Abstract
Galectins are a family of β-galactoside-binding lectins that are important modulators of homeostasis in the central nervous system (CNS). Galectin-1 is a pivotal regulator of microglia activation that alters the immune balance from neurodegeneration to neuroprotection and could have therapeutic relevance in HIV associated neurocognitive disorders (HAND). We have previously shown that galectin-1 treatment decreased oxidative stress in microglia and hypothesize that the mechanism underlying this phenomenon is the cross regulatory interactions between Nitric oxide (NO) and Arginase I activity in microglia. We induced microglial activation and examined the effect of galectin-1 on the expression of various M1/M2 microglial phenotypic markers. Since, TNF-α is associated with activation of microglial cells involved in pathogenesis of neurodegenerative diseases, we treated HIV transfected human microglial cell cultures (CHME-5/HIV) with TNF-α followed by treatment with galectin-1, to examine the galectin-1 mediated neuro-modulatory response. Our results show that treatment of CHME-5/HIV microglia with galectin-1 reduced TNF-α induced oxidative stress by ~40%, and also significantly reduced iNOS gene expression and NO production while correspondingly increasing arginase-1, cationic amino acid transporter (CAT-1) gene expression and arginase activity. Galectin-1 treatment results in shifting microglia polarization from M1 toward the beneficial M2 phenotype which may prevent neurodegeneration and promote neuroprotection. Thus, our data suggests that galectin-1 treatment reduces neuroinflammation in the CNS microenvironment via the modulation of the NO-arginase network in microglia and thus could play a neuroprotective role in HAND. Further, the therapeutic potential of galectin-1 could be enhanced by conjugation of galectin-1 onto gold nanoparticles (Au-NP), resulting in a nanogold-galectin-1 (Au-Gal-1) multivalent complex that will have more clinical translational efficacy than free galectin-1 by virtue of increasing the payload influx.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
HIV enters the brain early in the course of infection and a recent report suggests that HIV can replicate and mutate in the brain as early as four months after initial infection (Sturdevant et al. 2015). HIV is sequestered within compartments in the brain forming reservoirs and provides a foundation for neurocognitive impairment resulting in a spectrum of mild to moderate HIV associated neurocognitive disorders (HAND) (Schnell et al. 2010; Valcour et al. 2012; Ances and Ellis 2007; Fischer-Smith and Rappaport 2005). Activation of microglia in HIV infected subjects may be the result of direct infection or their interactions with infected cells and/or viral proteins, which contributes to the development of HAND. HIV-1 encephalitis (HIVE) is also characterized by the presence of infected macrophages and microglia within the brain resulting in astrogliosis and neuronal damage (Nath et al. 1999; Kaul et al. 2001). Both HIVE and HAND have been correlated with microglial activation rather than with viral load (Glass et al. 1995; Gray et al. 2000) suggesting that the key determining factor of neurological impairment may be the activation status of microglia. Microglial activation, thus precedes astroglial activation and neuronal loss (Borgmann and Ghorpade 2015; Rezai-Zadeh et al. 2009; Rock et al. 2004). Activated microglia secrete proinflammatory cytokines, up-regulate surface receptors, generate reactive oxygen species (ROS) and reactive nitrogen species (RNS), secrete excitatory amino acids, and secrete chemokines. These activation products result in activation of other resident cells in the CNS which allows for the recruitment of inflammatory cells into the brain that contribute to neurotoxicity and neuronal apoptosis (Nonaka and Fukuda 2012; Hanisch 2013).
Galectin-1 is a β-galactoside-binding protein that has diverse biological activities and plays an important role in neuroinflammation. Several studies have shown that galectin-1 expression is altered in neurological diseases and is correlated with neuro-regeneration (Wada et al. 2003; McGraw et al. 2005). Increased levels of galectin-1 were observed in patients with neurological disorders as compared to healthy control subjects (Lutomski et al. 1997). Galectin-1 is an important modulator of central nervous system (CNS) homeostasis, contributing to neuroinflammation as well as playing a neuroprotective role (Egnaczyk et al. 2003) depending on host environmental factors. Galectin-1 specifically is known to modulate microglial function in vitro, by attenuating secretion of pro-inflammatory cytokines (TNF-α and nitric oxide (NO)) in response to TNF-α stimulation and enhancing production of anti-inflammatory cytokines such as IL-10 and TGF-β (Almkvist and Karlsson 2004).
In our previous studies, we examined the role of galectin-1 in HIV pathogenesis in the context of drug abuse, wherein, we observed that galectin-HIV interactions contribute to HIV infectivity, and that drugs of abuse such as heroin and morphine significantly potentiate galectin-1 expression facilitating HIV pathogenesis. We showed that silencing galectin-1 gene expression using gold nanorod (GNR)-galectin-1 siRNA complexes significantly attenuated neuro-inflammation in HIV-1 infected monocyte derived macrophages that play a key role in the neuro-pathogenesis of HIV (Reynolds et al. 2006, 2007, 2012a, 2012b). Recent observations show that galectin-1 can regulate microglial function by suppressing the pro-inflammatory responses in several CNS neurodegenerative diseases (Starossom et al. 2012); and can inhibit the proliferation of astrocytes, attenuates astrogliosis and down-regulates the expression of iNOS and IL-1β after cerebral ischemia (Qu et al. 2011). We recently showed that galectin-1 plays a neuroprotective role by maintaining blood brain barrier (BBB) integrity and reversing the neuro-inflammatory effects induced by methamphetamine treatment in brain microvascular endothelial cells that constitute the BBB (Parikh et al. 2015). Therefore, in the current study, we examined the mechanism that may underlie the effect of galectin-1 on the microglial phenotypic switch from M1 to M2, which may signal the initiation of neuroprotection.
The brain is vulnerable to oxidative stress due to its increased dependence on oxidative metabolism for obtaining energy; both ROS and NO produced upon microglia activation contribute to oxidative stress which results in cytotoxic damage to neurons and other CNS cells (Ghasemi and Fatemi 2014). Arginase-1 (Arg-1) is a cytosolic enzyme that catalyzes the hydrolysis of l-arginine to urea and l-ornithine (Morris 2007). Arg-1 competes with inducible nitric oxide synthase (iNOS) for l-arginine, reducing the production of NO. Arg-1 is directly involved in the suppression of inflammation (Campbell et al. 2013; Pesce et al. 2009) and it is anticipated that in CNS disorders, increased Arg-1 expression is correlated with improved outcomes (Fenn et al. 2014; Kroner et al. 2014; Miró-Mur et al. 2015). We hypothesize that galectin-1 is neuroprotective and can inversely regulate iNOS and arginase activities by down-regulating iNOS activity and NO production and up-regulating arginase activity. The current study evaluated the in vitro effects of galectin-1 treatment on TNF-α induced neuroinflammation in HIV transfected primary human microglial cells. Furthermore, we investigated if NO-arginase signaling was the mechanism that underlies galectin-1 mediated neuroprotective effects in HIV-1 infected microglial cells. Our data suggest that galectin-1 is an important regulator of neurodegeneration and neuroprotection, and could thus be an important therapeutic agent/target in the treatment of neuroinflammation associated with HAND and other neurological disorders. In the current study, we observed that galectin-1 was neuroprotective and therefore to enhance the neurotherapeutic potential of galectin-1, we also evaluated if a nanoformulation of galectin-1 could enhance its bioavailability. Our data shows that gold nanoparticle - galectin-1 (Au-Gal-1) nanocomplex significantly enhanced its bioavailability and thus increases its potential for clinical translation as it could provide sustained neuroprotection in the CNS.
Materials and Methods
Cell Culture Conditions
CHME-5/HIV cells (generously provided by Dr. Jonathan Karn, CWRU, Cleveland, OH) are primary human microglial cells immortalized with SV40 T antigen (CHME-5 cells) that were co-transfected with an HIV LTR reporter and the HIV Tat gene. The CHME-5 cell line was originally created by transfecting human fetal microglia with SV40 T antigen (Janabi et al. 1995) and is used as an experimental control. The cell line was maintained in Dulbecco’s Minimal Essential Medium, high glucose (DMEM HG) supplemented with 5% fetal bovine serum (Cat #16140071, FBS, qualified, heat inactivated, US origin, ThermoFisher Scientific, Grand Island, NY) and 1% penicillin/streptomycin (Cat # 10378016, Penicillin-Streptomycin-Glutamine (100X), ThermoFisher Scientific, Grand Island, NY).
CHME-5/HIV (3 × 10(Block et al. 2007) cells /ml) were cultured in a 6 well tissue culture plate overnight and grown to 80% confluency, cells were subsequently treated with TNF-α (50 ng/ml, Cat # P01375, Recombinant Human TNF-α, R & D Systems Minneapolis, MN) overnight, followed by treatment with or without galectin-1 (1 μM, Cat # 1152-GA Recombinant Human Galectin-1 R & D Systems Minneapolis, MN) in triplicate for 24 h. To evaluate the specificity of the galectin-1 mediated modulation of arginase-1 we treated cells with the irreversible Arginase inhibitor (2S)-(+)-Amino-5-iodoacetamidopentanoic acid (10 μM Cat # 35748–65-3; Santacruz Biotech Inc.). Cells were washed in phosphate buffer saline (PBS), harvested and used for gene expression studies/activity assays. Untreated CHME-5/HIV cells were used as controls. 50 ng/ml TNF-α was used for stimulation and activation of HIV transcription in CHME-5/HIV cells as previously described (Wires et al. 2012). Activated cells were then treated with 1 μM galectin-1 for 24 h and TUNEL staining was done to quantitate microglial apoptosis. 1 μM galectin-1 was chosen as an optimal in vitro concentration for all experiments based on our earlier previous studies (Parikh et al. 2015) and other reports in literature that have used similar or higher doses in vitro (Zanon et al. 2015; Correa et al. 2003). Galectin-1 has a biphasic modulation on cell growth, depending on the in vitro dose used. High doses of recombinant galectin-1 inhibit cell proliferation while low doses can be mitogenic (Zanon et al. 2015, Fischer et al. 2005). The galectin-1 dose we used in vitro was nontoxic with greater than 95% cell viability of microglial cells observed using the MTS assay.
Cell Viability/ MTS Assay
CHME-5/HIV (10,000 cells/100 μL/well) were plated in a 96 well culture plate and were treated with TNF-α (50 ng/ml) overnight followed by treatment with or without 1 μM galectin-1 for 24 h in triplicate. Cell viability was assessed using a CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay (MTS assay) (Cat # G5421, Promega Corporation, Madison WI). Viability was measured 24 h post-treatment by adding 10 μL of 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H–tetrazolium (MTS)/phenazine ethosulfate (PMS) solution. Plates were incubated at 37 °C for 2 h in the presence of the MTS/PMS stable solution, and read at an absorbance of 490 nm using a BioTeK (BioTeK U. S, Winooski, VT) plate reader.
RNA Extraction
Cytoplasmic RNA was extracted using Trizol reagent (ThermoFisher Scientific, Grand Island, NY). The amount of RNA was quantitated using a Nano-Drop ND-1000 spectrophotometer (Nano-Drop™, Wilmington, DE) and isolated RNA was stored at –80 °C until used.
Synthesis of Gold Nanoparticle-Galectin-1 Complex
To extend the bioactivity of Galectin-1 over a longer time period, Galectin-1 was complexed with Gold nanoparticles to form a Gold Nanoparticle-Galectin-1 Complex (Au-Gal-1). Briefly, GNPs (19.1 nm) dispersed in water were purchased from Biotool.com (Houston, TX, Cat # 710106), their peak SPR was 514–520 nm, with a size dispersity (%PDI) of <10% and concentration equivalent to 2.5 mg/ml. Recombinant Galectin-1 protein (10 μg) (R&D systems Cat # 1152-GA/ CF (carrier free) which was reconstituted in sterile 1 ml 1X PBS was added to 1 mL of gold nanoparticle solution while stirring and the mixture gently stirred for 10 min. The solution was then centrifuged at 12,000 g at 4 °C for 30 min to remove unbound excess proteins and the pellet was resuspended in 1X PBS. The protein concentration in the supernatant was measured using a micro BCA assay kit (Thermo Scientific Pierce, Rockford, IL, USA) and the amount of protein bound to the surface of gold nanoparticles was calculated using the following formula: Protein bound to gold nanoparticles = Total protein added – protein remaining in the supernatant solution (Jiang et al. 2008).
Measurement of ROS and RNS
ROS and RNS are well established molecules responsible for the deleterious effects of oxidative stress. Accumulation of free radicals coupled with an increase in oxidative stress has been implicated in the pathogenesis of HAND (Louboutin and Strayer 2014). We measured total free radicals present in CHME-5/HIV cells treated with 50 ng/ml TNF-α and/or 1 μM galectin-1 in cell lysates using a commercially available in vitro ROS/RNS Assay Kit (Cat # STA-347, Cell Biolabs Inc., San Diego, CA) that measures total ROS and RNS, including hydrogen peroxide, nitric oxide, peroxyl radical, and peroxynitrite anion. The assay employs a proprietary quenched fluorogenic probe, dichlorodihydrofluorescin DiOxyQ (DCFH-DiOxyQ), which is a specific ROS/RNS probe that is based on similar chemistry to 2′, 7′-dichlorodihydrofluorescein diacetate. Briefly, the DCFH-DiOxyQ probe is first primed with a quench removal reagent, and subsequently stabilized in the highly reactive DCFH form. In this reactive state, ROS and RNS species react with DCFH, which is rapidly oxidized to the highly fluorescent 2′, 7′-dichlorodihydrofluorescein (DCF). The amount of DCF in the sample is determined based on the relative fluorescence units (RFU) obtained using a DCF standard curve and the fluorescence intensity obtained is proportional to the total ROS/RNS levels within the sample. The detection sensitivity limit is 10 pM for DCF and 40 nM for hydrogen peroxide.
Real Time, Quantitative PCR (Q-PCR)
Q-PCR was used to quantitate iNOS, CAT-1, and Arg −1 gene expression in CHME5/ HIV cultures. Approximately 1 × 10(Borgmann and Ghorpade 2015) cells/ml CHME5/HIV were treated with 50 ng/ml TNF-α overnight followed by treatment with or without 1 μM galectin-1 for 24 h. RNA was extracted then reverse transcribed to cDNA using a reverse transcriptase kit (Promega Inc., Madison, WI). Relative abundance of each mRNA species was quantitated by Q-PCR using specific primers and the Brilliant® SYBR® green Q-PCR master mix (Stratagene Inc., La Jolla, CA). The following were primer sequences used for Q-PCR: iNOS, forward primer 5′ TCCAGAAGCAGAATGTGACC-3′, reverse primer 5′-GGACCAGCCAAATCCAGT-3′, CAT-1, forward primer 5′-CCAACGTCAATGATAGGACC-3′, reverse primer 5′-CTGGTCCAGCTGCATCATGA-3′; Arg-1, forward primer 5′-CTTAAAGAACAAGAGTGTGATG-3′, reverse primer 5′-TTCTTCCTAGTAGATAGCTGAG-3′; CD86, forward primer 5′-GACCGTTGTGTGTGTTCTGG-3′, reverse primer 5′-GATGAGCAGCATCACAAGGA-3′; Jak1, forward primer 5′-CTCTCTGTCACAACCTCTTCGC-3′ reverse primer 5′- TTGGTAAAGTAGAACCTCATGCG-3′; Stat3, forward primer 5′- CACCTTGGATTGAGAGTCAAGAC-3′ reverse primer 5′- AGGAATCGGCTATATTGCTGGT-3′; IL-1β, forward primer 5′- CACAGCAGCACATCAACAAG-3; reverse primer 5′- GTGCTCATGTCCTCATCCTG-3′ and β-actin, (house-keeping gene) forward primer 5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′, reverse primer 5′-AGTCATAGTCCGCCTAGAAGCATTTGCGGT-3. To provide precise quantification of the initial target in each PCR reaction, the amplification plot was examined. Relative expression of mRNA species for each gene of interest was calculated using the comparative threshold cycle number (CT) method (Bustin 2002; Radonić et al. 2004). Briefly, for each sample, a difference in CT values (∆CT) is calculated for each mRNA by taking the mean CT of duplicate tubes and subtracting the mean CT of the duplicate tubes for the reference RNA (β-actin) measured on an aliquot from the same RT reaction. The ∆CT for the treated sample is then subtracted from the ∆CT for the untreated control sample to generate a ∆∆CT. The mean of these ∆∆CT measurements is then used to calculate the levels in the targeted cytoplasmic RNA relative to the reference gene and normalized to the control as follows: Relative levels or Transcript Accumulation Index =2-∆∆CT. This calculation assumes that all PCR reactions are working with 100% efficiency. All PCR efficiencies were found to be >95%; therefore, this assumption introduces minimal error into the calculations. All data were controlled for quantity of RNA input and by performing measurements on an endogenous reference gene, β-actin.
Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick End Labeling Assay
DNA fragmentation represents a characteristic hallmark of apoptosis. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) is an established method for detecting DNA fragments. The TUNEL assay was performed to visualize DNA damage in CHME-5/HIV cells. Briefly, 1 × 104 cells CHME-5/HIV were cultured in 35 mm culture dishes with glass bottom wells and were treated with 50 ng/ml TNF-α overnight followed by treatment with 1 μM galectin-1 for 24 h. Untreated cells were used as controls. Cells were fixed with 4% methanol-free paraformaldehyde in PBS for 10 min at RT. After fixation, cells were washed with PBS, permeabilized with a 0.2% Triton X-100 solution for 5 min, and washed twice in PBS, then 100 μL of equilibration buffer was added at RT and incubated for 5–10 min. Samples were washed with PBS and incubated with terminal deoxynucleotidyl transferase, recombinant (rTdT) buffer at 37 °C for 60 min inside the humidified chamber according to the manufacturer’s protocol (Biotool.com; CAT # B31115 TUNEL Apo-Green Detection Kit). The reaction was terminated by adding 100 μL of SSC for 15 min. The wells were washed 3 times, using PBS for 5 min to remove unincorporated fluorescein-12-dUTP nucleotides. Fragmented DNA was examined under an inverted fluorescence microscope (Carl Zeiss). For each sample, the total number of cells and the number of TUNEL-positive cells were quantified in 10 representative fields. The results were presented as a representation from a series of three separate experiments.
NO Colorimetric Assay Kit
CHME-5/HIV were treated with 50 ng/ml TNF-α overnight followed by treatment with or without 1 μM galectin-1 for 3–24 h and then the supernatant was harvested to analyze NO production using BioVision’s Nitric Oxide Colorimetric Assay kit (Cat # K262–200, BioVision, Inc. Milpitas, CA). The NO Colorimetric Assay kit provides an accurate, convenient measure of total nitrate/nitrite in a simple two-step process. The first step converts nitrate to nitrite utilizing nitrate reductase. The second step uses Griess Reagents to convert nitrite to a deep purple azo compound. The amount of the azochromophore accurately reflects nitric oxide amount in samples. The absorbance of the mixture was detected with an enzyme-linked immunosorbent assay (ELISA) at an optical density (OD) of 530 nm, with sodium nitrite as the standard. The detection limit of the assay is approximately 1.0 nmole nitrite/well, or 10 μM.
Arginase Activity Colorimetric Assay Kit
CHME-5/HIV were treated with 50 ng/ml TNF-α overnight followed by treatment with or without 1 μM galectin-1 for 24 h. Cells were harvested and lysed. Arginase activity was quantitated in the cell lysates using an Arginase Activity assay kit (Cat # K755–100, BioVision, Inc. Milpitas, CA). Arginase converts L-arginine into urea and L-ornithine and regulates arginine/ornithine concentration. Arginase activity in the sample is quantitated when arginase reacts with arginine and undergoes a series of reactions to form an intermediate that reacts stoichiometrically with OxiRed™ Probe to generate the colored product that can be colorimetrically detected at OD 570 nm. The sensitivity of the Arginase activity assay is less than 0.2 U/L.
Evaluating Bioactivity of Galectin-1 Using a Chemotaxis Assay
Cell migration assay kit (Trevigen, Gaithersburg, MD Cat # Catalog# 3465–024-K) with polyethylene terephthalate (PET) membrane filters of 8-μm membrane pores was used. Briefly, Serum starved 1 × 105/ml THP-1 cells (monocytic cell line THP1; ATCC® TIB202™) in 100 ul RPMI media and 0.1% FBS were incubated with AuNP, Gal-1 (10 μg/ml), Au NP-BSA (10μg/ml) and Au-NP Gal-1 (10μg/ml) for 30 min at 37 °C. Just before start of the chemotaxis assay, human RANTES (100 ng/ml) (R&D Systems) was added to 500 ul media placed in the bottom well of the Chemotaxis chamber, and the polyethylene terephthalate (PET) membrane filters was placed on top. A 100 μl of THP-1 cells were incubated with AuNP, Gal-1 (10 μg/ml), Au NP-BSA (10μg/ml) and Au-NP Gal-1 (10μg/ml) as described above were then placed on top of the filter. Plates were incubated for 3, 24 or 48 h in a humidified incubator at 37 °C with 5% CO2. At the end of the incubation period, cells remaining on top of the filter were absorbed off and the filter tops were carefully washed to ensure removal of nonmigrated cells. We aspirated bottom chamber, and wash each bottom well with 500 μl 1X Wash Buffer. 500 μl of cell dissociation solution containing Calcein-AM is added to the bottom chamber, and the PET filter cell migration device reassembled, and incubated at 37 °C for incubator for one hour, after which the inserts are tapped to ensure al the cells are dissociated into the lower chamber, the insert is removed and the plate read in a fluorescent plate reader at 485 nm excitation, and 520 nm emission to obtain relative fluorescence units (RFU). A standard curve was initially done to determine the RFU corresponding to increasing cell numbers. The percentage cell migration is then determine for each treatment condition based on number of cells migrated across the filter as determined by the RFU and comparisons are made between the media control vs treated samples.
Immunofluorescent Staining
CHME-5/HIV microglia are grown to 70% confluence in a glass bottom petri dish and treated with TNF-α (50 ng/ml) overnight, followed by treatment with or without galectin-1 (1 μM) for 24 h. Standard immuno fluorescent staining procedures were followed. Briefly, CHME-5/HIV microglia are then fixed for 10 min at 37 °C in 4% formaldehyde, followed by permabilization with ice-cold 100% methanol. Cells are then washed in 1X phosphate buffered saline (PBS) and treated with the following antibodies; anti-human Arginase-1 rabbit polyclonal antibody (Cat # GTX109242 GeneTex, Inc. Irvine, CA), anti human IL-1β rabbit polyclonal antibody (Cat # GTX109242 GeneTex, Inc. Irvine, CA) and anti-human Stat3 rabbit polyclonal antibody (Cat# GTX110587 GeneTex, Inc. Irvive CA). The secondary antibodies used were goat anti-Rabbit IgG (H + L) Secondary Antibody, Alexa Fluor 488 (green color) (Cat # A-11008, Molecular Probes Inc., Life Technologies, Grand Island, NY) and Goat anti-Rabbit IgG (H + L) Secondary Antibody, Cy5 (red color) (Cat # A10523, Molecular Probes Inc., Life Technologies, Grand Island, NY). DAPI (blue color) (Cat #D1306 Molecular Probes Inc., Life Technologies, Grand Island, NY) was used as the nuclear stain. The expression levels of Arginase-1, IL-1β and Stat-3 were quantitated based on the intensity of the fluorescent signal analyzed using the computer image analysis image J software (National Institutes of Health, Bethesda, MA, USA). Imaging was performed with the EVOS® FL Cell Imaging System (Life Technologies, Grand Island, NY).
Flow Cytometry Analysis
CHME-5/HIV microglia were treated with TNF-α (50 ng/ml) overnight at 37 °C, following which cells were fixed in 2% paraformaldehyde and permeabilized in 100% ice-cold methanol. Cells were washed twice in staining buffer (DPBS pH 7.2 with 0.2% BSA and 0.09% sodium azide) and labeled for flow cytometry. Flow cytometry antibodies used include PE conjugated Anti-Human CD86 (Clone IT2.2) (Cat # 12–0869-42; eBioscience Inc. San Diego, CA), a primary anti-human Stat3 rabbit polyclonal antibody (Cat# GTX110587 GeneTex, Inc. Irvive CA), Cy5 labelled Goat anti-Rabbit IgG (H + L) Secondary Antibody (Catalog#: A10523, ThermoFisher Scientific/Invitrogen) and fluorochrome-conjugated isotype control antibodies. Cells were stained for 30 min at room temperature, washed, and analyzed by flow cytometry (BD Biosciences LSRII Fortessa). Samples were gated on intact microglial cells by forward light scatter (FSC) vs right-angle light scatter (SSC). Data were acquired and analyzed with FlowJo software.
Statistical Analysis
All data are expressed as means ± SD and analyzed using Graphpad Prism software. Statistical analysis was done using an analysis of variance (One-way ANOVA) and comparisons were made between treated samples and untreated controls. A statistical significant difference was accepted when p < 0.05.
Results
Galectin-1 Treatment Decreased TNF-α Induced Neuroinflammatory Response
TNF-α stimulation of CHME-5/HIV cells resulted in microglial activation, which is a hallmark of increased neuroinflammation, and triggers cell apoptosis. We used TUNEL staining (Fig. 1a, b, c and d) to detect apoptotic cells that undergo extensive DNA degradation during the late stages of apoptosis in TNF-α stimulated CHME-5/HIV cells. Our results show that TNF-α stimulation induced DNA degradation and cell apoptosis as indicated by the increased TUNEL staining (Fig. 1b) and galectin-1 treatment significantly decreased cell apoptosis (Fig. 1c). Figure 1a shows the unstimulated control showing minimal TUNEL staining indicating no apoptosis. Image J quantitation of fluorescence signal intensity in pixel units indicates a significant increase (92%; p < 0.01) in TUNEL staining on TNF-α stimulation and a significant decrease (84%; p < 0.01) in the TUNEL staining in galectin-1 treated CHME-5/HIV cells. Statistical comparisons were made between TNF-α stimulated of CHME-5/HIV microglial cells that were treated with galectin-1 vs those not treated with galectin-1. These data demonstrate that TNF-α induced cell apoptosis, and subsequent galectin-1 treatment resulted in a reduction in cell apoptosis suggesting that galectin-1 plays a neuroprotective role in microglial cells.
a-c Effect of galectin-1 on cell apoptosis as measured by TUNEL staining in TNF-α stimulated HIV transfected microglial cells (CHME-5/HIV). DNA fragmentation was detected using a TUNEL Apo-Green Detection Kit. The number of TUNEL-positive cells were quantified in 10 representative fields. Representative images show b-c increased TUNEL staining in TNF-α treated CHME-5/HIV cells, which was significantly decreased by treatment with galectin-1 as compared to the unstimulated control a. Imaging was performed with the EVOS® FL Cell Imaging System. d The results presented in the histogram are a representation of three separate experiments and show the Image J quantitation of fluorescence signal intensity in pixel units
Galectin-1 Treatment Decreases ROS/RNS Activity
Treatment of CHME-5/HIV cells with TNF-α results in increased oxidative stress. ROS include superoxide (O2), hydrogen peroxide (H2O2) and hydroxyl radical (OH), which under physiological conditions are generated at low levels and play important roles in signaling and metabolic pathways. However, increased oxidative stress due to TNF-α treatment results in the generation of ROS which are potentially neurotoxic for microglia, contributing to neuroinflammation. We measured the total free radical presence using a commercially available in vitro ROS Assay Kit and our results (Fig. 2) show that treatment of CHME5/HIV with 50 ng/ml TNF-α increased ROS activity (1579 ± 111.7 RFU; p < 0.01) as compared to the untreated control (797.3 ± 103.1 RFU). Treatment of CHME5/HIV cells with 1 μM galectin-1 resulted in ROS values of 842.4 ± 103.7 RFU which were not significantly different than the untreated control (797.3 ± 103.1 RFU). Treatment of the TNF-α stimulated CHME5/HIV cells with 1 μM galectin-1 decreased oxidative stress by 40% (943.3 ± 142.4 RFU; p < 0.01). These data demonstrate that galectin-1 significantly reduces oxidative stress induced by TNF-α treatment.
Effect of Galectin-1 on ROS production. CHME5/HIV cells were treated with TNF-α (50 ng/ml) overnight followed by treatment with galectin-1 (1 μM) for 24 h. Cells were lysed and the total free radical presence in cell lysates was measured using a commercially available in vitro ROS Assay Kit. Our data show treatment of CHME5/HIV cells with TNF-α (50 ng/ml) resulted in an increase in oxidative stress as compared to the untreated control. Treatment of CHME5/HIV cells with galectin-1 (1 μM) decreased oxidative stress when compared to TNF-α treated cells. Results are expressed as the mean ± SD from n = 3 separate experiments. A p value of <0.05 was considered statistically significant
Galectin-1 Treatment Decreases NO Production
An important molecule released by activated glial cells is bioactive free radical NO which acts as both a neuromodulator and a neurotransmitter. Excess production of NO by glial cells causes neuronal cell injury/death (Louboutin and Strayer 2014; Lull and Block 2010). We speculate that Galectin-1 downregulates the classic metabolic pathway of L-arginine by inhibition NO production and iNOS expression, and enhances arginase activity via the alternative metabolic pathway of L-arginine. To this effect, we examined the time kinetics of the effect of galectin-1 treatment on NO production by CHME-5/HIV cells over a 3, 6, 12 and 24 h time period. Additionally, we used an irreversible arginase inhibitor (2S)-(+)-Amino-5-iodoacetamidopentanoic acid to evaluate the specificity of the galectin-1 mediated modulation of arginase expression in CHME-5/HIV cells.
Our results, (Fig. 3) expressed as Nitrate μM units, show that at 3 h TNF-α treatment increased NO production (66% increase) in CHME5/HIV microglia (86.34 ± 1.78 μM; p < 0.0001) as compared to untreated controls (30.85 ± 0.64 μM). Treatment with 1 μM galectin-1 and arginase inhibitor alone showed a slight, but insignificant increase in NO production (42.14 ± 1.2 μM; and 43.7 ± 0.97 μM respectively) as compared to the untreated controls (30.85 ± 0.64 μM). However, the TNF-α induced CHME5/HIV microglia where treated with galectin-1(1 μM) a significant inhibition (83% decrease, P < 0.0001) in NO production (15.14 ± 0.32 vs 86.34 ± 1.78 μM) was observed. Addition of the arginase inhibitor (10 μM), to the TNF-α induced CHME5/HIV microglia treated with galectin-1 (1 μM) resulted in marginal increase in NO production (19.75 ± 0.77 μM).
Effect of galectin-1 on NO production. CHME5/HIV cells were stimulated with TNF-α (50 ng/ml) overnight followed by treatment with galectin-1 (1 μM) for 3, 6, 12 and 24 h. Cells were lysed and NO levels were measured using commercially available Griess Reagent kit. Our data show that TNF-α treatment significantly increased NO production expression in CHME5/HIV cells and galectin-1 treatment significantly decreased NO production when compared to the TNF-α treated cells. Our data shows that galectin-1 significantly decreased NO production as early as 3 h post treatment and the effect was consistent over a period of 24 h with a plateau reached at about 12 h. post treatment. Results are expressed as the mean ± SD from n = 3 separate experiments. A p value of <0.05 was considered statistically significant
At 6 h, TNF-α treatment increased NO production (56% increase) in CHME5/HIV microglia (79.684 ± 2.01 μM; p < 0.002) as compared to untreated controls (35.29 ± 0.73 μM). Treatment with 1 μM galectin-1 and arginase inhibitor alone showed a slight, but insignificant increase in NO production (43.5 ± 1.19 μM; and 43.17 ± 1.11 μM respectively) as compared to the untreated controls (35.29 ± 0.73 μM). However, the TNF-α induced CHME5/HIV microglia where treated with galectin-1(1 μM) a significant inhibition (76% decrease, P < 0.0001) in NO production (19.58 ± 1.55 vs 79.684 ± 2.01 μM) was observed. Addition of the arginase inhibitor (10 μM), to the TNF-α induced CHME5/HIV microglia treated with galectin-1 (1 μM) resulted in marginal increase in NO production (25.31 ± 0.32 μM).
At 12 h, TNF-α treatment increased NO production (60% increase) in CHME5/HIV microglia (83.89 ± 1.73 μM; p < 0.0001) as compared to untreated controls (33.84 ± 0.70 μM). Treatment with 1 μM galectin-1 and arginase inhibitor alone showed a slight, but insignificant increase in NO production (47.2 ± 1.42 μM; and 41.33 ± 1.69 μM respectively) as compared to the untreated controls (33.84 ± 0.70 μM). The TNF-α induced CHME5/HIV microglia that where treated with galectin-1(1 μM) showed a significant inhibition (77% decrease, P < 0.0001) in NO production (20.70 ± 1.23 vs 83.89 ± 1.73 μM). Addition of the arginase inhibitor (10 μM), to the TNF-α induced CHME5/HIV microglia treated with galectin-1 (1 μM) resulted in significant increase in NO production (32.96 ± 1.38 μM).
At 24 h, TNF-α treatment increased NO production (56% increase) in CHME5/HIV microglia (81.45 ± 1.70 μM; p < 0.0001) as compared to untreated controls (32.86 ± 0.69 μM). Treatment with 1 μM galectin-1 and arginase inhibitor alone showed a slight, but insignificant increase in NO production (41.0 ± 1.05 μM; and 43.03 ± 1.22 μM respectively) as compared to the untreated controls (32.86 ± 0.69 μM). However, the TNF-α induced CHME5/HIV microglia where treated with galectin-1(1 μM) a significant inhibition (73% decrease, P < 0.0001) in NO production (22.07 ± 1.55 vs 81.45 ± 1.70 μM) was observed. Addition of the arginase inhibitor (10 μM), to the TNF-α induced CHME5/HIV microglia treated with galectin-1 (1 μM) resulted in marginal increase in NO production (32.01 ± 1.41 μM). Our data shows that galectin-1 significantly decreased NO production as early as 3 h post treatment and the effect was consistant over a period of 24 h with a plateau reached at about 12 h post treatment.
Galectin-1 Treatment Decreases iNOS Gene Expression
Co-induction of iNOS and arginase is a mechanism to limit NO production and to limit neuroinflammation. (Chicione et al. 2011). TNF-α stimulation of CHME-5/HIV cells significantly increased iNOS gene expression and treatment with galectin-1 inhibited iNOS gene expression in these microglial cells. Our results show that TNF-α treatment increased iNOS gene expression by 290% in CHME5/HIV microglia (TAI = 3.9 ± 0.81 vs 1.00 ± 0.005; p < 0.0001) as compared to their respective untreated controls (Fig. 4). Treatment of the TNF-α stimulated CHME-5/HIV cells with 1 μM galectin-1 resulted in a 66% decrease in CHME5/HIV cells (TAI = 1.33 ± 0.47 vs 3.9 ± 0.81; p < 0.01) respectively when compared to the TNF-α stimulated iNOS gene expression levels (Fig. 4). Treatment with 1 μM galectin-1 alone in CHME5/HIV cells showed iNOS gene expression levels comparable to the untreated control (TAI = 0.97 ± 0.27 vs 1.00 ± 0.005; p = NS).
Effect of Galectin-1 on iNOS gene expression. CHME5/HIV cells were stimulated with TNF-α (50 ng/ml) overnight followed by treatment with galectin-1 (1 μM) for 24 h, RNA was extracted, reverse transcribed and the gene expression levels of iNOS were measured using real time Q-PCR. Data demonstrate that TNF-α treatment significantly increased iNOS gene expression in CHME5/HIV cells and galectin-1 significantly decreased iNOS gene expression as compared to the TNF-α treated cells. Results are expressed as the mean ± SD from n = 3 separate experiments. A p value of <0.05 was considered statistically significant difference
Galectin-1 Treatment Decreases Arg-1 Gene Expression
Arginase and iNOS share a common substrate, L-arginine. It has been reported that inhibition of iNOS increased urea production, while inhibiting arginase expression increased NO production (Peranzoni et al. 2007; Munder 2009; Nieves and Langkamp-Henken 2002). Our immunofluorescence data shows that an overnight TNF-α stimulation of CHME-5/HIV cells significantly increases Arg-1 gene expression and additional treatment with galectin-1 (1 μM) further increased Arg-1 gene expression in CHME-5/HIV microglial cells (Fig. 5a, b). Our gene expression results show that TNF-α treatment increased Arg-1 gene expression by 190% in CHME5/HIV microglia (TAI = 2.9 ± 0.79 vs 1.00 ± 0.03; p < 0.0001) as compared to their respective untreated controls (Fig. 5c). Treatment of the TNF-α stimulated CHME-5/HIV cells with 1 μM galectin-1 resulted in a 151% (TAI = 4.38 ± 1.43 vs 2.9 ± 0.79; p < 0.0001) increase in Arg-1 gene expression in CHME5/HIV cells when compared to the Arg-1 gene expression levels obtained on TNF-α stimulation (Fig. 5c). Treatment with 1 μM galectin-1 alone in CHME5/HIV cells showed Arg-1 gene expression levels comparable to the untreated control (TAI = 1.19 ± 0.17 vs 1.00 ± 0.03; p = NS).
Effect of Galectin-1 on Arginase-1 expression. a CHME5/HIV microglial cells grown to 70% confluence on glass bottom petri dishes were stimulated with TNF-α (50 ng/ml) overnight followed by treatment with b galectin-1 (1 μM) for 24 h. Immunofluorescent staining done to determine Arginase −1 expression levels in microglial cells stimulated with TNF-α (50 ng/ml) with or without Galectin-1 treatment. Our data suggests that Galectin-1 treatment increased arginase −1 expression. c CHME5/HIV cells were stimulated with TNF-α (50 ng/ml) overnight followed by treatment with galectin-1 (1 μM) for 24 h. RNA was extracted, reverse transcribed and the gene expression levels of Arginase-1 were measured using real time Q-PCR. Data demonstrate that TNF-α treatment significantly increased Arginase-1 gene expression in CHME5/HIV cells and treatment with galectin-1 further significantly increased iNOS gene expression as compared to the TNF-α treated cells. Results are expressed as the mean ± SD from n = 3 separate experiments. A p value of <0.05 was considered statistically significant difference
Galectin-1 Increases Arginase Activity
The current paradigm on the interaction between arginase and iNOS in the context neuroinflammation suggests that increased NOS and arginase activity results in competition between these two metabolic pathways for the common substrate, L-arginine. We measured arginase activity in cells lysates from CHME-5/HIV cells treated with TNF-α and/or galectin-using a commercially available arginase activity assay kit. Our results show that treatment of CHME-5/HIV cells with TNF-α increased arginase activity. Our results (Fig. 6) show that treatment of CHME5/HIV with 50 ng/ml TNF-α increased arginase activity (33%) (133.03 ± 2.79 mU/μl; p = 0.004) as compared to the untreated control (100.05 ± 0.07 mU/μl). Treatment of the TNF-α stimulated CHME5/HIV cells with 1 μM galectin-1 further increased arginase activity (61%) (341.0 ± 15.56 mU/μl; p = 0.003) in CHME5/HIV cells. Treatment with 1 μM galectin-1 alone showed significant increase in arginase activity (168.67 ± 5.19 mU/μl) as compared to the untreated controls (100.05 ± 0.07 mU/μl). Treatment with arginase inhibitor (10 μM) alone showed decrease in arginase activity with mean values of 45.68 ± 3.00 mU/μl (p = 0.002) significantly lower than the untreated controls (100.05 ± 0.07 mU/μl). Addition of the arginase inhibitor (10 μM), to the TNF-α induced CHME5/HIV microglia treated with galectin-1 (1 μM) resulted in decrease in arginase activity [206.5 ± 4.95 mU/μl (p = 0.007)]. A significant increase in arginase activity i.e. 208.0 ± 4.41 mU/μl (p = 0.018) when compared to galectin-1 alone, was observed when comparison was made between unstimulated CHME5/HIV microglia treated with galectin-1 (1 μM) alone and galectin-1 plus arginase inhibitor treated cells. These data suggests that galectin-1 enhances arginase activity and that these effects are reversed by use of an arginase inhibitor.
Effect of Galectin-1 on Arginase activity. CHME5/HIV cells were treated with TNF-α (50 ng/ml) overnight followed by treatment with 1 μM galectin-1 for 24 h. Cells were lysed and the total arginase activity was measured in cell lysates using a commercially available arginase assay kit. Data demonstrate that treatment of CHME5/HIV cells with TNF-α increases arginase activity as compared to the untreated control. Treatment of CHME5/HIV cells with galectin-1 (1 μM) further increased arginase activity when compared to TNF-α treated cells. The galectin-1 mediated increase in Arginase-1 activity significantly decreased on treatment with an irreversible arginase inhibitor(2S)-(+)-Amino-5-iodoacetamidopentanoic acid (10 μM). Results are expressed as the mean ± SD from n = 3 separate experiments. For individual treatments, statistically comparisons were made with the untreated control, and for combination treatments comparisons were made with the appropriate relevant control. A p value of <0.05 was considered statistically significant
Galectin-1 Increases Cationic Amino Acid Transporter (CAT-1) Gene Expression
CAT-mediated L-arginine transport limits both iNOS-derived NO production as well as arginase activity (Rath et al. 2014). We observed a significant increase in CAT-1 gene expression synonymous with increased arginine activity when treated with galectin-1. Our data demonstrate that TNF-α stimulation increased CAT-1 gene expression by 96% in CHME5/HIV microglia (TAI = 1.96 ± 0.22 vs 1.00 ± 0.17; p < 0.01), further treatment of TNF-α stimulated CHME5/HIV microglia with 1 μM galectin-1 increased CAT-1 gene expression by 214% (TAI = 4.2 ± 0.93 vs 1.96 ± 0.22; p < 0.001) in CHME5/HIV cells (Fig. 7). Treatment with 1 μM galectin-1 alone in CHME5/HIV cells showed CAT-1 gene expression levels comparable to the untreated control (TAI = 1.2 ± 0.19 vs 1.00 ± 0.17; p = NS). These data demonstrate that galectin-1 increases arginine uptake by increasing CAT-1 gene expression and modulates the levels of NOS and NO production which reverses TNF-α induced oxidative stress.
Effect of Galectin-1 on CAT-1 expression. CHME5/HIV cells were stimulated with TNF-α (50 ng/ml) overnight followed by treatment with galectin-1 (1 μM) for 24 h. RNA was extracted, reverse transcribed and the gene expression levels of CAT-1 were measured using real time Q-PCR. Data demonstrate that TNF-α treatment significantly increased CAT-1 gene expression in CHME5/HIV cells and additional treatment with galectin-1 further significantly increased CAT-1 gene expression as compared to the TNF-α treated cells. Results are expressed as the mean ± SD from n = 3 separate experiments. A p value of <0.05 was considered statistically significant difference
Effect of Galectin-1 Treatment on M1 Vs M2 Microglial Phenotype
Microglial activation is associated with neuroinflammatory response. The activation states of microglia are categorized as M1 pro-inflammatory phenotype that facilitates neurodegeneration and an M2 anti-inflammatory phenotype that facilitates neuroprotection. Since the morphological changes in microglia are associated with their functional activities, we evaluated the effect of galectin-1 on the microglial phenotype using immunofluorescence, the gene and protein expression of specific M1/M2 microglial markers. On comparative analysis of M1 and M2 specific phenotypic markers, our immunofluorescence results (Fig. 8a, b) shows an increased expression of IL-1β in stimulated CHME5/HIV microglia not treated with galectin-1, and an increased expression of STAT-3 in TNF-α stimulated CHME5/HIV microglia treated with galectin-1. Figure 8c shows relative gene expression data showing increased expression of CD86 and IL-1β in TNF-α stimulated CHME5/HIV microglia and increased expression of Jak1 and STAT-3 in TNF-α stimulated CHME5/HIV microglia treated with galectin-1. Our flow cytometric analysis results show that treatment with galectin-1 resulted in augmentation of the M2 phenotype (Figs. 9 and 10).
Effect of Galectin on M1/M2 phenotypic markers in stimulated CHME5/HIV microglial cells. CHME5/HIV microglial cells grown to 70% confluence on glass bottom petri dishes were stimulated with TNF-α (50 ng/ml) overnight followed by treatment with Galectin-1. TNF-α (50 ng/ml) stimulated cells not treated with Galectin were used as the comparator. Microglia are then fixed for 10 min at 37 °C in 4% formaldehyde, followed by permabilization with ice-cold 100% methanol. Immunofluorescent staining done to determine the expression levels of IL1β and STAT-3 in TNF-α stimulated with and without galectin-1 treatment. Our data shows that treatment with galectin-1 resulted in a transition from an M1 to M2 microglial phenotype. a TNF-α (50 ng/ml) stimulated microglia showing M1 phenotype as indicated by increased IL-1β staining (green color) and moderate STAT-3 staining (red color). c TNF-α stimulated microglia treated with galectin-1 (1 μM) for 24 h, showing a predominantly M2 phenotype as indicated by decreased IL-1β staining (green color) and increased STAT-3 staining (red color). DAPI (blue color) was used as the nuclear stain. c To confirm the transition of TNF-α stimulated CHME5/HIV microglia from an M1 to M2 phenotype on galectin-1 treatment, gene expression studies were done. CHME5/HIV cells were stimulated overnight with TNF-α (50 ng/ml) and treated with galectin-1 for 24 h. RNA was extracted, reverse transcribed and the gene expression levels of CD86, IL-1β, Jak1 and STAT-3 were quantitated using real time Q-PCR. Our results demonstrate that galectin-1 treatment resulted in an increased expression of M2 phenotypic markers such as Jak1 and STAT-3 while microglia that were not treated with galectin-1 expressed significantly higher CD86, IL-1β, gene expression levels indicative of M1 phenotype. Results are expressed as the mean ± SD from n = 3 separate experiments. A p value of <0.05 was considered statistically significant difference
Flow cytometry analysis of CD86 expression on CHME5/HIV microglial cells. CHME5/HIV cells were stimulated overnight with TNF-α (50 ng/ml) and treated with galectin-1 (1 μM) for 24 h. CHME5/HIV microglia were stained with CD86 (PE). a Unstained microglia (inlet showing gated microglia) b Unstained TNF-α stimulated microglia c Isotype control d Untreated control e TNF-α stimulated f galectin-1 treated TNF-α stimulated. Data shows that CD86 is the biomarker for the M1 phenotype and that galectin-1 treatment decreased CD86 expression. Results shown are representative images of three experiments with similar results
Flow cytometry analysis of STAT-3 expression on CHME5/HIV microglial cells. CHME5/HIV cells were stimulated overnight with TNF-α (50 ng/ml) and treated with galectin-1 (1 μM) for 24 h. CHME5/HIV microglia were stained with STAT-3 (Cy5). a Unstained microglia (inlet showing gated microglia) b Unstained TNF-α stimulated microglia c Isotype control d Untreated control e TNF-α stimulated f galectin-1 treated TNF-α stimulated. Data shows that STAT-3 is the biomarker for the M2 phenotype and that galectin-1 treatment increases STAT-3 expression. Results shown are representative images of three experiments with similar results
Absorption of Galectin-1 onto Gold Nanoparticles Allows Increased Bioavailability of Galectin-1
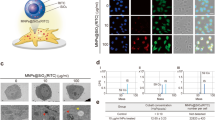
A simple nanoparticle-ligand complex of gold nanoparticles and galectin-1 (AuNP-Gal1) was formed in which recombinant human galectin-1 was conjugated onto a commercially available gold nanoparticle of 19.1 nm size, whose surface plasmon resonance (SPR) peak was between 514 and 520 nm (Fig. 11a). Transmission electron microscopy (TEM) demonstrated that AuNP-Gal1 had a uniform spherical structure and size of approximately 21 nm (Fig. 11b) and a polydispersity index (PDI) of <10% indicating homogeneity of size. Absorption of galectin-1 onto the surface of the Au-NP allows greater bioavailability of galectin-1 as compared to free galectin-1. Our results showed that 74% of the total galectin-1 added to the AuNP complexation process, bound to the Au-NP, while negligible amount of BSA bound to the Au-NP and was used as a control to determine binding efficiency of galectin-1. The specificity of galectin-1 bioactivity was confirmed using the chemotaxis assay, were we expected galectin-1 to limit cell migration. We examined the effect of AuNP- Gal-1 complex and free galectin-1 on monocytic cell migration in a chemotaxis assay and our data showed that the AuNP-Gal1 complex showed a 47% (p < 0.01), 81% (p < 0.003) and 86% (p < 0.001) decrease in cell migration as compared to free galectin-1 over an time period of 3, 24 and 48 h respectively (Fig. 11c).
a-b. Characterization of Au-NP: a Surface plasmon resonance (SPR) peak of Au NP is between 514 and 520 nm. b Transmission electron microscopy (TEM) of AuNP-Gal1 shows uniform spherical structure and size of approximately 21 nm and a polydispersity index (PDI) of <10% indicating homogeneity of size. c Validation of the biological effects of AuNP-Gal-1 in a commercial chemotaxis assay. THP-1 cells were incubated with AuNP, Gal-1 (10 μg/ml), Au NP-BSA (10μg/ml) (control) and Au-NP Gal-1 (10μg/ml) for 30mins at 37 °C and then added to the top of the PET filter. Plates were incubated for 3, 24 or 48 h in a humidified incubator at 37 °C with 5% CO2. At the end of the incubation period, cells migrated to the bottom of the inserts were dissociated into solution containing Calcein-AM and fluorescence measured at 485 nm excitation, and 520 nm emission to obtain relative fluorescence units (RFU) and the percentage cell migration calculated based on comparisons with the media control. Results are expressed as the mean ± SD from n = 3 separate experiments. A p value of <0.05 was considered statistically significant
Discussion
Several members of the galectin family are expressed by cells of the CNS and are believed to be important modulators of CNS homeostasis and neuroinflammation (Chen et al. 2014; Starossom et al. 2012; Nonaka and Fukuda 2012). Galectins regulate microglial activation through modulation of signaling pathways and may prevent neurodegeneration and promote neuroprotection. Microglia, serve crucial functions as the scavenger system of the CNS and facilitate repair and homeostasis in the CNS. Chronic activation of microglia can result in neuronal apoptosis and neurodegeneration which precedes astroglial activation and neuronal loss. Microglia become “activated” by inflammatory mediators such as secretion of pro-inflammatory cytokines, up-regulating surface receptors, activation of reactive oxygen species and nitrogen intermediates, excitatory amino acids, and chemokine modulation, contributing to a wide range of CNS pathologies, by including progression of HAND. A recent report suggested that galectin-1 regulates the polarization of microglia in vitro and in vivo after cerebral ischemia or after exposure to TNF-α (Tang and Le 2016). Microglia have two distinct phenotypes, M1 and M2. M1 microglia release pro-inflammatory cytokines that facilitate the neurodegeneration process, while M2 microglia release anti-inflammatory mediators that facilitate neuroprotection (Saijo and Glass 2011; Guang Luo and Chen 2012; Cherry et al. 2014; Tang and Le 2016).
Galectins are a family of glycan-binding proteins or ‘lectins’ wherein the extracellular lectin-glycan interactions function by trapping glycoprotein receptors at the cell surface and subsequent modulation of downstream cell signaling events, thereby promoting cell proliferation and survival (Boscher et al. 2011; Lau et al. 2007; Partridge et al. 2004). Interactions between galectins and glycoproteins are largely multivalent and, therefore, depend on critical glycan concentrations (Dam et al. 2005; Lee and Lee 2000). The glycoproteins are central to biological effects of the galectins, as they include cytokine receptors, nutrient transporters and adhesion receptors, all of which are crucial sensors of the physiological environment at the cell surface. The physiological effects of galectins are thus complex and vary depending on local concentration, intracellular or extracellular localization, and also on the cell type. Lower concentrations of galectin-1 inhibit Th1 responses, and this effect is diminished or lost at high concentrations, while intracellular and extracellular galectin concentration may prevent or induce cell apoptosis (Barrionuevo et al. 2007). There is also substantial cross-talk between various members of the galectin family during an inflammatory response and both HIV-1 and drug abuse can contribute to activation of pro-inflammatory and pro-apoptotic genes which can present a major obstacle to promoting neuroprotection and galectin-1 can down regulate the expression levels of these genes, thereby regulating immune balance from neurodegeneration to neuroprotection (Barrionuevo et al. 2007; Sato et al. 2012).
In conclusion, loss of Gal-1 leads to an abrogation of CG induced second-phase edema in the mouse paw. This reduced inflammatory response is associated with decreased expression of iNOS and IL-1β, in conjunction with an increased expression of TGFβ and Gal-9 and increased apoptosis of infiltrated leukocytes. We wish to speculate that a Gal-1/Gal-9 cross talk may be operative during the course of a resolving model of acute inflammation, a feature that may also have repercussions in chronic settings, though future studies will address this hypothesis in detail.
In the context of HIV-1 infection, galectin-1 promotes infection by HIV-1 by mediating viral attachment to host cell surface glycans; it enhances HIV-1 adsorption kinetics on monocyte-derived host macrophages, which facilitates HIV-1 infectivity by shortening the time required to establish an infection (Ouellet et al. 2005, 2015; St –Pierre et al. 2010, 2012; Mercier et al. 2008; Reynolds et al. 2012a, b). However, given that galectin-1 is a pivotal regulator of microglial activation, it may play a crucial role in controlling inflammatory responses and maintaining CNS homeostasis (Aalinkeel and Mahajan 2016). We have recently shown that the brain microvascular endothelial cells of the BBB release galectin-1. When the integrity of the BBB is compromised under neuroinflammatory conditions, galectin-1 modulates an inflammatory response and confers a remodeling capacity in a compromised BBB (Parikh et al. 2015). The goal of our study was thus to evaluate if galectin-1 reduces TNF-α induced neuroinflammation in HIV-1 transfected microglial cell line CHME5/HIV and examine if the NO-arginase signaling network mediates these neuroprotective effects. These studies will highlight signaling pathways that impact microglial cell dysfunction and outline the role of galectin-1 in mediating CNS homeostasis and limiting neurotoxicity.
TNF-α stimulation increases phagocytic activity of microglia, and is associated with activation of microglial cells involved in the pathogenesis of neurodegenerative diseases (Lull and Block 2010). We observed that TNF-α treatment increased microglial activation in CHME5/HIV cells (data not shown) which facilitates cell apoptosis. We quantitated cell apoptosis by TUNEL staining (Fig. 1a, b and c) in TNF-α treated CHME5/HIV microglial cells and observed a significant decrease in TUNEL positive cells when these cells were treated with galectin-1 indicating that galectin-1 is able to limit the neuroinflammatory response. Recent studies also suggested that galectin-1 regulates microglial function by preferentially binding to and deactivating M1 microglia thereby suppressing the proinflammatory responses associated with CNS demyelinating diseases (Saijo and Glass 2011; Guang Luo and Chen 2012; Cherry et al. 2014; Tang and Le 2016). Galectin-1 can not only modulate the levels of pro-inflammatory cytokines such as IFN-γ and TNF-α, but can also increases the release of anti-inflammatory cytokines such as IL-10 and IL-4 which can result in preferential transition of microglia to M2 phenotype contributing to CNS homeostasis (Cherry et al. 2014; Tang and Le 2016).
Recent studies suggest that alterations in the expression levels of Arg I and iNOS in the peripheral T cells and peripheral nodes of HIV infected patients are associated with HIV disease progression (Zhang et al. 2016). However not much is known about the impact of the NO-arginase signaling network in the neuropathogenesis of HIV. Data from this study shows that galectin-1 can regulate the expression levels of Arg I and iNOS in microglia and it is likely that these neuromodulatory effects will have a significant impact on neuroprotection. L-arginine can regulate immune response and is a substrate of Arg I, and NOS (Rath et al. 2014). Arg I is involved inflammatory response and hydrolyzes L-arginine into L-ornithine and urea, and NOS catalyzes the conversion of L-arginine into citrulline and NO, which is also an important effector and regulator of immune function (Munder 2009). Arg I regulates the bioavailability of L-arginine, which is involved in inflammation-triggered immune dysfunction, immunosuppression and immune responses to infectious agents (Peranzoni et al. 2007; Munder 2009; Nieves and Langkamp-Henken 2002). We observed that TNF-α stimulation increased NO production and iNOS and Arg 1 gene expression in CHME5/HIV microglia, and treatment with galectin-1 reversed the TNF- α effects by significantly decreasing NO production, iNOS and Arg-1 gene expression. Our data demonstrate that microglial activation increases iNOS-derived NO production, which activates pro-apoptotic pathways such as the caspases, which increase cell apoptosis as observed by TUNEL staining. Since iNOS is a stress response protein its upregulation in response to an inflammatory stimulus in microglial cells is expected. On the other hand, arginases are believed to be the first step in the generation of proline and polyamines, which are crucial for cell proliferation. Polyamines are critical mediators of cell growth and division because of their capacity to bind directly to DNA and to modulate DNA-protein interactions (Peranzoni et al. 2007). Induction of arginase in response to an inflammatory stimulus is thus vital for CNS repair after neuronal injury (Nieves and Langkamp-Henken 2002). Thus it is not surprising that a TNF-α induced proinflammatory stimulus results in increased levels of iNOS, arginase 1 and NO in CHME5/HIV microglia. Our observation that galectin-1 significantly decreases iNOS expression and NO production suggests that targeting galectin-1 may represent a new approach to treat neuroinflammation.
Activated microglia can induce significant and highly detrimental neurotoxic effects by the excess production of a large array of cytotoxic factors such as superoxide, NO. Although microglial activation is necessary and crucial for host defense and neuron survival, the over activation of microglia results in deleterious and neurotoxic consequences (Block et al. 2007). Intracellular ROS is crucial for both microglial pro-inflammatory function and microglial survival and acts as second messenger capable of modifying gene expression through effects on kinase cascades and transcription factor activation as we and others have previously shown, thus increased levels of intracellular ROS resulted in neuroinflammation, microglial apoptosis and neurotoxic consequences. (Parikh et al. 2015; Lull and Block 2010; Block et al. 2007; Rojo et al. 2014). We observed that TNF-α treatment significantly increased ROS/RNS activity (Fig. 1e). ROS under physiological conditions are generated at low levels and play important roles in signaling and metabolic pathways. Increased oxidative stress as a consequence of stimulation results in the generation of ROS, which is potentially toxic to microglial cells further contributing to neuroinflammation. Classic microglial activation results in the production of both extracellular and intracellular ROS. The ability of galectin-1 to decrease oxidative stress in CHME-5/HIV cells may prevent these microglia from progressing to apoptosis and reduce neurodegradation in the CNS.
L-arginine is transported into cells via the cationic amino acid transporters (CAT) (Cui et al. 2011). Arginine uptake is mediated by CAT-1 and coincides with induction of NOS for the production of NO (Cui et al. 2011; Zani and Bohlen 2005; Chicione et al. 2011). We observed a significant increase in CAT-1 gene expression (Fig. 2d) synonymous with increased arginine activity when treated with galectin-1 (Fig. 2c). We believe that CAT-mediated L-arginine transport can limit both iNOS-derived NO production as well as arginase activity. Galectin-1 is emerging as one of these effectors of endogenous anti-inflammation. Galectin-1 can also affect arginase indirectly by acting as a general anti-inflammatory agent. Arginase plays a regulatory role in NO production and although arginase and iNOS catalyze the common substrate L-arginine, their metabolilites may have opposing biological effects (Chang et al. 1998, 2000). Several endogenous compounds that mediate inflammation modulate arginase activity, and most of them also affect NO production (Sonoki et al. 1997). Galectin-1 could be released during an inflammatory response to achieve homeostasis by autocrine or paracrine mechanisms. Galectin-1 downregulates the classic metabolic pathway of L-arginine by inhibition NO production and iNOS expression, and enhances arginase activity via the alternative metabolic pathway of L-arginine. Elucidating the role for Galectin-1 in inflammation provides an alternative cellular mechanism to understand the immunoregulatory effects of this carbohydrate-binding protein and help delineate its use as a novel therapeutic strategy in chronic neuroinflammatory processes.
In conclusion, microglia play an important role in several neurodegenerative diseases but their role in HIV neuropathogenesis is critical as microglia are a source of latent HIV (Borgmann and Ghorpade 2015; Rezai-Zadeh et al. 2009; Rock et al. 2004). In our study, TNF-α treatment caused an expected neuroinflammatory response in HIV-1 transfected CHME5 microglia, which resulted in microglial activation and induction of iNOS, arginase I, CAT-1 expression leading to an increase in NO production and decreased cellular proliferation and activation of cell apoptosis. Our results indicate that galectin-1 may be neuroprotective, therefore, manipulation of endogenous galectin-1 levels in the CNS could improve the neuroinflammation associated with HAND and may be a novel therapeutic target to modulate cell proliferation and/or apoptosis in the CNS cells. Our study also highlights the importance of M1/M2 dynamics in neuroinflammation. Differences in carbohydrate specificities among various member of the galectin family can give rise to opposing effects on neuropathogenesis (Leffler et al. 2004). Our data confirm that Galectin-1 treatment results in shifting microglia polarization from M1 toward the beneficial M2 phenotype which may prevent neurodegeneration and promote neuroprotection.
The translational potential of galectin-1 as a neurotherapeutic could be enhanced if its bioavailability is increased and a nanoformulation of Galectin-1 will improve the clinical translational potential of this biomolecule. We synthesized a galectin-AuNP nanocomplex, and evaluated its efficacy as a potential nanotherapeutic by confirming the specificity of its galectin-1 bioactivity using a chemotaxis assay. The results of our chemotaxis assay suggests that the ability of galectin-1 to form a nanocomplex with the AuNP, not only increased its bioavailability, but also potentially increased sensitivity by virtue of the increased number of galectin-1 protein molecules that can bind to a single gold nanoparticle and therefore showed a significantly limited in cell migration as compared to free galectin-1. The galectin-1 protein contains free cysteine residues with the potential to form covalent bonds, and dimerization can also occur via the formation of noncovalent bonds forms by extended β-sheet interactions across two monomeric subunits and is bioactive in both monomeric and dimeric forms. This ability of galectin-1 to form covalent and non-covalent bonds enhances its ability to complex with not only with gold nanoparticles, but also other types of nanoparticles, enhancing its efficacy as a potential nano-neurotherapeutic.
References
Aalinkeel R, Mahajan SD (2016) Neuroprotective role of galectin-1 in CNS pathophysiology associated with HIV-associated neurocognitive disorders (HAND). Neural regeneration research. Neural Regen Res 11(6):896–897
Almkvist J, Karlsson A (2004) Galectins as inflammatory mediators. Glycoconj J 19:575–581
Ances BM, Ellis RJ (2007) Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol 27:86–92
Barrionuevo P, Beigier-Bompadre M, Ilarregui JM, Toscano MA, Bianco GA, Isturiz MA, Rabinovich GA (2007) A novel function for galectin-1 at the crossroad of innate and adaptive immunity: galectin-1 regulates monocyte/macrophage physiology through a nonapoptotic ERK-dependent pathway. J Immunol 178:436–445
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8:57–69
Borgmann K, Ghorpade A (2015) HIV-1, methamphetamine and astrocytes at neuroinflammatory crossroads. Front Microbiol 6:1143
Boscher C, Dennis JW, Nabi IR (2011) Glycosylation, galectins and cellular signaling. Curr Opin Cell Biol 23:383–392
Bustin SA (2002) Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol 29(1):23–39
Campbell L, Saville CR, Murray PJ, Cruickshank SM, Hardman MJ (2013) Local arginase 1 activity is required for cutaneous wound healing. J Invest Dermatol 133:2461–2470
Chang C, Liao J, Kuo L (1998) Arginase modulates nitric oxide production in activated macrophages. Am J Physiol Heart Circ Physiol 274:342–348
Chang C, Zoghi B, Liao JC, Kuo L (2000) The involvement of tyrosine kinases, cyclic AMP/protein kinase a, and p38 mitogenactivated protein kinase in IL-13-mediated arginase I induction in macrophages: its implications in IL-13-inhibited nitric oxide production. J Immunol 165:2134–2141
Chen HL, Liao F, Lin TN, Liu FT (2014) Galectins and neuroinflammation. Adv Neurobiol 9:517–542
Cherry JD, Olschowka JA, O’Banion MK (2014) Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation 11:98
Chicione LG, Stenger MR, Cui H, Calvert A, Evans RJ, Keith English B, Liu Y, Nelin LD (2011) Nitric oxide suppression of cellular proliferation depends on cationic amino acid transporter activity in cytokine-stimulated pulmonary endothelial cells. Am J Phys Lung Cell Mol Phys 300(4):L596–L604
Correa SG, Sotomayor CE, Aoki MP, Maldonado CA, Rabinovich GA (2003) Opposite effects of galectin-1 on alternative metabolic pathways of L-arginine in resident, inflammatory, and activated macrophages. Glycobiology 13(2):119–128
Cui H, Chen B, Chicoine LG, Nelin LD (2011) Overexpression of cationic amino acid transporter-1 increases nitric oxide production in hypoxic human pulmonary microvascular endothelial cells. Clin Exp Pharmacol Physiol 38(12):796–803
Dam TK, Gabius H-J, André S, Kaltner H, Lensch M, Brewer CF (2005) Galectins bind to the multivalent glycoprotein asialofetuin with enhanced affinities and a gradient of decreasing binding constants. Biochemistry 44:12564–12571
Egnaczyk GF, Pomonis JD, Schmidt JA, Rogers SD, Peters C, Ghilardi JR, Mantyh PW, Maggio JE (2003) Proteomic analysis of the reactive phenotype of astrocytes following endothelin-1 exposure. Proteomics 3:689–398
Fenn AM, Hall JCE, Gensel JC, Popovich PG, Godbout JP (2014) IL-4 signaling drives a unique arginase+/IL-1β + microglia phenotype and recruits macrophages to the inflammatory CNS: consequences of age-related deficits in IL-4Rα after traumatic spinal cord injury. J Neurosci 34:8904–8917
Fischer C, Sanchez-Ruderisch H, Welzel M, Wiedenmann B, Sakai T, Andre S, Gabius HJ, Khachigian L, Detjen KM, Rosewicz S (2005) Galectin-1 interacts with the {alpha}5{beta}1 fibronectin receptor to restrict carcinoma cell growth via induction of p21 and p27. J Biol Chem 280:37266–37277
Fischer-Smith T, Rappaport J (2005) Evolving paradigms in the pathogenesis of HIV-1-associated dementia. Expert Rev Mol Med 7:1–26. doi:10.1017/s1462399405010239
Ghasemi M, Fatemi A (2014) Pathologic role of glial nitric oxide in adult and pediatric neuroinflammatory diseases. Neurosci Biobehav Rev 45:168–182
Glass JD, Fedor H, Wesselingh SL, McArthur JC (1995) Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol 38:755–762
Gray F, Adle-Biassette H, Brion F, Ereau T, le Maner I, Levy V, Corcket G (2000) Neuronal apoptosis in human immunodeficiency virus infection. J Neurovirol 6(Suppl 1):S38–S43
Guang Luo X, Chen SD (2012) The changing phenotype of microglia from homeostasis to disease. Transl Neurosci 1:9. doi:10.1186/2047-9158-1-9
Hanisch UK (2013) Functional diversity of microglia – how heterogeneous are they to begin with? Front Cell Neurosci 7:65
Janabi N, Peudenier S, Héron B, Ng KH, Tardieu M (1995) Establishment of human microglial cell lines after transfection of primary cultures of embryonic microglial cells with the SV40 large T antigen. Neurosci Lett 195(2):105–108
Jiang W, Kim BY, Rutka JT, Chan WC (2008) Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol 3:145–150
Kaul M, Garden GA, Lipton SA (2001) Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410:988–994
Kroner A, Greenhalgh AD, Zarruk JG, Passos dos Santos R, Gaestel M, David S (2014) TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron 83:1098–1116
Lau KS, Partridge EA, Grigorian A, Silvescu CI, Reinhold VN, Demetriou M, Dennis JW (2007) Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell 129:123–134
Lee RT, Lee YC (2000) Affinity enhancement by multivalent lectin–carbohydrate interaction. Glycoconj J 17:543–551
Leffler H, Carlsson S, Hedlund M, Qian Y, Poirier F (2004) Introduction to galectins. Glycocon 19:433–440
Louboutin J-P, Strayer D (2014) Role of oxidative stress in HIV-1-associated neurocognitive disorder and protection by Gene delivery of antioxidant enzymes. Antioxidants 3(4):770–797
Lull ME, Block ML (2010) Microglial activation & chronic neurodegeneration. Neurotherapeutics. 7(4):354–365
Lutomski D, Fouillit M, Bourin P, Mellottee D, Denize N, Pontet M, Bladier D, Caron M, Joubert-Caron R (1997) Externalization and binding of galectin-1 on cell surface of K562 cells upon erythroid differentiation. Glycobiology 7:1193–1199
McGraw J, Gaudet AD, Oschipok LW, Kadoya T, Horie H, Steeves JD, Tetzlaff W, Ramer MS (2005) Regulation of neuronal and glial galectin-1 expression by peripheral and central axotomy of rat primary afferent neurons. Exp Neurol 195:103–114
Mercier S, St-Pierre C, Pelletier I, Ouellet M, Tremblay MJ, Sato S (2008) Galectin-1 promotes HIV-1 infectivity in macrophages through stabilization of viral adsorption. Virology 371:121–129
Miró-Mur F, Pérez-de-Puig I, Ferrer-Ferrer M, Urra X, Justicia C, Chamorro A, Planas AM (2015) Immature monocytes recruited to the ischemic mouse brain differentiate into macrophages with features of alternative activation. Brain Behav Immun 48:18–33
Morris SM (2007) Arginine metabolism: boundaries of our knowledge. J Nutr 137:1602S–1609S
Munder M (2009) Arginase: an emerging key player in the mammalian immune system. Br J Pharmacol 158:638–651
Nath A, Conant K, Chen P, Scott C, Major EO (1999) Transient exposure to HIV-1 tat protein results in cytokine production in macrophages and astrocytes. A hit and run phenomenon. J Biol Chem 274:17098–17102
Nieves CJ Jr, Langkamp-Henken B (2002) Arginine and immunity: a unique perspective. Biomed Pharmacother 56:471–482
Nonaka M, Fukuda M (2012) Galectin-1 for neuroprotection? Immunity 37(2):187–189
Ouellet MS, Pelletier I, Bounou S, Roy J, Hirabayashi J, Sato S, Tremblay MJ (2005) Galectin-1 acts as a soluble host factor that promotes HIV-1 infectivity through stabilization of virus attachment to host cells. J Immunol 174:4120–4126
Ouellet M, St-Pierre C, Tremblay MJ, Sato S (2015) Effect of galectins on viral transmission. Methods Mol Biol 1207:397–420
Parikh NU, Aalinkeel R, Reynolds JL, Nair BB, Sykes DE, Mammen MJ, Schwartz SA, Mahajan SD (2015) Galectin-1 suppresses methamphetamine induced neuroinflammation in human brain microvascular endothelial cells: Neuroprotective role in maintaining blood brain barrier integrity. Brain Res 1624:175–187
Partridge EA, Le Roy C, Di Guglielmo GM, Pawling J, Cheung P, Granovsky M, Nabi IR, Wrana JL, Dennis JW (2004) Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science 306:120–124
Peranzoni E, Marigo I, Dolcetti L, Ugel S, Sonda N, Taschin E, Mantelli B, Bronte V, Zanovello P (2007) Role of arginine metabolism in immunity and immunopathology. Immunobiology 212:795–812
Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, Smith AM, Thompson RW, Cheever AW, Murray PJ, Wynn TA (2009) Arginase-1 expressing macrophages suppress Th2 cytokine driven inflammation and fibrosis. PLoS Pathog 5:e1000371
Qu WS, Wang YH, Ma JF, Tian DS, Zhang Q, Pan DJ, Yu ZY, Xie MJ, Wang JP, Wang W (2011) Galectin-1 attenuates astrogliosis-associated injuries and improves recovery of rats following focal cerebral ischemia. J Neurochem 116(2):217–226
Radonić A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A (2004) Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun 313(4):856–862
Rath M, Müller I, Kropf P, Closs EI, Munder M (2014) Metabolism via arginase or nitric oxide synthase: two competing arginine pathways in macrophages. Front Immunol 5:532
Reynolds JL, Mahajan SD, Sykes D, Nair MP (2006) Heroin-induces differential protein expression by normal human astrocytes (NHA). Am J Infect Dis 2(2):49–57
Reynolds JL, Mahajan SD, Sykes DE, Schwartz SA, Nair MP (2007) Proteomic analyses of methamphetamine (METH)-induced differential protein expression by immature dendritic cells (IDC). Biochim Biophys Acta 1774(4):433–442
Reynolds JL, Law WC, Mahajan SD, Aalinkeel R, Nair B, Sykes DE, Mammen MJ, Yong KT, Hui R, Prasad PN, Schwartz SA (2012a) Morphine and galectin-1 modulate HIV-1 infection of human monocyte-derived macrophages. J Immunol 188(8):3757–3765
Reynolds JL, Law WC, Mahajan SD, Aalinkeel R, Nair B, Sykes DE, Yong KT, Hui R, Prasad PN, Schwartz SA (2012b) Nanoparticle based galectin-1 gene silencing, implications in methamphetamine regulation of HIV-1 infection in monocyte derived macrophages. J NeuroImmune Pharmacol 7(3):673–685
Rezai-Zadeh K, Gate D, Town T (2009) CNS infiltration of peripheral immune cells: D-day for neurodegenerative disease? J NeuroImmune Pharmacol 4(4):462–475. doi:10.1007/s11481-009-9166-2
Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, Peterson PK (2004) Role of microglia in central nervous system infections. Clin Microbiol Rev 17(4):942–964
Rojo AI, McBean G, Cindric M, Egea J, López MG, Rada P, … Cuadrado A (2014) Redox control of microglial function: molecular mechanisms and functional significance. Antioxid Redox Signal, 21(12):1766–1801
Saijo K, Glass CK (2011) Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol 11:775–787. doi:10.1038/nri3086
Sato S, Ouellet M, St-Pierre C, Tremblay MJ (2012) Glycans, galectins, and HIV-1 infection. Ann N Y Acad Sci 1253:133–148
Schnell G, Price RW, Swanstrom R, Spudich S (2010) Compartmentalization and clonal amplification of HIV-1 variants in the cerebrospinal fluid during primary infection. J Virol 84:2395–2407. doi:10.1128/JVI.01863-09
Sonoki T, Nagasaki A, Gotoh T, Takiguchi M, Takeya M, Matsuzaki H, Mori M (1997) Co-induction of nitric-oxide synthase and arginase I in cultured rat peritoneal macrophages and rat tissues in vivo by lipopolysaccharide. J Biol Chem 272:3689–3693
Starossom SC, Mascanfroni ID, Imitola J, Cao L, Raddassi K, Hernandez S, Bassil R, Croci DO, Cerliani JP, Delacour D, Wang Y, Elyaman W, Khoury SJ, Rabinovich GA (2012) Galectin-1 deactivates classically-activated microglia and protects from inflammation-induced neurodegeneration. Immunity 37(2):249–263
St-Pierre C, Ouellet M, Tremblay MJ, Sato S (2010) Galectin-1 and HIV-. Methods Enzymol 480:267–294
St-Pierre C, Ouellet M, Giguère D, Ohtake R, Roy R, Sato S, Tremblay MJ (2012) Galectin-1-specific inhibitors as a new class of compounds to treat HIV-1 infection. Antimicrob Agents Chemother 56(1):154–162
Sturdevant CB, Joseph SB, Schnell G, Price RW, Swanstrom R, Spudich S (2015) Compartmentalized replication of R5 T cell-tropic HIV-1 in the central nervous system early in the course of infection. PLoS Pathog 11(3):e1004720. doi:10.1371/journal.ppat.1004720
Tang Y, Le W (2016) Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol 53:1181–1194
Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S et al (2012) Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis 206:275–282
Wada M, Ono S, Kadoya T, Kawanami T, Kurita K, Kato T (2003) Decreased galectin-1 immunoreactivity of the skin in amyotrophic lateral sclerosis. J Neurol Sci 208:67–70
Wires ES, Alvarez D, Dobrowolski C, Wang Y, Morales M, Karn J, Harvey BK (2012) Methamphetamine activates nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and induces human immunodeficiency virus (HIV) transcription in human microglial cells. J Neurovirol 18(5):400–410
Zani BG, Bohlen HG (2005) Transport of extracellular l-arginine via cationic amino acid transporter is required during in vivo endothelial nitric oxide production. Am J Physiol Heart Circ Physiol 289(4):H1381–H1390
Zanon CF, Sonehara NM, Girol AP, Gil CD, Oliani SM (2015) Protective effects of the galectin-1 protein on in vivo and in vitro models of ocular inflammation. Mol Vis 21:1036–1050
Zhang N, Deng J, Wu F, Lu X, Huang L, Zhao M (2016) Expression of arginase I and inducible nitric oxide synthase in the peripheral blood and lymph nodes of HIV-positive patients. Mol Med Rep 13(1):731–743. doi:10.3892/mmr.2015.4601
Acknowledgements
The authors who like to acknowledge the funding support from the Dr. Louis Sklarow Memorial Trust 2015-2016 (Mahajan SD) and the New York State Department of Health (DOH) funded Empire Clinical Research Investigator Program (ECRIP) (Schwartz SA). The authors are extremely grateful to Dr. Jonathan Karn, Chairman and Reinberger Professor of Molecular Biology, Case Western Reserve University, Cleveland, Ohio and Director of the CASE Center for AIDS Research for the generous donation of the CHME-5/HIV cell line.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
Authors declare no conflict of interests.
Rights and permissions
About this article
Cite this article
Aalinkeel, R., Mangum, C.S., Abou-Jaoude, E. et al. Galectin-1 Reduces Neuroinflammation via Modulation of Nitric Oxide-Arginase Signaling in HIV-1 Transfected Microglia: a Gold Nanoparticle-Galectin-1 “Nanoplex” a Possible Neurotherapeutic?. J Neuroimmune Pharmacol 12, 133–151 (2017). https://doi.org/10.1007/s11481-016-9723-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-016-9723-4