Abstract
During early brain development, microglial activation can negatively impact long-term neuroimmune and cognitive outcomes. It is well-known that significant alcohol exposure during early gestation results in a number of cognitive deficits associated with fetal alcohol spectrum disorders (FASD). Additionally, microglia are activated following high levels of alcohol exposure in rodent models of FASD. We sought to examine whether moderate prenatal alcohol exposure (70 mg/dL blood alcohol concentration) activates microglia in the fetal rat brain, and whether moderate fetal alcohol exposure has long-term negative consequences for immune function and cognitive function in the rat. We also measured inflammation within the placenta and maternal serum following moderate alcohol exposure to determine whether either could be a source of cytokine production in the fetus. One week of moderate prenatal alcohol exposure produced a sex-specific increase in cytokines and chemokines within the fetal brain. Cytokines were also increased within the placenta, regardless of the sex of the fetus, and independent of the low levels of circulating cytokines within the maternal serum. Adult offspring exposed to alcohol prenatally had exaggerated cytokine production in the brain and periphery in response to lipopolysaccharide (25 μg/kg), as well as significant memory deficits precipitated by this low-level of inflammation. Thus the immune system, including microglia, may be a key link to understanding the etiology of fetal alcohol spectrum disorders and other unexplored cognitive or health risks associated with even low levels of fetal alcohol exposure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microglia are the innate immune cells of the brain, which respond to insults and injury via the release of immune molecules such as cytokines and chemokines. Their primary goal is to return the brain to homeostasis following these events; however, activation of microglia can also result in neuronal dysfunction and neuronal cell death through their excessive release of immune molecules. Microglial activation and cytokine production have been associated with a number of cognitive and neurological disorders including Alzheimer’s disease (Fassbender et al. 2004; Yang et al. 2014), neurodevelopmental disorders including autism spectrum disorder and schizophrenia (Estes and McAllister 2015; Monji et al. 2013), as well as a number of other physiological states and diseases. Alcohol exposure at binge levels can activate microglia in the rodent brain, a novel finding that may have significant implications for the physiological and neurodevelopmental effects of prenatal alcohol exposure (Drew and Kane 2014; Qin et al. 2008).
Microglial cells in the developing brain are particularly sensitive to even small disturbances in homeostasis (Bilbo and Schwarz 2012). Microglia have been localized to most areas of the embryonic brain in both humans and rodents as early as 8 weeks of gestation and embryonic day (E) 10 respectively (Chen et al. 2008; Rezaie et al. 1999, 2005). Microglia play a key role in a number of important processes in the developing brain including cell proliferation, dendritic spine pruning, and they carry out the clearance of apoptotic cells during early brain development (Antony et al. 2011; Cunningham et al. 2013; Squarzoni et al. 2014). Given their importance in these neurodevelopmental processes, activation of microglia at this time can have significant consequences for long-term neural function and this has been demonstrated via a number of models of perinatal infection or immune activation (Bilbo and Schwarz 2009).
There is no question that binge drinking during pregnancy is detrimental to the development of the exposed infants. Fetal Alcohol Spectrum Disorder (FASD) is the leading cause of preventable mental retardation in children, and costs the U.S. billions of dollars each year (Lupton et al. 2004). In addition to the physical defects that prenatal alcohol exposure causes, these children more often than not have problems with learning, memory, language, and attention, among other health and behavioral problems (Lewis et al. 2015; Mattson et al. 2006; Wyper and Rasmussen 2011). Despite the vast amount of research conducted on this topic, there is still considerable debate on whether or not any amount of alcohol is safe to drink during pregnancy. A few studies have suggested that low levels or moderate levels of drinking during early pregnancy have no adverse effects on a child health’s and neurodevelopment (Falgreen Eriksen et al. 2012; Skogerbo et al. 2012). In fact, one study suggests that drinking alcohol in moderation during pregnancy may improve children’s cognitive abilities and decrease hyperactivity (Kelly et al. 2012). With the recent findings that alcohol activates microglia in the brain, we sought to determine whether low levels of fetal alcohol exposure during early gestation would result in inflammation within the developing brain, placenta, or the maternal immune system and whether low levels of alcohol exposure would have significant effects on immune function of affected offspring later in life.
Materials and Methods
Animals
Albino outbred Sprague-Dawley rats from Harlan Laboratories (Indianapolis, IN) were used for all of the experiments described below. They were housed in clear polypropylene cages on a 12:12-h light: dark cycle maintained at constant temperature and humidity, and had ad libitum access to food and water. All animals were kept in the University of Delaware’s Office of Laboratory Animal Medicine (OLAM) facility in accord with Institutional Animal Care and Use Committee.
Breeding, Treatment, and Blood Alcohol Concentrations
At the beginning of each experiment, female rats were bred individually with males. The presence of a sperm plug indicated day of conception, or E1. Beginning on E10, pregnant females were administered 2 g/kg of ethanol or the equal volume of water (0.5 mL/100 g) between 8 am to 9 am and again 4 h later, between 12 pm to 1 pm. Treatment of the dams was performed using flexible gavage needle (Instech, PA, Cat. No. 1-FTP-18-75). In our experience, using these flexible feeding needles for the treatment of rats is extremely safe, effective and produces very little stress for the rats as it requires only brief restraint lasting less than 5 s.
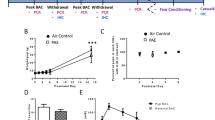
From one cohort of rats, blood samples were obtained from treated dams on E10 via tail snips for subsequent BAC analysis. Blood was collected at 30 min, 1 h, 2 h, and 4 h following the first dose of alcohol or water and again 30 min, 2 h, 4 h, and 20 h after the second dose of alcohol or water. The blood samples were centrifuged (15,000 rpm/15 min) and the plasma was collected and stored at −80 °C. Plasma was analyzed for BAC using an Analox GL5 Alcohol Analyzer (Analox Instruments, Boston, MA). The final BACs obtained using this alcohol administration paradigm are shown in Fig. 1a.
Intragastric gavage of 2 g/kg ethanol as model of low dose fetal alcohol exposure. a Pregnant dams were administered either alcohol (2 g/kg) or water in equal volume at hours 0 and 4. Blood samples were collected from tail snips at 30 min, 1 h, 2 h, and 4 h after the first dose, and 30 min, 2 h, 4 h, and 20 h after the second dose. This dosage of alcohol elevated the blood alcohol concentration to approximately 70 mg/dl for 8 h. b Pregnant dams were weighed daily throughout gestation. Dams treated with alcohol gained significantly less weight during pregnancy, with significant differences at time points E14 – E20 (at E16, *p = 0.038; at E20, *p = 0.015). c On postnatal days 1 and 2, maternal behavior was recorded over 1 h. There was no difference in maternal behaviors observed between alcohol-treated and water-treated dams
A second cohort of pregnant females was treated with this administration paradigm from embryonic day (E) 10 through 16. These dates were selected for alcohol exposure because this is the age at which microglial progenitor cells first migrate into the rat central nervous system from the periphery (Chan et al. 2007; Cuadros and Navascues 1998 ). We weighed all dams before, during and after treatment in each experiment and found that dams treated with alcohol gained less weight during pregnancy than control rats (E16 weight alcohol vs. water: p = 0.038; Fig. 1b) and by E20 there was still a significant, though slight, difference in weight between alcohol treated dams and water treated controls (p = 0.015).
Experiment 1: Effect of Low Level Prenatal Alcohol Exposure on Inflammation in the Fetal Brain, Placenta, and the Maternal Immune System
In Experiment 1.1, we examined the effects of prenatal alcohol exposure on the fetal brain by analyzing the mRNA levels of a number of pro-inflammatory cytokines and chemokines using a quantitative real-time PCR (qPCR) array (SABiosciences, MD, ‘Inflammatory Cytokines and Receptors,’ Cat. No. PARN-001Z). 15 pregnant dams were assigned to one of three treatment groups (n = 5 dams/group): no treatment, 2 g/kg ethanol, or water in equal volume, as described above, twice a day from E10 to E16. Samples were collected from these pregnant dams on E17, 24 h after the last treatment, from each pup including the tail, placenta, and hippocampus/cortex, resulting in six groups, n = 8 pups in each treatment group. For all experiments, including those listed below, no more than 1–2 pups of a single sex were used from each litter within each treatment group to control for potential litter effects. Maternal serum and spleen were also collected from all dams.
Experiment 2: Impact of Low Level Prenatal Alcohol Exposure on the Adult Immune Response to Lipopolysaccharide
Pregnant dams were treated with either 2 g/kg of ethanol or water in equal volume, twice a day. Given that we did not see very striking differences between untreated and water treated controls in our earlier experiments, we did not use untreated dams for these subsequent experiments to reduce the number of animals used in subsequent experiments. The pups were allowed to mature to P90, at which point they were treated with either 25 μg/kg of lipopolysaccharide (LPS) or equal volume saline (n = 8/group). The medial prefrontal cortex (mPFC), hippocampus (HP), and spleen were collected 4 h post treatment to analyze the mRNA levels of pro-inflammatory cytokines produced in brain regions important for cognition in response to an immune challenge in adult rats previously exposed to low levels of prenatal alcohol.
Experiment 3: Impact of Low Level Prenatal Alcohol Exposure and Mild Adult Immune Activation on Recognition Memory
Pregnant dams treated with either 2 g/kg of ethanol or water in equal volume, twice a day. The pups were allowed to mature to P90, at which point the Novel Object Recognition (NOR) testing began. See below for further details on behavioral testing. All behavioral testing was done in the morning between 8 am to 12 pm.
Maternal Behavior Observations and Weaning
At the time of birth, we found no significant effect of maternal treatment on the size of the litters (t13 = 0.069; p = 0.79) or the sex ratio of the litters. We also examined pup weight 1 day after birth and found no significant effect of maternal treatment on the weight of either male or female pups (F3,194 = 0.56; p 0.813). Offspring were raised with their mothers until P21 at which point the pups were weaned into clear polypropylene cages with 3–4 same-sex, same-treatment rats per cage. At P40, animals were separated into same-sex, same-treatment pair housing and left undisturbed until experiments began.
In Experiment 2, maternal behavior was observed without manipulation to determine whether prenatal treatment would affect maternal care immediately postpartum. On P1 and again on P2, the behavior of each dam was categorized each minute over an hour (10 am–11 am) each day by two observers blinded to treatment group. The behaviors were categorized as either nursing/hovering, licking/grooming, or other (non-maternal behaviors). The total number of observed behaviors in each category were taken as a percent of the total number of events observed (60 events, 1 per minute) and these were averaged across the 2 days of observation and subsequently compared across groups using an unpaired t-test (alcohol-treated, n = 6 and water-treated, n = 6). On average, new mothers spent the majority of the hour (approximately 70–77 % of the hour) nursing and hovering. We found no significant effect of alcohol treatment on any postpartum maternal behaviors observed [nursing/hovering: t11 = 0.22; p = 0.827; licking/grooming: t11 = −0.40; p = 0.69; other: t11 = −0.07; p = 0.939; Fig. 1c].
Lipopolysaccharide Injections
Experiment 2 and 3 used lipopolysaccharide (LPS) derived from Escherichia coli 0111:B4 which was obtained from Sigma-Aldrich (Cat. No. L2630). Sterile, pyrogen-free Dulbecco’s phosphate buffered saline (DPBS) was used to dilute the LPS to a concentration of 25 μg LPS/mL. Rats were treated with an intraperitoneal (i.p.) injection of either 1 mL/kg of LPS (final dose of 25 μg/kg LPS) or DPBS as a control.
Novel Object Recognition Task
Examination of NOR Memory Following a Mild Immune Challenge during a 24 h Delay
In this experiment, we tested the effect of a mild immune challenge on recognition memory using the NOR task with a 24 h delay. This paradigm is similar to what is described in (Ennaceur and Delacour 1988) and (Westbrook et al. 2014), though with slightly longer habituation trials and different objects. Briefly, rats received two habituation sessions in the chamber (Stoelting Co., 45 × 45 cm, black Plexiglas walls, grey plastic floor) devoid of objects for 10 min each day on Days 1 and 2. On Day 3, rats were placed into the same chamber with two identical objects located approximately 15 cm from the top and left/right walls. Rats were allowed to explore these objects for 5 min. Immediately after the 5 min exploration phase, all rats were injected with either saline (1 ml/kg) or LPS (25 μg/kg) and returned to their home cage. This low dose of LPS produces an acute immune response that we hypothesized would interfere with the recognition memory consolidation, thus increasing the likelihood of memory deficits, particularly in rats that were exposed to fetal alcohol. Twenty-four hours later, the rats were placed back into the same chamber, now containing one of the previous objects and one new object. The objects used in the task were a white, plastic over-the-door hook and a yellow rubber duck, both of which were faced towards the rat as he was placed into the chamber. The test phase lasted 3 min during which time the rats were video recorded (Clover Electronics CM625 with Panasonic PLZ727 lens). The groups tested were water-treated or alcohol-exposed males given saline or LPS (n = 10/group) on Day 3, and water-treated or alcohol-exposed females given saline or LPS (n = 10/group) on Day 3. An experimenter blind to the treatment later watched the videos and hand-scored the total time spent exploring either object using a stop watch. Exploration of each object was defined as actively sniffing, pawing at, or whisking with its snout directed toward the object and less than approximately 2 cm away from the object (Ennaceur 2010). The percent of time spent exploring the novel object was taken as a ratio of the time spent exploring both objects combined (“discrimination ratio”) as described in (Blaser and Heyser 2015).
Euthanasia, Perfusion, and Tissue Collection
Rats were euthanized via administration of an overdose of the barbiturate Euthasol® (ANADA 200–071) through i.p. injection. Once under heavy anesthesia, a blood sample was collected from each rat via cardiac puncture before being perfused with a cold 0.9 % saline solution via cardiac puncture to remove blood and peripheral immune cells from the brain. Following perfusion, pups for Experiment 1.1 were taken from the dam and the hippocampus/cortex, tail, and placenta was extracted from each pup at E17. In Experiment 2, using adult rats, the whole hippocampus (HP), medial pre-frontal cortex (mPFC), liver, and spleen was collected. After extraction, all samples were flash frozen on dry ice and stored at −80 °C for further processing.
Real-Time PCR
Levels of pro- and anti-inflammatory cytokine and chemokine messenger RNA (mRNA) were analyzed using quantitative real-time PCR (qPCR). Extraction of mRNA from tissue samples was done using Isol-RNA Lysis Reagent (Cat. No. FP2302700, 5 PRIME). Using the QuantiTect® Reverse Transcription Kit (Cat. No. 205310, Qiagen), the samples were subjected to DNase treatment to remove genomic DNA before creating a 1000 ng/μL concentration of RNA that was converted to complimentary DNA (cDNA). In Experiment 1.1, a PCR Array from SABioscience/Qiagen (Cat. No. PARN-011Z) was used to analyze relative gene expression between E17 samples. This array was used in the first experiment in order to examine a broader scope of inflammatory molecules in the fetal brain exposed to alcohol. The quantitative threshold amplification cycle number (Cq) for all genes were analyzed using the real-time PCR machine (BioRad CFX96). The expression of 5 housekeeping genes [beta actin, beta-2 microglobulin, Hypoxanthine phosphoribosyltransferase 1(Hprt1), lactate dehydrogenase A, and ribosomal protein P1] were also run for all samples. We found no significant effects of sex or treatment on the Cq value obtained for each of the 5 housekeeping genes. Thus the average of all five Cq values was calculated for each sample, and from this, the 2-ΔΔCq method was used to calculate relative gene expression for the genes of interest.
In Experiment 2, relative gene expression in adult brain and spleen tissue was determined using targeted qPCR. RealMasterMix™ Fast SYBR Kit (Cat. No. 2200830, 5 PRIME) was prepared in a 10 μL reaction on a CFX96Touch™ real-time PCR machine. Primer sequences can be found in Supplementary Table 1. The IL-6 primer was a QuantiTect® Primer Assay Rn_Il6_1_SG (Cat. No. QT00182896, Qiagen) obtained from Qiagen and diluted according to the protocol for real-time PCR. All additional primers were obtained through Integrated DNA Technologies and diluted to a working concentration of 13 μM. GAPDH was used as a housekeeping gene, as it did not differ significantly between treatment groups. Samples were blinded to treatment group and run on real-time PCR plates in duplicate. The average quantitative threshold amplification cycle number (Cq) was determined from the duplicates, and the 2-ΔΔCq method was used to calculate relative gene expression.
In addition to brain and peripheral tissue RNA extraction, we extracted DNA from the tail samples of E17 pups to determine the sex of the pups using PureLink Genomic DNA Mini Kit (Cat. No. K182001, Invitrogen). We examined the qPCR expression of the male-specific gene (sex-determining region of the Y chromosome; SRY gene). Samples were run in duplicate and samples that had an average Cq value of less than 25 were classified as males, and those with a Cq value over 25 were classified as females.
Multiplex Analysis of Maternal Serum Samples
In Experiment 1.1, maternal serum was collected on E17, 24 h after the last dose of alcohol. The protein levels of IL-1β, IL-6, TNFα, IL-10, IP-10, CCL3 (MIP-1α), GRO-KC, and CCL2 (MCP-1) in undiluted maternal serum were analyzed from untreated dams, water-treated control dams and alcohol-exposed dams (n = 5/group) using multiplex assay by an experimenter blind to treatment group (Milliplex® Rat analyte kit).
Statistical Analyses
Maternal behaviors, identified above, were analyzed using a two-tailed t-test to examine potential differences between water and alcohol treated dams. Multiplex data from maternal serum were analyzed using one-way ANOVA. In Experiment 1, elative gene expression data from E17 was analyzed using a 2 × 2 ANOVA with sex and fetal alcohol exposure as factors. For Experiment 2, relative gene expression values were analyzed using a 2x2x2 ANOVA with sex, prenatal alcohol treatment, and adulthood LPS treatment as the three factors. In Experiment 3, the NOR discrimination ratios were analyzed following convention (Akkerman et al. 2012) using one-sample t-test to compare discrimination ratios of each group to a discrimination ratio indicating no discrimination (0.5). To compare across groups for the NOR task, the discrimination ratios were analyzed using a 2x2x2 ANOVA with sex, prenatal alcohol exposure, and adult immune challenge as factors. Tukey’s post hoc test was used to further analyze significant main effects and interactions, in order to determine individual group differences. All graphs show the mean relative gene expression ± SEM for each treatment group.
Results
Experiment 1.1: Effect of Low Level Prenatal Alcohol Exposure on Inflammation in the Fetal Brain, Placenta, and Maternal Immune System
Hippocampus-cortex tissue was collected from pups on E17 to analyze levels of pro- and anti-inflammatory molecules and microglial activation markers. Of the 84 genes originally examined, only 19 genes were significantly affected by prenatal alcohol exposure. We also found a main effect of sex in the expression pattern for a number of cytokines and chemokines in the fetal brain. All significant results are summarized in Table 1. The genes that were not affected by prenatal alcohol exposure or sex are listed in Supplementary Table 2. We found a main effect of alcohol exposure on a very select group of cytokines, chemokines, and receptors including, CCL3, CCL6, CCL9, CCR2, CCR4 (the receptor for CCL3), CCR6, CXCL9, CXCL11, IL10ra, IL21, IL5, Lta, Osm, TNFα, and TNFrsf10. Post hoc tests revealed that only CCL6, CCR6, IL21, IL10ra, and TNFα were increased significantly in the hippocampus-cortex of both males and females following alcohol exposure relative to both control groups (p < 0.05). In contrast, there were significant interactions of sex and treatment for a number of immune molecules including CCL2, CCL5, CCL9, CXCL10, IL5, and RGD1561905 (also known as complement component 5). Post hoc tests revealed that expression of CCL2 and IL5 was significantly decreased in alcohol exposed males compared to both same-sex control groups (p < 0.05). In contrast, the expression of CCL2, CCL5, CCL9, CXCL10, and IL5 were significantly increased in alcohol exposed females relative to same-sex control groups (p < 0.05). The expression of CCL2 and IL5 were significantly attenuated in males following treatment with water alone (p < 0.05 relative to untreated controls). Similarly, post hoc comparisons also revealed a significant increase in CCL5 in water treated males (p < 0.05 relative to untreated controls). In spite of these “treatment effects,” alcohol consistently produced more robust changes in cytokine and chemokine expression than was measured in water-treated controls in both male and female fetuses. Thus low levels of fetal alcohol exposure during early brain development produced a unique inflammatory response within the fetal rat brain that is, for a number of genes, sex-specific.
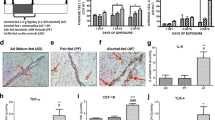
We also analyzed the expression of a number of cytokines in the placenta. Of the genes that were analyzed, we found that IL-6 and TNFsf4 genes were significantly elevated in the placentae of males and females exposed to alcohol (IL-6 Main Effect of Treatment: F2,41 = 9.12; p = 0.001; TNFsf4 Main Effect of Treatment: F2,43 = 4.62; p = 0.016; Fig. 2a). Interestingly, we found no effect of sex on these cytokines, indicating that the sex of the fetus does not impact the cytokine response in the placenta. We found no main effect of treatment (p = 0.768) or interaction of treatment and sex (p = 0.143) on the expression of IL-1β, or on the expression of TNFα in the placenta (Main Effect of Treatment: p = 0.751 and Interaction of Sex and Treatment: p = 0.125) (Fig. 2a).
Effect of low dose prenatal alcohol exposure on placenta gene expression and circulating cytokines in maternal serum. On E17, 24 h after the last dose of alcohol or water, placental tissue and maternal serum was collected for analysis. a Placenta tissue was analyzed for pro-inflammatory cytokines using real-time PCR (n = 8/group). Pups exposed to alcohol during gestation had significantly increased levels of IL-6 and TNFsf4 in their placentae (* Main effect of treatment; p < 0.05). There were no main effects or interactions of treatment and sex for IL-1β and TNFα expression in the placenta. b Serum samples from dams collected at E17 were analyzed for circulating inflammatory cytokines (n = 5/group). Low, but detectable levels of IL-10, CCL3, and TNFα did not differ significantly across treatment groups. Cytokines IP-10 and CCL2 were detected at higher levels, however there was no effect of treatment across groups
We also analyzed the levels of circulating cytokines in the serum of the dams treated with either alcohol or water. Serum was collected from the dams 24 h after the last alcohol dose. A number of cytokines were below detectable levels in maternal serum, regardless of treatment, including IL-6 (< 73.2 pg/ml), GRO-KC (< 14.6 pg/ml), and IL-1β (< 2.4 pg/ml) (data not shown). IL-10, CCL3, and TNFα were all detected at very low levels, but were not significantly different across treatment groups (IL-10: F2,20 = 0.83; p = 0.452; CCL3: F2,20 = 0.06; p = 0.941; and TNFα: F2,20 = 0.812; p = 0.87; Fig. 2b). IP-10 and CCL2 were detected at higher levels, but these levels were also not different across treatment groups (IP-10: F2,20 = 0.52; p = 0.603; CCL2: F2,20 = 0.59; p = 0.564; Fig. 2b).
Given that prenatal alcohol exposure is associated neural cell death, we also analyzed the expression of two cell-death activators that regulate apoptosis, Cell death-inducing DFFA-like effector B (Cideb) 1 and Cideb2, on E17 revealed a significant main effect of treatment on the expression of Cideb1 (F2,47 = 3.05; p = 0.05; Fig. 3). Post hoc tests revealed that prenatal alcohol exposure increased the expression of Cideb1 in the hippocampus/cortex of both males and females relative to the control groups (p < 0.05). We found no effects or interactions of treatment or sex on the expression of Cideb2 in the hippocampus/cortex on E17 (Fig. 3).
Impact of low dose fetal alcohol exposure on the expression of the cell-death markers Cideb1 and 2 in the hippocampus/cortex on E17. Following 1 week of alcohol exposure, the hippocampus / cortex were collected from fetuses at E17, 24 h after the last dose of alcohol or water (n = 8/group). There was a significant main effect of treatment on Cideb1 expression and post hoc tests revealed that alcohol exposure specifically increased Cideb1 levels of cDNA in both males and females compared to both control groups (*p < 0.05). There were no significant effects of treatment or sex on Cideb2 in the hippocampus / cortex at this age
Experiment 2: Impact of Low Level Prenatal Alcohol Exposure on the Adult Immune Response to Lipopolysaccharide
In order to examine the long-term effects of alcohol exposure on the function of the immune system, offspring previously exposed to either water or alcohol during gestation were treated with a low dose of lipopolysaccharide (LPS) in adulthood to elicit a mild immune response.
In the hippocampus, we found a significant interaction of sex, prenatal alcohol exposure, and adult LPS treatment on IL-1β expression in the hippocampus (F1,51 = 4.569; p = 0.037, Fig. 4a). Specifically, alcohol exposed males had exaggerated IL-1β expression in the hippocampus following LPS treatment (p < 0.05 relative to all other groups). We also found a significant interaction of prenatal alcohol exposure and LPS immune challenge on the expression of IL-6 in the hippocampus (F1,50 = 6.081; p = 0.017; Fig. 4b). Rats exposed to alcohol prenatally had significantly higher levels of IL-6 in the hippocampus compared to controls (p < 0.05); though this effect seems to largely be the result of exaggerated IL-6 specifically in alcohol-exposed males treated with LPS as adults. We found no significant main effects or interactions of sex, prenatal alcohol exposure, or LPS on the expression of CD11b, a marker of microglial activation, in the adult hippocampus (Fig. 4c).
Long-term consequences of fetal alcohol exposure on adult neuroimmune function in the hippocampus and cortex. Rats exposed to alcohol or water during gestation were exposed to a second immune challenge, 25 μg/kg lipopolysaccharide (LPS), at postnatal day (P) 90. Tissue was collected 4 h after LPS administration to investigate relative gene expression of inflammatory molecules in the hippocampus and prefrontal cortex (n = 8/group). a–c In the hippocampus, there was a significant interaction between sex, prenatal alcohol exposure, and adult LPS treatment on levels of IL-1β, specifically males exposed to alcohol and LPS had increased expression of IL-1β (**p < 0.01 compared to all other groups, *p < 0.05 compared to saline-treated controls). There was an interaction of prenatal alcohol exposure and adult LPS treatment on levels of IL-6 such that alcohol and LPS-exposed rats had increased expression of IL-6 (**p < 0.01 compared to all other groups, *p < 0.05 compared to saline-treated controls). There were no main effects or interactions on levels of CD11b. d–F. In the mPFC, there were main effects of prenatal alcohol exposure and LPS exposure on levels of IL-1β (**p < 0.01 compared to all other groups, *p < 0.05 compared to saline-treated controls). Levels of IL-6 were significantly increased by LPS treatment (*p < 0.05). There was also a main effect of prenatal treatment on the expression of CD11b in the mPFC in that males and females exposed to alcohol during gestation had higher levels of CD11b expression (*p < 0.05 compared to water-treated controls)
In the medial prefrontal cortex, we found a significant main effect of prenatal alcohol exposure and LPS immune challenge on the expression of IL-1β in the prefrontal cortex (F1,55 = 5.727; p = 0.020; F1,55 = 53.604; p < 0.001 respectively; Fig. 4d), indicating that the two factors are additive. IL-6 was significantly elevated in the prefrontal cortex by LPS (F1,54 = 37.27; p < 0.001; Fig. 4e), but was not affected by sex or prenatal alcohol exposure. We found a significant main effect of prenatal alcohol exposure on CD11b expression in the adult PFC (F1,56 = 8.130; p = 0.006; Fig. 4f). Specifically, CD11b was increased in both male and female rats that were exposed to alcohol prenatally relative to water-treated controls (p < 0.05), suggesting a long-term change in microglia in the mPFC caused by prenatal alcohol exposure.
Finally, we analyzed the expression of cytokines in spleen samples collected from these rats in order to determine the effects of prenatal alcohol exposure and subsequent activation on peripheral immune function and thus whether the effects that we saw in the hippocampus and mPFC were specific to the brain. Similar to the effects in the brain, analysis of IL-1β mRNA expression in the spleen revealed an interaction of prenatal alcohol exposure and adulthood LPS challenge (F1,56 = 8.084; p = 0.006; Fig. 5a). While LPS treatment significantly increased IL-1β in the spleen, levels of IL-1β were increased significantly more in male and female rats that had previously been exposed to prenatal alcohol (p < 0.05 compared to all other groups). We found a main effect of LPS treatment on IL-6 in the spleen (IL-6: F1,56 = 34.77; p < 0.001; Fig. 5b), but no interactions with either sex or prenatal alcohol exposure. Analysis of TNFα revealed an interaction of sex and LPS treatment (F1,56 = 4.107, p = 0.047; Fig. 5c), in that LPS increased TNFα expression at this particular time point significantly more in males than in females.
Long-term consequences of fetal alcohol exposure on adult immune function in the spleen. Rats exposed to alcohol or water during gestation were exposed to a second immune challenge, 25 μg/kg lipopolysaccharide (LPS), at postnatal day (P) 90. Four hours after LPS treatment, spleen tissue was collected to investigate relative gene expression of inflammatory molecules (n = 8/group). a There was an interaction of prenatal alcohol exposure and LPS treatment on levels of IL-1β; specifically, males and females exposed to alcohol and LPS had increased expression levels of IL-1β (**p < 0.01 compared to all other treatment groups; *p < 0.05 compared to saline-treated controls). b Levels of expression of IL-6 were impacted by a main effect of LPS treatment (*p < 0.001). C. There was also an interaction between sex and LPS treatment on the expression of TNFα, in that males exposed to LPS had higher levels of TNFα in the spleen (**p < 0.05 compared to all other groups, *p < 0.05 compared to saline-treated controls)
Experiment 3: Impact of Low Level Prenatal Alcohol Exposure and Mild Adult Immune Activation on Recognition Memory
To assess the effects of low level prenatal alcohol exposure and mild immune dysregulation on a simple memory task, we assessed recognition memory using the Novel Object Recognition (NOR) task. Briefly, all rats were allowed to explore two identical objects in an arena for 5 min before being administered either LPS (25 μg/kg) or saline. Twenty four hours later, the rats were returned to the arena and allowed to freely explore the familiar object or a new object to test recognition memory.
We found no main effect of sex on the NOR task described above (F1,79 = 0.08; p = 0.778; Fig. 6). We found a significant main effect of fetal alcohol exposure (F1,79 = 4.42; p = 0.039) and a main effect of immune activation (F1,79 = 5.07; p = 0.027), which was further confirmed by analysis of the discrimination ratio of each group to chance (no discrimination). Specifically, males and females exposed to low alcohol levels prenatally and then challenged as adults with a mild dose of LPS showed no discrimination of the two objects. These data indicate that even low levels of immune activation can produce cognitive deficits in offspring previously exposed to low levels of fetal alcohol.
Impact of fetal alcohol exposure and immune activation on adult recognition memory. Rats exposed to either alcohol or water during gestation were raised to P60 at which point they were tested using a Novel Object Recognition paradigm (n = 10/group). Rats were habituated to the chamber for 10 min on Day 1 and Day 2. On Day 3, animals were placed into a chamber with 2 identical objects and allowed to explore for 5 min. Immediately after the exploration/sample phase, the rats were treated with either LPS (25 μg/kg) or saline. 24 h later, on Day 4, the same rats were placed in a chamber with one of the previous objects and one novel object. The mean discrimination ratio (± SEM) of time spent exploring the novel object is shown. *p < 0.05 relative to chance (no discrimination). We found an interaction of prenatal alcohol exposure and LPS treatment in that males and females exposed to alcohol and LPS showed significantly decreased exploration of the novel object
Discussion
This study sought to determine the immediate and long-term consequences of low level prenatal alcohol exposure on the fetal neuroimmune system, while also considering the effects of low level alcohol exposure on the maternal immune system and placenta. Despite recent findings that low doses of alcohol consumption during pregnancy produce no harmful cognitive effects in children (Falgreen Eriksen et al. 2012; Kelly et al. 2012; Skogerbo et al. 2012), it had never been previously examined whether low levels of fetal alcohol exposure affect neuroimmune function, and whether changes in neuroimmune function would significantly affect cognitive function in offspring. To that end, we administered alcohol to pregnant dams from E10-E16, a time during which microglia first colonize the fetal rat brain (Chan et al. 2007; Cuadros and Navascues 1998). We administered alcohol at a low dose, sufficient to raise blood alcohol concentrations to approximately 70 mg/dL, or 70 mg% for about 8 h, consistent with other reports (Bielawski and Abel 2002; Livy et al. 2003). We felt this dose and treatment paradigm were physiologically relevant as they may reflect the BAC that may be produced from a pregnant woman drinking early in gestation and having just one or two drinks in a sitting, though exact human BAC correlates are dependent on an individual (Centers for Disease Control and Prevention 2015).
Our findings reveal important changes in the fetal neuroimmune system caused by low levels of alcohol exposure during early gestation. Specifically, alcohol significantly altered the expression of many pro and anti-inflammatory cytokines and chemokines in the fetal brain. These data indicate that that the fetal brain responds to low levels of fetal alcohol exposure with a change in the expression of well-known inflammatory molecules. We initially hypothesized that low level alcohol exposure would result in the activation of microglia, based on previous research demonstrating that alcohol is an effective activator of microglia in the neonatal rodent brain (Drew et al. 2015; Kane et al. 2011, 2013). Unique to this study, however, we also predicted that low level alcohol exposure would result in peripheral cytokine production in the mother and cytokine production in the placenta that may in turn influence the developing fetus. The importance of the placental barrier between mother and baby is well-known and dysfunction of the placenta, for a number of reasons, has been associated with poor health and neurodevelopmental outcomes in children (Baker and Sibley 2006). We found that low level prenatal alcohol exposure increased the expression of IL-6 and TNFSF4 in the placenta. Interestingly, though, the effects of alcohol on inflammatory gene expression in the placenta and the fetal brain were independent of the maternal immune system, as alcohol-exposed dams had low or unaffected levels of cytokines and chemokines circulating in their serum. To date, very few studies have fully examined the impact of fetal alcohol exposure on inflammation in both the maternal and fetal compartments in rodents. Our results suggest that alcohol induces inflammatory gene expression de novo in both the fetal brain and the placenta and that these effects are not the secondary result of maternal immune activation. Our results also reveal that the placenta and the fetal brain may be more sensitive to or more responsive to low levels of alcohol exposure than the peripheral immune system of the mother.
We also found that female pups exposed to alcohol during gestation had significantly higher levels of inflammatory gene expression in the brain compared to their male counterparts. In contrast, control males often had higher expression levels of the same gene than control females. Notably, these sex differences in inflammatory gene expression within the fetal brain were observed on E17, prior to the secretion of sex-specific hormones, which occurs just before birth in rodents (Corbier et al. 1992; Weisz and Ward 1980). Males and females have very different immune profiles across neurodevelopment (Schwarz et al. 2012), demonstrating that sex can influence neural development and function, even at the earliest stages of development, via the production of immune molecules. One interpretation of our data presented here is that females may be more vulnerable to the effects of low level fetal alcohol exposure, resulting in exaggerated cytokine expression in the female brain; however, an alternate interpretation of the data is that females have a robust and appropriate neuroimmune response to the alcohol exposure but that males do not. Given our findings in the adult offspring, discussed below, one might also conclude that males and females both respond with an appropriate, yet distinct, immune response to low levels of prenatal alcohol exposure, which results in similar and significant consequences for immune and cognitive function later in life.
An important implication of this prenatal immune activation is its relevance to what is known as the “two-hit hypothesis.” This hypothesis states that immune cells, like microglia, may be primed by events that activate the immune system during early development such that when a subsequent immune challenge occurs, the re-activation of these cells is exaggerated (Bilbo and Schwarz 2009). A number of studies now suggest that the “two-hit hypothesis” and immune activation may play a crucial role in the etiology of neurological and cognitive disorders, including Alzheimer’s disease and schizophrenia among others (Feigenson et al. 2014; Zhu et al. 2007). We hypothesized that exposure to alcohol during gestation may similarly prime fetal microglia and alter their response to subsequent immune challenges, thereby resulting in cognitive deficits later in life. We tested the “two-hit hypothesis” in our model by administering a very low dose of LPS to adult offspring that had been exposed to either alcohol or water during gestation. Our findings from this experiment support this hypothesis and indicate that the rats exposed to both low levels of alcohol prenatally and LPS in adulthood had exaggerated expression of pro-inflammatory cytokines (specifically IL-1β and IL-6) in the brain as well as in the periphery (IL-1β in the spleen). In particular, males exposed to prenatal alcohol then challenged with LPS as adults had higher levels of IL-1β expression than their female counterparts, suggesting an interaction of the fetal alcohol exposure and sex on the expression of this particular cytokine. Notably, the effect of fetal alcohol exposure on IL-1β expression in the male offspring was seen across brain regions and in the periphery, specifically the spleen, suggesting that the “priming” effect of fetal alcohol exposure was not limited to microglial cells alone. This is quite fascinating given that the spleen does not develop until after E17 in rodents (Losco 1992), and suggests that somehow fetal alcohol exposure alters the development of primordial cells or structures that will ultimately determine this important immune organ and its associated function.
The hippocampus and cortex are critical for cognition, memory, and decision making, but are also particularly vulnerable to immune challenges that result in elevated cytokine levels (Guan and Fang 2006). While cytokines are best known for the role they have in producing classic “sickness behaviors” (Kent et al. 1992), the role of these molecules have also been studied in the context of a number of cognitive and psychiatric disorders (Bilbo and Schwarz 2012; Skaper et al. 2014; Streit et al. 2014). Similarly, alcohol exposure is also linked to neurodegeneration and cognitive dysfunction resulting from microglial activation and its associated neuroinflammation (Crews and Nixon 2009; Yang et al. 2014). To determine whether low level fetal alcohol exposure increases the likelihood of cognitive dysfunction caused by subsequent immune activation, we tested object recognition memory in our model of prenatal alcohol exposure. We found that both male and female rats exposed to prenatal alcohol had significant deficits in recognition memory, but only in the presence of immune activation. This same immune challenge had no effect on control offspring. These findings, in addition to others (Goodfellow and Lindquist 2014; Jablonski and Stanton 2014; Murawski et al. 2012), indicate that alcohol consumption during pregnancy has significant consequences for cognitive outcomes in the offspring. Notably, our findings further highlight that even low levels of fetal alcohol exposure can negatively impact cognitive function in the offspring, particularly in combination with other events that may activate the immune system. Thus, any amount of alcohol exposure during prenatal development has the potential to increase the risk of any number of cognitive or psychiatric disorders associated with altered cytokine expression in adulthood.
Our research highlights the importance of considering the immune system as a target of fetal alcohol exposure, with significant consequences for long-term immune and cognitive function. In particular, we have demonstrated that even though low levels of alcohol consumption during pregnancy did not result in detectable levels of maternal immune activation, this same level of alcohol exposure did have significant immunological consequences for the fetus and placenta. Similarly, cognitive deficits can be precipitated by exaggerated cytokine production in adult offspring that had been exposed prenatally to low levels of alcohol exposure. While further research should be conducted to better understand the relationship between immune activation and cognitive or neural dysfunction, our results suggest that even low levels of alcohol exposure during early embryonic development can have both immediate and long-term consequences on the neuroimmune system of the offspring. Importantly while these equivalent levels of alcohol consumption during pregnancy are not likely to cause explicit fetal alcohol syndrome, our data highlight that there is still significant cause for concern, as consuming any amount of alcohol at any point during gestation, even early gestation, may affect a number of future health and cognitive outcomes associated with immune activation and inflammation in the offspring.
References
Akkerman S, Prickaerts J, Steinbusch HW, Blokland A (2012) Object recognition testing: statistical considerations. Behav Brain Res 232(2):317–322. doi:10.1016/j.bbr.2012.03.024
Antony JM, Paquin A, Nutt SL, Kaplan DR, Miller FD (2011) Endogenous microglia regulate development of embryonic cortical precursor cells. J Neurosci Res 89(3):286–298. doi:10.1002/jnr.22533
Baker P, Sibley C (2006) In: Baker P, Sibley C (eds) The placenta and neurodisability. Cambridge University Press, Cambridge
Bielawski DM, Abel EL (2002) The effect of administering ethanol as single vs. divided doses on blood alcohol levels in the rat. Neurotoxicol Teratol 24(4):559–562
Bilbo SD, Schwarz JM (2009) Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci 3:14. doi:10.3389/neuro.08.014.2009
Bilbo SD, Schwarz JM (2012) The immune system and developmental programming of brain and behavior. Front Neuroendocrinol 33(3):267–286. doi:10.1016/j.yfrne.2012.08.006
Blaser R, Heyser C (2015) Spontaneous object recognition: a promising approach to the comparative study of memory. Front Behav Neurosci 9:183. doi:10.3389/fnbeh.2015.00183
Centers for Disease Control and Prevention (CDC) (2015) Center for disease control and prevention: Alcohol and public health FAQs. Retrieved from http://www.cdc.gov/alcohol/faqs.htm
Chan WY, Kohsaka S, Rezaie P (2007) The origin and cell lineage of microglia: New concepts. Brain Res Rev 53(2):344–354
Chen H, Simar D, Lambert K, Mercier J, Morris MJ (2008) Maternal and postnatal overnutrition differentially impact appetite regulators and fuel metabolism. Endocrinology 149(11):5348–5356. doi:10.1210/en.2008-0582
Corbier P, Edwards DA, Roffi J (1992) The neonatal testosterone surge: a comparative study. Arch Int Physiol Biochim Biophys 100(2):127–131
Crews FT, Nixon K (2009) Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol (Oxford, Oxfordshire) 44(2):115–127. doi:10.1093/alcalc/agn079
Cuadros MA, Navascues J (1998) The origin and differentiation of microglial cells during development. Prog Neurobiol 56(2):173–189
Cunningham CL, Martinez-Cerdeno V, Noctor SC (2013) Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci 33(10):4216–4233. doi:10.1523/JNEUROSCI.3441-12.2013
Drew PD, Kane CJ (2014) Fetal alcohol spectrum disorders and neuroimmune changes. Int Rev Neurobiol 118:41–80. doi:10.1016/B978-0-12-801284-0.00003-8
Drew PD, Johnson JW, Douglas JC, Phelan KD, Kane CJ (2015) Pioglitazone blocks ethanol induction of microglial activation and immune responses in the hippocampus, cerebellum, and cerebral cortex in a mouse model of fetal alcohol spectrum disorders. Alcohol Clin Exp Res 39(3):445–454. doi:10.1111/acer.12639
Ennaceur A (2010) One-trial object recognition in rats and mice: methodological and theoretical issues. Behav Brain Res 215(2):244–254. doi:10.1016/j.bbr.2009.12.036
Ennaceur A, Delacour J (1988) A new one-trial test for neurobiological studies of memory in rats. Behav Brain Res 31(1):47–59
Estes ML, McAllister AK (2015) Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nature Reviews. Neuroscience 16(8):469–486. doi:10.1038/nrn3978
Falgreen Eriksen HL, Mortensen EL, Kilburn T, Underbjerg M, Bertrand J, Stovring H, et al. (2012) The effects of low to moderate prenatal alcohol exposure in early pregnancy on IQ in 5-year-old children. BJOG 119(10):1191–1200. doi:10.1111/j.1471-0528.2012.03394.x
Fassbender K, Walter S, Kuhl S, Landmann R, Ishii K, Bertsch T, et al. (2004) The LPS receptor (CD14) links innate immunity with alzheimer’s disease. FASEB J : Official Publication of the Federation of American Societies for Experimental Biology 18(1):203–205. doi:10.1096/fj.03-0364fje
Feigenson KA, Kusnecov AW, Silverstein SM (2014) Inflammation and the two-hit hypothesis of schizophrenia. Neurosci Biobehav Rev 38:72–93. doi:10.1016/j.neubiorev.2013.11.006
Goodfellow MJ, Lindquist DH (2014) Significant long-term, but not short-term, hippocampal-dependent memory impairment in adult rats exposed to alcohol in early postnatal life. Dev Psychobiol 56(6):1316–1326. doi:10.1002/dev.21210
Guan Z, Fang J (2006) Peripheral immune activation by lipopolysaccharide decreases neurotrophins in the cortex and hippocampus in rats. Brain Behav Immun 20(1):64–71
Jablonski SA, Stanton ME (2014) Neonatal alcohol impairs the context preexposure facilitation effect in juvenile rats: Dose-response and post-training consolidation effects. Alcohol (Fayetteville, N.Y.) 48(1):35–42. doi:10.1016/j.alcohol.2013.11.002
Kane CJ, Phelan KD, Han L, Smith RR, Xie J, Douglas JC, Drew PD (2011) Protection of neurons and microglia against ethanol in a mouse model of fetal alcohol spectrum disorders by peroxisome proliferator-activated receptor-gamma agonists. Brain Behav Immun 25(Suppl 1):S137–S145. doi:10.1016/j.bbi.2011.02.016
Kane CJ, Phelan KD, Douglas JC, Wagoner G, Johnson JW, Xu J, Drew PD (2013) Effects of ethanol on immune response in the brain: Region-specific changes in aged mice. J Neuroinflammation 10:66. doi:10.1186/1742-2094-10-66
Kelly YJ, Sacker A, Gray R, Kelly J, Wolke D, Head J, Quigley MA (2012) Light drinking during pregnancy: still no increased risk for socioemotional difficulties or cognitive deficits at 5 years of age? J Epidemiol Community Health 66(1):41–48. doi:10.1136/jech.2009.103002
Kent S, Bluthe RM, Dantzer R, Hardwick AJ, Kelley KW, Rothwell NJ, Vannice JL (1992) Different receptor mechanisms mediate the pyrogenic and behavioral effects of interleukin 1. Proc Natl Acad Sci U S A 89(19):9117–9120
Lewis CE, Thomas KG, Dodge NC, Molteno CD, Meintjes EM, Jacobson JL, Jacobson SW (2015) Verbal learning and memory impairment in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 39(4):724–732. doi:10.1111/acer.12671
Livy DJ, Parnell SE, West JR (2003) Blood ethanol concentration profiles: A comparison between rats and mice. Alcohol (Fayetteville, N.Y.) 29(3):165–171
Losco P (1992) Normal development, growth, and aging of the spleen. In: Mohr U, Dungwörth DL, Capen CC (eds) Pathobiology of the aging rat, vol 1. ILSI Press, Washington, D.C., pp. 75–94
Lupton C, Burd L, Harwood R (2004) Cost of fetal alcohol spectrum disorders. Am J Med Genet 127C(1):42–50. doi:10.1002/ajmg.c.30015
Mattson SN, Calarco KE, Lang AR (2006) Focused and shifting attention in children with heavy prenatal alcohol exposure. Neuropsychology 20(3):361–369
Monji A, Kato TA, Mizoguchi Y, Horikawa H, Seki Y, Kasai M, et al. (2013) Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuro-Psychopharmacol Biol Psychiatry 42:115–121. doi:10.1016/j.pnpbp.2011.12.002
Murawski NJ, Klintsova AY, Stanton ME (2012) Neonatal alcohol exposure and the hippocampus in developing male rats: effects on behaviorally induced CA1 c-fos expression, CA1 pyramidal cell number, and contextual fear conditioning. Neuroscience 206:89–99. doi:10.1016/j.neuroscience.2012.01.006
Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT (2008) Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation 5:10. doi:10.1186/1742-2094-5-10
Rezaie P, Patel K, Male DK (1999) Microglia in the human fetal spinal cord--patterns of distribution, morphology and phenotype. Brain Res Dev Brain Res 115(1):71–81
Rezaie P, Dean A, Male D, Ulfig N (2005) Microglia in the cerebral wall of the human telencephalon at second trimester. Cereb Cortex (New York, N.Y.: 1991) 15(7):938–949
Schwarz JM, Sholar PW, Bilbo SD (2012) Sex differences in microglial colonization of the developing rat brain. J Neurochem 120(6):948–963. doi:10.1111/j.1471-4159.2011.07630.x
Skaper SD, Facci L, Giusti P (2014) Neuroinflammation, microglia and mast cells in the pathophysiology of neurocognitive disorders: A review. CNS Neurol Disord Drug Targets 13(10):1654–1666
Skogerbo A, Kesmodel US, Wimberley T, Stovring H, Bertrand J, Landro NI, Mortensen EL (2012) The effects of low to moderate alcohol consumption and binge drinking in early pregnancy on executive function in 5-year-old children. BJOG 119(10):1201–1210. doi:10.1111/j.1471-0528.2012.03397.x
Squarzoni P, Oller G, Hoeffel G, Pont-Lezica L, Rostaing P, Low D, et al. (2014) Microglia modulate wiring of the embryonic forebrain. Cell Rep 8(5):1271–1279. doi:10.1016/j.celrep.2014.07.042
Streit WJ, Xue QS, Tischer J, Bechmann I (2014) Microglial pathology. Acta Neuropathol Commun 2:142. doi:10.1186/s40478-014-0142-6
Weisz J, Ward IL (1980) Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology 106(1):306–316. doi:10.1210/endo-106-1-306
Westbrook SR, Brennan LE, Stanton ME (2014) Ontogeny of object versus location recognition in the rat: acquisition and retention effects. Dev Psychobiol 56(7):1492–1506. doi:10.1002/dev.21232
Wyper KR, Rasmussen CR (2011) Language impairments in children with fetal alcohol spectrum disorders. J Popul Ther Clin Pharmacol = Journal De La Therapeutique Des Populations Et De La Pharamcologie Clinique 18(2):e364–e376
Yang JY, Xue X, Tian H, Wang XX, Dong YX, Wang F, et al. (2014) Role of microglia in ethanol-induced neurodegenerative disease: pathological and behavioral dysfunction at different developmental stages. Pharmacol Ther 144(3):321–337. doi:10.1016/j.pharmthera.2014.07.002
Zhu X, Lee HG, Perry G, Smith MA (2007) Alzheimer disease, the two-hit hypothesis: An update. Biochim Biophys Acta 1772(4):494–502
Acknowledgments
The authors would like to acknowledge the Klintsova Lab, including Karen Boschen and Kerry Cris, for assistance with the Analox machine; Kenneth Kirschner for assistance with the multiplex analysis; as well as Andrew Blades, Julie Gomez, Caitlin Posillico, and Jasmine Caulfield for their additional technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by P5P20GM103653-02.
Conflict of Interest
Laurne S. Terasaki declares that she has no conflicts of interest. Jaclyn M. Schwarz declares that she has no conflicts of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Electronic Supplementary Material
ESM 1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Terasaki, L.S., Schwarz, J.M. Effects of Moderate Prenatal Alcohol Exposure during Early Gestation in Rats on Inflammation across the Maternal-Fetal-Immune Interface and Later-Life Immune Function in the Offspring. J Neuroimmune Pharmacol 11, 680–692 (2016). https://doi.org/10.1007/s11481-016-9691-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-016-9691-8