Abstract
Neurodegenerative and neuropsychological disorders are becoming a greater proportion of the global disease burden; however the pathogenic mechanisms by which these disorders originate and contribute to disease progression are not well-described. Increasing evidence supports neuroinflammation as a common underlying component associated with the neuropathological processes that effect disease progression. This collection of articles explores the role of adaptive immunity in autoimmunity, neurodegeneration, neurotrauma, and psychological disorders. The section emphasizes the interactions of T cells with innate cellular responses within the CNS and the effects on neurological functions. One recurrent theme is that modified and aggregated self-proteins upregulate innate-mediated inflammation and provide a permissive environment for polarization of T cells to proinflammatory effector cells. Moreover, infiltration and reactivation of those T effector cells exacerbate neuroinflammation and oxidative stress to greater neurotoxic levels. Another recurrent theme in these disorders promotes diminished regulatory functions that reduce control over activated T effector cells and microglia, and ultimately augment proinflammatory conditions. Augmentation of regulatory control is discussed as therapeutic strategies to attenuate neuroinflammation, mitigate neurodegeneration or neuronal dysfunction, and lessen disease progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.

With diminishing prevalence of acute infectious disease and increasing aged population, a majority (54 %) of the global burden of disease (GBD) for the first time is associated with non-communicable diseases, and neurological disorders are rising to levels more prevalent than previously realized (Chin and Vora 2014). Neurological disorders from both infectious and non-infectious diseases, now account for over 11 % of the total deaths worldwide (Dua et al. 2006). Newer metrics such as disability-adjusted life years (DALYs), which consider not only the severity of the disorder, but also the chronicity, prevalence, and premature mortality, indicate that neurological disorders currently account for over 7 % the total GBD (Murray et al. 2012). Among the GBD for 1990–2010 by the World Health Organization (WHO), traditional neurodegenerative disorders such as Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS) comprised approximately 10 % of the total neurological burden, while stroke accounted for 58 % of the neurological DALYs. Mental and behavioral disorders accounted for 7.4 % of neurological burden, which included major depressive disorder (MDD) (2.5 %), anxiety disorders (1.1 %), drug/alcohol use (1.5 %), and schizophrenia (0.6 %). Epilepsy and migraine accounted for 9.9 % and 12.7 % of GBD, respectively, and other causes and disorders comprised 10.2 % of the neurological GBD. Exact metrics for GBD due to neurological injuries have proven difficult to obtain, presumably to under-reporting attributable to a variety of reasons that include lack of medical attention for minor injuries; inclusion into and reporting as multi-traumatic events; lack of reporting systems in many countries; or non-inclusion of incidences associated with war or civil unrest (Hyder et al. 2007). While WHO has defined parameters for evaluating neurological injuries and sequelae, the implementation of those parameters has not been uniformly applied world-wide, thus resulting in variable GBD estimates for trauma-associated neurological complications.
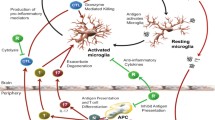
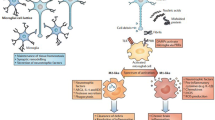
For many neurological disorders, etiologies are either complex or remain enigmatic. Recurrent neuropathological themes such as misfolded proteins, intraneuronal inclusions, neurodegeneration, chemical/neurotransmitter imbalances, and neuronal misfiring generate profound repercussions upon neuronal function and homeostasis, and provide a modicum of insight into possible mechanistic processes responsible for functional consequences. Inflammation represents a common neuropathological component that underlies many neurodegenerative disorders, stroke, peripheral and central neuronal injury, and neuropsychiatric disorders. The principal innate immune cell of the CNS is the microglia, but infiltrating monocytes and macrophages also can play a significant role. The cellular interactions of the immune and nervous systems are well-documented (Shie et al. 2011; Tian et al. 2012), and thus implicate the cytokine/chemokine network as a principal mechanistic component influencing the nervous system and the behaviors therefrom derived. As expected with tissue and neuronal injury/death and accompanying inflammation, cellular infiltration of many cell types into inflamed tissue sites are also a prominent feature.
T cells, as one infiltrating immune cell subset, are noted to surveille the CNS just as in other peripheral organs, but in smaller numbers than in the periphery (Rezai-Zadeh et al. 2009). Most mononuclear cells from normal cerebral spinal fluid are CD4+ T cells (70–80 %) with a central memory phenotype, while B lymphocytes and NK cells are found in low frequencies relative to their representation in blood (de Graaf et al. 2011). Under neurological stress, epitopes from self-proteins or modified self-proteins may initiate or become incorporated into the afferent immune response involving antigen presenting cells (APCs), such as dendritic cells or possibly microglia, and non-activated T cells (Carare et al. 2014). This may occur within the CNS or outside where antigens drain to the deep cervical lymph nodes and activate APCs to initiate afferent T cell responses (Laman and Weller 2013; Carare et al. 2014). Once in the circulation, activated T cells within the CNS vasculature flow by foci of neurological stress producing gradients of cytokines and chemoattractants that in turn induce endothelium to upregulate cell adhesion molecules and attract T cells. Attracted T cells undergo a complex process of adhesion molecule recognition and interaction that leads to slowing, rolling, crawling and capture along the endothelium with eventual extravasation to sites of inflammation (Lyck and Engelhardt 2012). Upon encountering cognate antigen in the context of MHC on APCs, antigen-specfic T cells undergo efferent reactivation to an effector T cell expressing effector-type specific cytokines. The effector T cell (Teff) type, cytokine milieu produced, and combined interactions with microglia, astrocytes, and neurons as well as the area of the brain where these interactions culminate, determine the eventual T cell-mediated influence on neurodegenerative and/or neuropsychological outcomes. Additional influences to clinical outcomes include the functions by several unique T cell lineages, such as forkhead box protein 3 (FoxP3)-expressing regulatory T cells (Tregs) and type 1 regulatory T cells (Tr1) that maintain immunological self-tolerance, suppress inflammatory responses, limit Teff cell responses, and mitigate neurodegenerative processes (Kleinewietfeld and Hafler 2014).
Legroux and Arbour inaugurate the special section on adaptive immunity in neurological disorders with the description of T cell phenotype and function in the prototypical autoimmune neurodegenerative disorder, multiple sclerosis (MS) (Legroux and Arbour 2015). As such, T cells are thought to be the major driver of pathology and disease progression in MS. Both CD4+ and CD8+ T cells from MS patients and in experimental allergic encephalomyelitis (EAE) recognize myelin antigens such as myelin basic protein (MBP), proteolipid protein (PLP), and myelin oligodendrocyte glycoprotein (MOG), but also other neuronal peptides. Triggering of autoreactive T cell responses and functional differentiation of self-antigen specific T cells to encephalitic states induce a proinflammatory immune cascade that includes infiltrates not only of T cells, but also B cells, macrophages, NK cells, granulocytes and reactive microglia (Weissert 2013). A critical step in this process is the permissive state for encephalitogenic T cells to breach the BBB and enter the brain parenchyma. After extravasation and entrance into the CNS, encephalitogenic T cells are reactivated to yield a storm of cytokines that is not only neurotoxic, but also can hyperactivate innate immune responses to neurotoxic levels. More recently, support has increased for Th17 cells as a principal component for MS progression,; however, a role for Th1 cells also has been noted since disease severity diminishes with adoptive transfer of Th17 cells alone compared to severity with transfer of Th1 and Th17 cell types (El-behi et al. 2010). The report proposes that T cell-glia crosstalk represents other mechanisms(s) that are necessary in MS for disease initiation and progression (Legroux and Arbour 2015).
Gender disparity in incidences of neurological disorders has been well-documented (Hanamsagara and Bilbob 2015), and is particularly increased for females in MS and myasthenia gravis (MG); however the mechanism(s) by which gender differences are established is not entirely clear. One notion is that the more robust immune function of females affords a predilection to autoimmune disorders (Selmi 2008). These gender differences are attributed to the role of gender-specific hormones and the differential effects bestowed upon the immune system (Hanamsagara and Bilbob 2015). In EAE, androgen therapy ameliorates or lessens disease progression with enhanced production of IL-10. Dr. Reddy and colleagues using a model of EAE and I-As dextramers, show that in vitro treatment with the androgen, dihydrotestosterone (DHT) reduces numbers of T cells that recognize cognate PLP-I-As dextramers, diminishes myelin-specific T cell-mediated responses as well as IFN-γ and IL-17 production, and increases IL-10 expression (Jia et al. 2015). Activation of caspases accompanied by increased T cell apoptosis and autophagy in stimulated T cells treated with DHT suggest a mechanism by which androgens reduce T cell-mediated immunity by triggering death programs and may afford a novel therapeutic strategy for inflammatory-driven neurological disorders.
Myasthenia gravis (MG) is an autoimmune neuromuscular disorder mediated by circulating auto-antibodies that target post-synaptic acetylcholine receptors (AChRs) at neuromuscular junctions and inhibit acetylcholine-mediated signaling. Drs. Zhang and Shao explore the role of Th17 cells and increased IL-17 production in MG, which appear critical in the development of experimental autoimmune MG (Wang et al. 2013; Zhang and Shao 2015).They posit that a Th17/IL-17 cascade is initially triggered which drives chronic inflammation and autoimmune disease progression. One negative regulator of innate and adaptive immunity is tumor necrosis factor (TNF)-α-induced protein 8-like-2 (TNFAIP8L2 or TIPE2), which is thought to control the initiation of immune responses and figures prominently in autoimmune initiation. This is suggested by deficiencies that lead to increased sensitivity to TLR signaling, levels of inflammatory cytokines, and inflammatory diseases. Therein, they report the reduced expression of TIPE2 by MG patients correlates with increased levels of IL-6, iL-17, and IL-21 in serum and in TLR-4 engaged peripheral blood mononuclear cells (PBMC) as well as increases in expression of RORγt, a transcription factor specific for Th17 development. Overexpression of TIPE2 by transfection of TIPE2-AAV constructs in MG patients’ PBMCs reverses TLR-4-mediated overexpression of the Th17 profile. This provides convincing evidence for the involvement of TIPE2 to reduce thresholds for innate signaling and serve as a mechanism in MG. Th17 involvement underscores one etiological scenario involving the loss of immune tolerance due to activation of pro-inflammatory thymic T cells that recognize dominant AChR epitopes, presented by thymic APCs, and are easily driven by low thresholds necessary for activation of afferent immune responses.
Alzheimer’s disease (AD) is the most common neurodegenerative disorder and accounts for a majority of dementia cases worldwide. Misfolded tau proteins and amyloidogenic proteins provide a mechanism by which APCs in peripheral lymphoid tissues are able to induce antigen specific T cells. In their article, Drs. McManus, Mills, and Lynch provide a narrative of T cell involvement in AD after induction of peripheral lymphoid T cells (McManus et al. 2015). They describe the initial entry of T cells into the brain and the conditions that facilitate increased penetration into the brain of AD patients due to the loss of BBB integrity and the diminished expression and interactions of tight junction proteins such as ZO-1, claudin-5 and occludin, resulting primarily from actions of inflammatory cytokines. Increased permeability of the BBB allows greater infiltration of T cells and interactions with glial cells within brain parenchyma. In the presence of the proinflammatory milieu, microglia increase amyloidogenic and APC functions to drive T cells to higher levels of inflammatory activation. The authors also provide a line of evidence for the putative role of astrocytes as APCs. With increased T cell infiltration and microglial/APC activation in the presence of plentiful levels of Aβ and misfolded tau protein, reactivation of antigen-specific effector T cells elevate T cell- and microglia-induced neurotoxic factors to levels that exacerbate hippocampal neuronal loss. In light of the unsuccessful vaccine trial with Aβ using Th1 adjuvants, the authors advance strategies for use of Th2 cells and Aβ antigens in the context of adjuvants that induce Th2 cells.
Parkinson’s disease (PD) is the most common neurodegenerative movement disorder and second most common neurodegenerative disorder after AD. Characteristic neuropathological hallmarks of PD include misfolded α-synuclein; Lewy body-neuronal inclusions comprised primarily of α-synuclein and ubiquitin; neuroinflammation; and ultimate loss of dopaminergic neurons and dopamine with corresponding motor dysfunction. The treatise by Drs. González, Contreras, and Pacheco on immunity in PD initially focuses on evidence for the importance of T cells in the CNS and their association with neuroinflammatory responses in PD and animal models (Gonzalez et al. 2015). Therein, they point out that in post-mortem brains of PD patients and animal models, ratios of CD4+ and CD8+ T cells are inverted from those typically found in the peripheral blood. In animal models of PD, CD4+ T cells have greater influence on neuropathological processes and disease progression than do CD8+ T cells. Of particular note are CD4+ Teffs with Th1 and Th17 expression profiles. This notion is underscored by numbers of peripheral CD4+ Teffs from PD patients that directly correlate with increased severity of motor dysfunction (Saunders et al. 2012). Gonzalex and colleagues explain that CD4+ Teffs activate and shift microglia to M1 proinflammatory status that in turn increases the extent of neuroinflammation and oxidative stress which elevates inflammatory mediators to levels that injure and kill dopaminergic neurons. CD4+ Teffs are thought to recognize neo-epitopes on self-proteins that are modified by inflammation and oxidative processes. One candidate modified self-protein is posited as nitrated α-synuclein that accumulates in neurons, is expelled from injured and dying neurons, and activates microglia and APCs in the periphery to induce pathogenic Teffs. They assert that regulation of proinflammatory processes in PD and PD models are under the purview of CD4+ regulatory T cells (Tregs) which attenuate neuroinflammatory processes and are neuroprotective. The authors propose a role of neurotransmitter-receptor interactions which also regulate immune cells. The expression of dopamine receptors on naïve, central memory, and effector memory CD4+ T cell subset (Kustrimovic et al. 2014) and that loss of dopamine in affected areas of the brain in PD leads to dysregulated adaptive and innate immunity with upregulation of proinflammatory responses is supportive of the authors' notion. The role for GABA is also discussed in the context of attenuating inflammation by induction of M2-type microglia, heightened Treg function and anti-inflammatory cytokines.
Proportionally, stroke is one of the largest contributors to the GBD and the largest among neurological disorders. Immune activation can affect acute pathogenic processes in stroke; however, the exact mechanisms of those processes remain largely undiscovered. Dr. Tang and colleagues provide a review of evidence that inflammatory responses associated with stroke are key to secondary brain damage and neurodegeneration, particularly in the context of repair and recovery (Klebe et al. 2015). They indicate that current evidence suggests macrophages and/or microglia are activated by initial injury and tissue destruction that accompany stroke, and carry out their function to clear tissue debris and partake in repair processes. They discuss the states of activation associated with macrophages/microglia and conclude that many of the markers and functions that define fully differentiated M1 and M2 types are not necessarily expressed after stroke. In fact, the innate immune response may take on a broader spectrum of marker expression and function that may be temporally- or spatially-defined depending on the extent of damage and brain region affected. Similar to the myeloid lineages, helper Teffs represent a range of pro-inflammatory and anti-inflammatory subsets that either augment or counter myeloid function (Gill and Veltkamp 2015). The authors indicate that microglia become activated shortly after stroke, peak within the week, and are resolved to basal levels within a few weeks (Klebe et al. 2015). A few days subsequent to microglial activation, macrophages and T cells infiltrate and peak by day 5, however, infiltrating Teff subsets that exhibit purely inflammatory or reparative phenotypes are not consistently reported. In models of stroke, limiting Teff function or augmenting anti-inflammatory responses by adoptive transfer of Tregs, neural stem cells, or pharmacological intervention tend to be neuroprotective, which suggests that proinflammatory, oxidative stressful and neurotoxic processes play a key pathogenic role early in stroke. However, the exact nature of Teff or Treg cell differentiation in response to stroke as well as the cognate self-antigens have yet to be determined.
Neuronal injury, whether being peripheral nerves, motor neurons, or in the context of traumatic brain injury, represents a major source of neurological disorder burden. The issue in determining the clinical prevalence and extent of the problem is the inconsistency of global reporting. In the USA, the estimated prevalence of burden associated with brain and spinal cord injuries collectively approaches 2 million people. Nevertheless, mechanisms of post-injury neuronal loss are not completely understood. Dr. Jones and colleagues discuss mechanisms of T cell-mediated neuroprotection in axotomized peripheral nerves (Jones et al. 2015). In their model, the absence of T or B cells leads to diminished survival of neurons which is restored after lymphoid reconstitution with anti-inflammatory CD4+ Th2-type Teff cells, but not pro-inflammatory Th1 cells. Interestingly, both Th2 and Th1 cells play a role in elongation and regeneration of axonal nerves after axotomy. They indicate that naïve T cells are recruited into a site of injury in a chemokine-dependent fashion and undergo activation and reactivation by antigen in the context of MHCII to differentiate into the appropriate Teff type. They suggest that CD4+ Teff cell-microglia communication is responsible in large part for production of BDNF and IL-10 neurotrophic factors that support neuronal repair and regeneration. However, the antigen(s) necessary for recognition by T cells that drive neuroprotective processes in neuronal injury has yet to be determined.
Due in part to better differential diagnostic methods, reporting of mental disorders has increased in prevalence and incidence from past estimates. For instance, major depressive disorders (MDD) now account for 40 % of the GBD for all mental and substance abuse disorders (Whiteford et al. 2013), however a complete picture of pathogenesis in MDD has yet to be delineated. Herein, Drs. Toben and Baune provide a narrative on the role of adaptive and innate immunity in MDD (Toben and Baune 2015). They review the cytokine hypothesis of MDD, which posits multiple effects of cytokines on neuroendocrine function, neuronal firing, neurotransmitter metabolism, and neurogenesis. In general, proinflammatory factors such as TNFα, IL-6, and C reactive protein appear more associated with depressive events (Gibney and Drexhage 2013). They impart that administration of LPS, IFN-γ, IL-2, TNFα or IL-1β to humans or in animal models leads to increased incidence of MDD and IL-6 levels, however deficiencies in IL-1β expression is neuroprotective. In depressed patients, in vitro T cell-mediated responses are found to be diminished; however, the mechanisms by which T cells and subsets contribute to the behavior have yet to be elucidated. The authors' meta-analyses on T cells in MDD confirm that in vitro T cell function was generally diminished in MDD, however no study assessed whether any specific T cell lineage or subset is associated with that functional diminution. The possibility that some T cells may have a greater predilection to apoptosis is suggested by increased numbers of CD4+ T cells that exhibit increased CD95 (FAS), BAX, oxidative stress mediators, and fragmented nuclei. The authors point that low levels of 5- hydroxytryptamine (5-HT) characterize MDD, but also act as an immune modulator for which 5-HT receptors are expressed by Tregs. Notably, depressed patients exhibit lower Treg numbers and higher expression levels of mRNA for mediators associated with pro-inflammatory processes. These immune aberrations are underscored after antidepressant therapy for 6 weeks results in increased Treg numbers and decreased expression of IL-1β and IL-6.
Schizophrenia (SCZ) is a severe chronic mental disorder of unknown etiology, but is generally thought to primarily involve an imbalance of dopamine and glutamate neurotransmitters, and may include others. It accounts for 7.4 % of the GBD for mental and substance abuse disorders (Whiteford et al. 2013). The narrative by Dr. Debnath provides evidence supporting an immune component in SCZ as demonstrated by multiple genome wide association studies (GWAS) studies (Debnath 2015). A current hypothesis posits that neuroinflammation and possibly neuronal degeneration play a key role in SCZ (Gibney and Drexhage 2013). He reports that a network of activated T cells and T cell subsets are present in SCZ patients as found in CSF and at high densities in post-mortem hippocampus. Thus, the possibility is broached that an underlying infection to which T cells are responding may be associated with development of SCZ. Indeed, increased CD4/CD8 ratios, higher numbers of Th17 cells, and increased expression of CD28 and CD152 (CTLA-4) have been found in drug-naïve SCZ patients, but were reduced after treatment with anti-psychotic drugs (APDs). Increased T cell-mediated immunity and evidence of processes and sequelae normally associated with autoimmune responses raise the possibility of an autoimmune-mediated development of SCZ (Gibney and Drexhage 2013; Ermakov et al. 2015). The authors show convincingly that APD treatment also has an effect on T cell profile relative to number; however the direction of change and subsets are not entirely consistent with all APD modalities and may represent the differential effects on peripheral immunity by different drugs or drug combinations, many of which have not been screened for effects on T cell-mediated immunity.
Finally, the perspective by Drs. Gendelman and Mosley discuss how the aberrancies of the innate and adaptive immune systems result in a spectrum of immune responses that correspondingly yield neurological outcomes ranging from fulminant autoimmunity with reactivity to unmodified self-proteins such as myelin components, to neurodegenerative disorders wherein responses to modified, misfolded and aggregated self-proteins such as Aβ, tau, α-synuclein, and SOD1 underlie the neuropathological processes (Gendelman and Mosley 2015). The functional unit of these aberrant responses is concentrated primarily in the interactions of Teffs and microglia which ultimately produce a microenvironment of inflammation and oxidative stress that augments self-protein modifications and further drives aberrant responses to levels that injure and kill neurons. Since control over these inflammatory processes is afforded by regulatory lymphocytes such as Tregs, they argue that new and unique therapeutic strategies should target increasing regulatory functions that attenuate inflammatory and oxidative processes, diminish proteinopathies, and mitigate neurodegenerative programs.
In the context of severity, chronicity, and disability, neurodegenerative and neuropsychological disorders represent a growing significant burden on global disease. The incidence and prevalence of these disorders are expected to increase with continued diminution of infectious diseases and increasing age of the population. While the etiologies of these diseases are not entirely delineated, common proinflammatory processes seem to play a prominent role in disease progression and clinical outcomes. Moreover, the modification and aggregation of self-proteins underlie neuropathologies associated with several neurodegenerative disorders. Cross-talk between Teffs and Tregs of the adaptive immune arm and microglia and macrophages of the innate immune arm typically control surveillance, debris removal, and repair. Apparent aberrancies in these processes and lack of adequate control, either due to continual exposure to modified self-proteins or diminished regulatory mechanisms, promote immune responses that exacerbate T cell and microglia function to levels that injure and kill neurons. Even for neuropsychological disorders, previously thought primarily due to neurotransmitter imbalances or neuronal misfiring, the specter of immune dysregulation that supports proinflammatory T cells, microglia, and cytokines seems exceedingly prominent. While T cells are associated with these neurological disorders, the identities of T cell subsets responsible for pathogenic activity, the antigens recognized by the T cells, and the particular cytokines that precipitate differential neurological responses have yet to be clearly delineated. In light of the neurotoxic proinflammatory and autoimmune processes in many of these disorders, and provided the opportunity to adequately control these processes, strategies to remediate diminished Treg and/or dysregulated Teff function as therapeutic targets should be vigorously examined.
References
Carare RO, Hawkes CA, Weller RO (2014) Afferent and efferent immunological pathways of the brain. Anatomy, function and failure. Brain Behav Immun 36:9–14
Chin JH, Vora N (2014) The global burden of neurologic diseases. Neurology 83:349–351
Debnath M (2015) Adaptive immunity in schizophrenia: functional implications of T cells in the etiology, Course and Treatment. J Neuroimmune Pharmacol. doi:10.1007/s11481-015-9626-9
Dua T, Garrido Cumbrera M, Mathers C, Saxena S (2006) Chapter 2. Global buden of neurological disorders: estimates and projections. In: Neurological Disorders: Public Health Challenges, pp 26–39. Geneva, Switzerland: World Health Organization Press.
de Graaf MT, Smitt PA, Luitwieler RL, van Velzen C, van den Broek PD, Kraan J, Gratama JW (2011) Central memory CD4+ T cells dominate the normal cerebrospinal fluid. Cytometry B Clin Cytom 80:43–50
El-behi M, Rostami A, Ciric B (2010) Current views on the roles of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. J NeuroImmune Pharmacol 5:189–197
Ermakov EA, Smirnova LP, Parkhomenko TA, Dmitrenok PS, Krotenko NM, Fattakhov NS, Bokhan NA, Semke AV, Ivanova SA, Buneva VN, Nevinsky GA (2015) DNA-hydrolysing activity of IgG antibodies from the sera of patients with schizophrenia. Open Biol 5:150064
Gendelman HE, Mosley RL (2015) A perspective on roles played by innate and adaptive immunity in the pathobiology of neurodegenerative disorders. J NeuroImmune Pharmacol
Gibney SM, Drexhage HA (2013) Evidence for a dysregulated immune system in the etiology of psychiatric disorders. J NeuroImmune Pharmacol 8:900–920
Gill D, Veltkamp R (2015) Dynamics of T cell responses after stroke. Curr Opin Pharmacol 26:26–32
Gonzalez H, Contreras F, Pacheco R (2015) Regulation of the neurodegenerative process associated to Parkinson’s disease by CD4+ T-cells. J NeuroImmune Pharmacol. doi:10.1007/s11481-015-9618-9
Hanamsagara R, Bilbob SD (2015) Sex differences in neurodevelopmental and neurodegenerative disorders: focus on microglial function and neuroinflammation during development. J Steroid Biochem Mol Biol. doi:10.1016/j.jsbmb.2015.09.039
Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC (2007) The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation 22:341–353
Jia T, Anandhan A, Massilamany C, Rajasekaran RA, Franco R, Reddy J (2015) Association of autophagy in the cell death mediated by dihydrotestosterone in autoreactive T cells independent of antigenic stimulation. J NeuroImmune Pharmacol. doi:10.1007/s11481-015-9633-x
Jones KJ, Lovett-Racke AE, Walker CL, Sanders VM (2015) CD4 + T cells and neuroprotection: relevance to motoneuron injury and disease. J NeuroImmune Pharmacol. doi:10.1007/s11481-015-9625-x
Klebe D, McBride D, Flores JJ, Zhang JH, Tang J (2015) Modulating the immune response towards a neuroregenerative peri-injury milieu after cerebral hemorrhage. J NeuroImmune Pharmacol. doi:10.1007/s11481-015-9613-1
Kleinewietfeld M, Hafler DA (2014) Regulatory T cells in autoimmune neuroinflammation. Immunol Rev 259:231–244
Kustrimovic N, Rasini E, Legnaro M, Marino F, Cosentino M (2014) Expression of dopaminergic receptors on human CD4+ T lymphocytes: flow cytometric analysis of naive and memory subsets and relevance for the neuroimmunology of neurodegenerative disease. J NeuroImmune Pharmacol 9:302–312
Laman JD, Weller RO (2013) Drainage of cells and soluble antigen from the CNS to regional lymph nodes. J NeuroImmune Pharmacol 8:840–856
Legroux L, Arbour N (2015) Multiple sclerosis and T lymphocytes: an entangled story. J NeuroImmune Pharmacol. doi:10.1007/s11481-015-9614-0
Lyck R, Engelhardt B (2012) Going against the tide–how encephalitogenic T cells breach the blood-brain barrier. J Vasc Res 49:497–509
McManus RM, Mills KH, Lynch MA (2015) T cells-protective or pathogenic in Alzheimer’s disease? J NeuroImmune Pharmacol. doi:10.1007/s11481-015-9612-2
Murray CJ et al. (2012) Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet 380:2197–2223
Rezai-Zadeh K, Gate D, Town T (2009) CNS infiltration of peripheral immune cells: D-day for neurodegenerative disease? J NeuroImmune Pharmacol 4:462–475
Saunders JA, Estes KA, Kosloski LM, Allen HE, Dempsey KM, Torres-Russotto DR, Meza JL, Santamaria PM, Bertoni JM, Murman DL, Ali HH, Standaert DG, Mosley RL, Gendelman HE (2012) CD4+ regulatory and effector/memory T cell subsets profile motor dysfunction in Parkinson's disease. J NeuroImmune Pharmacol 7:927–938
Selmi C (2008) The X in sex: how autoimmune diseases revolve around sex chromosomes. Best Pract Res Clin Rheumatol 22:913–922
Shie FS, Chen YH, Chen CH, Ho IK (2011) Neuroimmune pharmacology of neurodegenerative and mental diseases. J NeuroImmune Pharmacol 6:28–40
Tian L, Ma L, Kaarela T, Li Z (2012) Neuroimmune crosstalk in the central nervous system and its significance for neurological diseases. J Neuroinflammation 9:155
Toben C, Baune BT (2015) An act of balance between adaptive and maladaptive immunity in depression: a role for T lymphocytes. J NeuroImmune Pharmacol. doi:10.1007/s11481-015-9620-2
Wang J, Zheng S, Xin N, Dou C, Fu L, Zhang X, Chen J, Zhang Y, Geng D, Xiao C, Cui G, Shen X, Lu Y, Wang J, Dong R, Qiao Y, Zhang Y (2013) Identification of novel MicroRNA signatures linked to experimental autoimmune myasthenia gravis pathogenesis: down-regulated miR-145 promotes pathogenetic Th17 cell response. J NeuroImmune Pharmacol 8:1287–1302
Weissert R (2013) The immune pathogenesis of multiple sclerosis. J NeuroImmune Pharmacol 8:857–866
Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJ, Vos T (2013) Global burden of disease attributable to mental and substance use disorders: findings from the global burden of disease study 2010. Lancet 382:1575–1586
Zhang Y, Shao Z (2015) TIPE2 play a negative role in TLR4-mediated autoimmune T helper 17 cell responses in patients with myasthenia gravis. J Neuroimmune Pharmacol
Acknowledgments
I would like to thank and applaud the authors for providing a stellar assemblage of articles for this special section. Dr. Howard Gendelman deserves our heartfelt appreciation for providing the creativity, support and opportunity that made this section possible. Finally and most assuredly, Ms. Robin Taylor deserves our utmost thanks for providing expert editorial leadership and generally keeping us on track. This work was supported in part by National Institutes of Health grants R01 NS070190, P01 DA028555, P20 GM103480, R01 NS034239, and R01 NS077873.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that he has no conflict of interest.
Rights and permissions
About this article
Cite this article
Lee Mosley, R. Adaptive Immunity in Neurodegenerative and Neuropsychological Disorders. J Neuroimmune Pharmacol 10, 522–527 (2015). https://doi.org/10.1007/s11481-015-9640-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-015-9640-y