Abstract
Parkinson's disease (PD) is recognized as the most common neurodegenerative movement disorder and results in debilitating motor deficits. The accumulation and spread of neurotoxic synuclein aggregates in the form of Lewy bodies is a key pathological feature of PD. Chronic activation of the NLRP3 inflammasome by protein aggregates is emerging as a major pathogenic mechanism in progressive neurodegenerative disorders and is considered an important therapeutic target. Recently the ketone body, β-hydroxy butyrate (BHB), was shown to efficiently inhibit the NLRP3 inflammasome in macrophages, and in vivo models of inflammatory disease. Furthermore, BHB can readily cross the blood brain barrier suggesting that it could have therapeutic benefits for the management of PD. In this study, we evaluated if BHB could inhibit chronic microglial inflammasome activation induced by pathological fibrillar synuclein aggregates. Interestingly, we found that BHB treatment almost completely blocked all aspects of inflammasome activation and pyroptosis induced by ATP and monosodium urate (MSU) crystals, consistent with previously published reports in macrophages. Surprisingly however, BHB did not inhibit inflammasome activation and release of IL-1β or caspase-1 induced by synuclein fibrils. Our results demonstrate that BHB does not block the upstream pathways regulating inflammasome activation by synuclein fibrils and suggest that synuclein mediated inflammasome activation proceeds via distinct mechanisms compared to traditional NLRP3 activators such as ATP and MSU.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is recognized as the second most common neurodegenerative disease, and is characterised by a profound loss of dopaminergic neurons in the substantia nigra, leading to dopamine deficiency and subsequent motor deficits (Sulzer 2007; Ozawa 2010). The accumulation and spread of Lewy body aggregates, consisting primarily of misfolded alpha synuclein, is considered a central hallmark of the disease although the precise pathological mechanisms involved remain to be defined (Luk et al. 2012; Peelaerts et al. 2015). Chronic activation of the NLRP3 inflammasome in the CNS has emerged as an important mechanism by which misfolded protein aggregates can trigger persistent neuroinflammation and subsequent neurodegeneration in the CNS (Heneka et al. 2013; Heneka et al. 2014; Walsh et al. 2014; Heneka et al. 2015). The NLRP3 inflammasome has been shown to be activated by multiple misfolded and prionoid protein aggregates including β-amyloid, synuclein and prions (Heneka et al. 2013; Gustin et al. 2015; Gustot et al. 2015). Crucially, NLRP3 knockout mice are protected against neuropathology in multiple neurodegenerative disease models suggesting that the NLRP3 inflammasome could be a major disease-modifying therapeutic target (Heneka et al. 2013; Heneka et al. 2015).
Recently, the ketone body β-hydroxybutyrate (BHB) was shown to be an effective inhibitor of the NLRP3 inflammasome in response to multiple activation stimuli including ATP, silica and monosodium urate (MSU) crystals (Youm et al. 2015). Given that BHB is capable of effectively crossing the blood brain barrier, NLRP3 inhibition by BHB could be a major therapeutic strategy for PD and other neurodegenerative disorders in which chronic inflammasome activation has been shown to drive disease pathology and progression. Further, previous studies in the MPTP model of PD suggested that BHB can confer partial neuroprotection and protect against motor deficits (Kashiwaya et al. 2000; Tieu et al. 2003). However, the effects of BHB on synuclein pathology, or on inflammasome activation by pathological synuclein aggregates in microglia have not been studied to date. Therefore, we evaluated if BHB can inhibit inflammasome activation by fibrillar synuclein aggregates as well as traditional NLRP3 activators such as ATP and MSU in primary microglial cultures. Surprisingly, we found that while BHB effectively blocked microglial NLRP3 activation induced by ATP and MSU, it had no inhibitory effects on inflammasome activation by fibrillar synuclein aggregates. In contrast, the well-characterized and highly specific caspase-1 inhibitor, VX-765, effectively blocked NLRP3 activation and IL-1β release by synuclein fibrils. These findings suggest that inflammasome activation triggered by fibrillar synuclein aggregates proceeds by distinct upstream mechanisms compared to traditional NLRP3 activators such as ATP and MSU. Crucially, our results indicate that BHB may not be effective at blocking inflammasome-mediated pathology in the CNS that is driven by misfolded protein aggregates.
Materials and Methods
Primary Microglia Cultures
Postnatal day mouse pups from day P0 to P1 were collected and washed with Dulbecco’s modified Eagle’s medium/F-12 nutrient mixture (DMEM-F12, GIBCO Catalogue number-11320) supplemented with 10% heat-inactivated fetal bovine serum (GIBCO), 50 U/mL penicillin, 50 μg/mL streptomycin, 2 mM l-glutamine, 100 μM non-essential amino acids, and 2 mM sodium pyruvate (Invitrogen). Trypsin (Sigma 0.25%) were added to the brains incubated at 37 °C for 30 min and neutralization of trypsin was done after 30 min by adding equal amount of media. Mouse brains were triturated and passed through a 70 μm nylon mesh cell strainer. Cells were then incubated in a CO2 incubator at 37 °C for 14–16 days. The separation of microglia was performed using a column-free magnetic separation system as previously described (Gordon et al. 2011).
Preparation of Fibrillar Synuclein Aggregates
Recombinant human synuclein was obtained from rPeptide Inc. (Bogart, GA) and in vitro fibril generation was performed by incubation at 37 °C with agitation in an orbital mixer for 5–7 days with sonication used to break down fibrillar aggregates as described previously (Luk et al. 2012; Herva et al. 2014). The generation of fibrillar Syn species was confirmed by transmission electron microscopy and Thioflavin T fluorescence prior to use.
Primary Microglia Treatment for Inflammasome Activation
For inflammasome activation experiments, 1 × 105 cells of primary microglia were plated in 96 well plate and 5 × 105 in 12 well plate with serum-free media overnight and primed with 200 ng/ml of ultrapure LPS for 3 h. Cells were then washed in serum-free medium after priming to remove any residual LPS and stimulated with conventional NLRP3 inflammasome activators ATP (5 mM) and MSU (250 μg/ml) or fibrillar Syn (10 μM) for the indicated time points. BHB was added 15 min prior to NLRP3 activation at a concentration of 10 mM which has been shown to effectively block NLRP3 activation by multiple particulate and soluble activation stimuli (Youm et al. 2015). At the end of treatment, the supernatant was collected and stored at −80 °C until analysis by ELISA, LDH assay or western blotting.
IL-1β and TNFα ELISA
The mouse IL-1β kit (R&D Systems, Catalog # DY008), IL-18 kit (R&D Catalog # S7625) and the BD Opti-EIA TNF kit (Catalog # 558534) were used to measure IL-1β, IL-18 and TNFα levels in the supernatants of activated microglia. Briefly, the capture antibody was diluted to the working concentration PBS without carrier protein and incubated overnight at 4 °C. The following day the plates were blocked with assay diluent (1% BSA or 10% FBS in PBS) for 1 h. Plates were then washed and 100 μl of standards and sample were added to the plates and incubated at room temperature for 2 h. Following a series of incubations and washes, detection antibody was added at the recommended working concentration for 1 h. After a series of washes, 100 μL of the working dilution of streptavidin-HRP were added to each well. The plate was covered and incubated for 20 min at room temperature. Again plates were washed and 100 μL of TMB substrate solution (BD Biosciences) was added to each well and incubated for 30 min at room temperature. Finally 50 μL of stop solution was added to each well and the optical density was determined using a SpectraMax microplate reader (Molecular Devices).
Western Blotting
At the end of each treatment supernatants were collected and concentrated by methanol-chloroform precipitation as previously described (Jakobs et al. 2013). The microglia cell lysates were prepared by using an equal volume of RIPA buffer (Pierce). The protein samples were separated using Bio-Rad 4–20% precast gels and then transferred onto nitrocellulose membranes. Membranes were blocked in the odyssey blocking buffer for 1 h. Primary antibodies for NLRP3 (Adipogen Cryo-2 clone), caspase-1 (Adipogen Casper-1 clone) and IL-1β (R&D Systems) were prepared by at a concentration of 1:000 as specified by the manufacturer. After incubation with Licor IR dye-labelled secondary antibodies (1:10,000) and washing steps, the membranes were scanned using Licor Odyssey CLX imaging system. For densitometric analysis, band intensities were normalized against the GAPDH loading control and represented as the fold change over vehicle-treated controls.
Quantification of Caspase-1 Mediated Pyroptosis
At the end of each treatment, supernatants were collected and LDH release was quantified using an LHD assay kit (TOX7-1KT) as per the manufacturer’s instructions. Caspase-1 dependent LDH release that was inhibited by the highly-specific small molecule caspase-1 inhibitor VX-765 (20 μM), was used as a readout for pyroptosis as previously described (Coll et al. 2015; Bezbradica et al. 2017).
Data Analysis
Statistical analysis was performed using GraphPad Prism 6.0 software. Data are represented as mean ± s.e.m. from at least 3 experiments. ANOVA followed by Tukey’s post-test was performed to compare all treatment groups. ** P < 0.01 and *** P < 0.001 denote statistically significant differences between indicated groups.
Results
BHB Inhibits ATP and MSU-Induced Inflammasome Activation in Primary Microglia
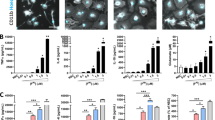
We first determined if BHB can inhibit inflammasome activation in primary microglial cells, by using the well-characterized NLRP3 activators ATP and MSU. These studies were performed to confirm this exciting new paradigm that was recently identified in human and mouse macrophages cells (Youm et al. 2015). ATP treatment caused robust NLRP3 activation and IL-1β release into the supernatant at 1 h in microglia. Consistent with previous results in macrophages, we found that 10 mM BHB effectively blocked NLRP3-mediated IL-1β release in primary murine microglia in response to ATP (Fig. 1a). Similarly, treatment with MSU crystals for 4 h induced significant IL-1β release that was inhibited by BHB treatment (Fig. 1b). We also found that BHB treatment could significantly block the release of the NLRP3-mediated pro-inflammatory cytokine, IL-18, from microglial activated with ATP and MSU (Fig. 1c). To further confirm these findings, we determined the secreted levels of active inflammasome components, cleaved caspase-1 and cleaved IL-1β in the supernatants. Consistent with our ELISA results for IL-1β, we found significantly reduced levels of cleaved caspase-1 and cleaved IL-1β the supernatants of BHB-treated cells (Fig. 1d,e). NLRP3 inflammasome activation by ATP typically induces caspase-1 dependent pyroptosis which involves rapid plasma-membrane rupture and release of pro-inflammatory intracellular contents (Bergsbaken et al. 2009; Zha et al. 2016). By using phase-contrast microscopy we observed that BHB treatment protected microglial cells from NLRP3-dependent pyroptosis. While ATP-treated cells showed typical pyroptotic morphology with signs of extensive plasma-membrane rupture, cells that were treated with BHB had almost normal cellular morphology and limited signs of pyroptotic cell death (Fig. 1f). To further confirm these qualitative findings, we determined caspase-1 dependent LDH release as a quantitative measure of pyroptosis (Coll et al. 2015; Zha et al. 2016; Bezbradica et al. 2017). We found that BHB treatment significantly reduced LDH release caused by ATP stimulation (Fig. 1g) confirming that BHB blocks inflammatory pyroptotic cell death in microglia. Nigericin was used as a positive control with the specific caspase-1 inhibitor VX-765 demonstrating that caspase-1 dependent LDH release upon pyroptosis (Fig. 1g). Collectively these results confirm that BHB can effectively inhibit NLRP3 inflammasome activation induced by ATP and MSU in microglia.
Ketone body Beta hydroxy butyrate inhibits NLRP3 activation by ATP and MSU in primary microglia. (a) Inflammasome activation measured by IL-1β release in primed microglia activated with ATP (5 mM) for 1 h and pre-treated with BHB (10 mM) for 15 min. (b) IL-1β release in primed microglia activated with MSU (250 μg/ml) for 4 h and pre-treated with BHB (10 mM) for 15 min. (c) IL-18 release in primed microglia activated with MSU (5 mM) for 4 h or ATP (5 mM) 1 h and pre-treated with BHB (10 mM) for 15 min (d) Western blot showing cleaved caspase-1 (p20) and cleaved IL-1β in supernatants of microglia treated with ATP and pre-treated with BHB. (e) Densitometric analysis of supernatant cleaved caspase-1 (p20), cleaved IL-1β (p17) western blots (f) Representative images of pyroptotic cellular morphology following ATP treatment in primed microglial cells. (g) LDH release assay for quantification of caspase-1 dependent pyroptosis, showing protection of LDH release in microglia pre-treated with BHB (10 mM) for 15 min and activated with ATP (5 mM). Nigericin (10 μM) treatment for 1 h was used as a positive control with the small molecule capsase-1 inhibitor VX-765 used to confirm caspase-1 dependent LDH-release. Data are represented as mean ± s.e.m. from at least 3 experiments. ***P < 0.001 and **P < 0.01 by one-way ANOVA and Tukey’s post-test
BHB Treatment Does Not Inhibit Microglial Inflammasome Activation Induced by Fibrillar Synuclein Aggregates
Having confirmed that BHB treatment can effectively inhibit inflammasome activation by ATP and MSU in microglia, we next determined the effect of BHB treatment on inflammasome activation induced by pathological fibrillar synuclein aggregates. Previous studies have reported that synuclein fibrils cause delayed, but robust inflammasome activation in THP1 monocytes (Gustot et al. 2015). However, the kinetics and mechanisms by which protein aggregates cause inflammasome activation are markedly different from those of traditional activators such as ATP and MSU and it is unclear if BHB can block inflammasome activation by protein aggregates. Therefore, we treated primed microglia with synuclein fibrils in the presence and absence of 10 mM BHB, the same dose which significantly blocks inflammasome activation by ATP and MSU in microglial cells (Fig. 1). We then evaluated the production of inflammasome-dependent and independent pro-inflammatory mediators that are generated by synuclein activation in microglia. Treatment with fibrillar synuclein resulted in substantial production of TNFα and IL-1β in primary microglia. We found that BHB treatment markedly inhibited the secretion of TNFα (Fig. 2a) which is known to proceed independently of canonical inflammasome activation pathways. Remarkably however, in contrast to our findings with other NLRP3 activators such as ATP and MSU, we found that BHB treatment had no inhibitory effect on synuclein-mediated IL-1β production in primary microglia (Fig. 2b). However, treatment with the specific caspase-1 inhibitor VX-765 almost completely reduced synuclein-mediated IL-1β release under the same conditions. We further confirmed these surprising findings by measuring the release of cleaved caspase-1 (p20) and cleaved IL1β (p17) in the supernatant of activated microglia stimulated with synuclein and BHB. Again, we found that BHB treatment did not inhibit the production of cleaved caspase-1 and cleaved IL1β into the supernatant (Fig. 2c,d). This was however blocked by VX-765 treatment under the same conditions. We also confirmed that both BHB and VX-765 did not change the expression levels of the pro-forms of caspase-1, IL-1β and NLRP3 in microglial cells upon treatment (Fig. 2c). Taken together, our unexpected results demonstrate for the first time that BHB treatment does not inhibit inflammasome activation triggered by pathological synuclein aggregates in microglia.
Beta hydroxy butyrate does not inhibit NLRP3 inflammasome activation by fibrillar synuclein aggregates. (a) ELISA for TNFα showing that synuclein mediated TNF production is substantially reduced by BHB (10 mM) treatment at 24 h. (b) IL-1β levels in the supernatant of microglia activated with synuclein fibrils for 24 h and pre-treated with BHB. (c) Western blot for cleaved caspase-1 (p20) and cleaved IL-1β (p17) levels in the supernatants of primed microglia treated with synuclein fibrils and BHB. (d) Densitometric analysis of cleaved caspase-1 and cleaved IL-1β western blots shown in panel C. Data are represented as mean ± s.e.m. from at least 3 experiments. ***P < 0.001 by one-way ANOVA, and Tukey’s post-test
Discussion
Several lines of evidence now suggest that chronic inflammasome activation by misfolded protein aggregates is a central pathophysiological mechanism in progressive neurodegenerative diseases including Alzheimer’s and Parkinson’s disease (Heneka et al. 2013; Walsh et al. 2014). Indeed NLRP3 knockout mice are protected against neuropathology and cognitive deficits in multiple models of progressive neurodegeneration, making it an attractive therapeutic target (Heneka et al. 2013). BHB has recently emerged as a promising endogenous inhibitor of the NLRP3 inflammasome by multiple activation mechanisms including extracellular ATP as well as particulate activators such as MSU crystals and silica (Youm et al. 2015). Given that BHB readily crosses the blood brain barrier, it could have significant therapeutic potential to mitigate NLRP3-driven neuropathology either through BHB supplements or by increasing endogenous BHB levels through ketogenic diets. However our unexpected results with primary microglia demonstrate that while BHB can efficiently block NLRP3 inflammasome activation by ATP and MSU, it does not inhibit inflammasome activation by fibrillar synuclein aggregates. Mechanistic studies in macrophages have indicated that BHB inhibits the NLRP3 inflammasome independently of Gpr109a or starvation mechanisms and that BHB blocks inflammasome activation by regulating upstream events that reduce K+ efflux (Youm et al. 2015). Our results suggest that inflammasome activation by synuclein fibrils proceeds independently of these upstream events modulated by BHB. Therefore, inflammasome activation triggered by synuclein, and possibly other pathological protein aggregates, occurs via distinct signaling mechanisms compared to traditional NLRP3 activators such as ATP and MSU. In fact, it has been shown that inflammasome activation in microglia by fibrillar beta-amyloid aggregates is NLRP3 and caspase-1 dependent but is independent of purinergic receptor signaling. This could explain the observed decrease in IL-1β following treatment with the specific caspase-1 inhibitor VX-765 in microglia activated with synuclein (Halle et al. 2008). Given that synuclein pathology and spread is considered central to disease progression in PD, our results indicate that BHB supplementation may not have beneficial effects in terms of blocking these key pathological processes during the course of the disease. Our results obtained in microglia however, do not rule out a direct neuroprotective role for BHB in neurons, as previously demonstrated using neurotoxicant models of PD (Kashiwaya et al. 2000; Tieu et al. 2003). However, these previous studies did not evaluate the effects of BHB in a model of synuclein pathology (Luk et al. 2012; Peelaerts et al. 2015). Our unexpected finding that BHB has no inhibitory effect on inflammasome-driven pathology initiated by synuclein aggregates also highlights the critical need for evaluation of promising therapeutic strategies such as BHB using in vivo models of synuclein pathology in addition to standard neurotoxicant models of PD.
References
Bergsbaken T, Fink SL, Cookson BT (2009) Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7:99–109
Bezbradica JS, Coll RC, Schroder K (2017) Sterile signals generate weaker and delayed macrophage NLRP3 inflammasome responses relative to microbial signals. Cell Mol Immunol 14:118–126
Coll RC et al (2015) A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 21:248–255
Gordon R, Hogan CE, Neal ML, Anantharam V, Kanthasamy AG, Kanthasamy A (2011) A simple magnetic separation method for high-yield isolation of pure primary microglia. J Neurosci Methods 194:287–296
Gustin A, Kirchmeyer M, Koncina E, Felten P, Losciuto S, Heurtaux T, Tardivel A, Heuschling P, Dostert C (2015) NLRP3 Inflammasome is expressed and functional in mouse brain microglia but not in astrocytes. PLoS One 10:e0130624
Gustot A, Gallea JI, Sarroukh R, Celej MS, Ruysschaert JM, Raussens V (2015) Amyloid fibrils are the molecular trigger of inflammation in Parkinson's disease. Biochem J 471:323–333
Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT (2008) The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol 9:857–865
Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT (2013) NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature 493:674–678
Heneka MT, Kummer MP, Latz E (2014) Innate immune activation in neurodegenerative disease. Nat Rev Immunol 14:463–477
Heneka MT, Golenbock DT, Latz E (2015) Innate immunity in Alzheimer's disease. Nat Immunol 16:229–236
Herva ME, Zibaee S, Fraser G, Barker RA, Goedert M, Spillantini MG (2014) Anti-amyloid compounds inhibit alpha-synuclein aggregation induced by protein misfolding cyclic amplification (PMCA). J Biol Chem 289:11897–11905
Jakobs C, Bartok E, Kubarenko A, Bauernfeind F, Hornung V (2013) Immunoblotting for active caspase-1. Methods Mol Biol 1040:103–115
Kashiwaya Y, Takeshima T, Mori N, Nakashima K, Clarke K, Veech RL (2000) D-beta-hydroxybutyrate protects neurons in models of Alzheimer's and Parkinson's disease. Proc Natl Acad Sci U S A 97:5440–5444
Luk KC, Kehm V, Carroll J, Zhang B, O'Brien P, Trojanowski JQ, Lee VM (2012) Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338:949–953
Ozawa Y (2010) Neurodegenerative disease: pieces of the Parkinson's puzzle. Nat Rev Neurosci 11:787
Peelaerts W, Bousset L, Van der Perren A, Moskalyuk A, Pulizzi R, Giugliano M, Van den Haute C, Melki R, Baekelandt V (2015) Alpha-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522:340–344
Sulzer D (2007) Multiple hit hypotheses for dopamine neuron loss in Parkinson's disease. Trends Neurosci 30:244–250
Tieu K, Perier C, Caspersen C, Teismann P, Wu DC, Yan SD, Naini A, Vila M, Jackson-Lewis V, Ramasamy R, Przedborski S (2003) D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest 112:892–901
Walsh JG, Muruve DA, Power C (2014) Inflammasomes in the CNS. Nat Rev Neurosci 15:84–97
Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D'Agostino D, Planavsky N, Lupfer C, Kanneganti TD, Kang S, Horvath TL, Fahmy TM, Crawford PA, Biragyn A, Alnemri E, Dixit VD (2015) The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med 21:263–269
Zha QB, Wei HX, Li CG, Liang YD, Xu LH, Bai WJ, Pan H, He XH, Ouyang DY (2016) ATP-induced Inflammasome activation and Pyroptosis is regulated by AMP-activated protein kinase in macrophages. Front Immunol 7:597
Author information
Authors and Affiliations
Contributions
RG conceived the project, designed experiments and supervised the study. VD and EA performed experiments and data analysis with assistance from KZ and RG. RG and VD wrote the manuscript. All authors edited and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Deora, V., Albornoz, E.A., Zhu, K. et al. The Ketone Body β-Hydroxybutyrate Does Not Inhibit Synuclein Mediated Inflammasome Activation in Microglia. J Neuroimmune Pharmacol 12, 568–574 (2017). https://doi.org/10.1007/s11481-017-9754-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-017-9754-5