereoradiographic 3D-reconstruction of the spine using a combined geometric and statistic model. Clin Biomech (Bristol, Avon) 19(3):240–247
Benameur S et al (2003) 3D/2D registration and segmentation of scoliotic vertebrae using statistical models. Comput Med Imaging Graph 27(5):321–337
Benameur S et al (2002) 3D biplanar statistical reconstruction of scoliotic vertebrae. Stud Health Technol Inform 91:281–285
Mitton D et al (2006) 3D reconstruction of the pelvis from bi-planar radiography. Comput Methods Biomech Biomed Eng 9(1):1–5
Le Bras A et al (2004) 3D reconstruction of the proximal femur with low-dose digital stereoradiography. Comput Aided Surg 9(3):51–57
Pomero V et al. (2003) Procede d’imagerie radiographique pour la reconstruction tridimensionnelle, dispositif et programme d’ordinateur pour mettre en œuvre ce procede Radiographic imaging method for three-dimensional reconstruction, device and computer software for carrying out said method
Mitton D et al (2000) 3D reconstruction method from biplanar radiography using non-stereocorresponding points and elastic deformable meshes. Med Biol Eng Comput 38(2):133–139
Mitulescu A et al (2001) Validation of the non-stereo corresponding points stereoradiographic 3D reconstruction technique. Med Biol Eng Comput 39(2):152–158
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Baudoin, A., Skalli, W., de Guise, J.A. et al. Parametric subject-specific model for in vivo 3D reconstruction using bi-planar X-rays: application to the upper femoral extremity. Med Biol Eng Comput 46, 799–805 (2008). https://doi.org/10.1007/s11517-008-0353-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-008-0353-8