Abstract
Because of their neuroprotective properties, cannabinoids are being investigated in neurodegenerative disorders, mainly in preclinical studies. These disorders also include amyotrophic lateral sclerosis (ALS), a degenerative disease produced by the damage of the upper and lower motor neurons leading to muscle denervation, atrophy and paralysis. The studies with cannabinoids in ALS have been conducted exclusively in a transgenic mouse model bearing mutated forms of human superoxide dismutase-1, the first gene that was identified in relation with ALS. The present study represents the first attempt to investigate the endocannabinoid system in an alternative model, the transgenic mouse model of TAR-DNA binding protein-43 (TDP-43), a protein related to ALS and also to frontotemporal dementia. We used these mice for behavioral and histological characterization at an early symptomatic phase (70–80 days of age) and at a post-symptomatic stage (100–110 days of age). TDP-43 transgenic mice exhibited a worsened rotarod performance at both disease stages. This was accompanied by a loss of motor neurons in the spinal cord (measured by Nissl staining) and by reactive microgliosis (measured by Iba-1 immunostaining) at the post-symptomatic stage. We also detected elevated levels of the CB2 receptor (measured by qRT-PCR and western blotting) in the spinal cord of these animals. Double-staining studies confirmed that this up-regulation occurs in microglial cells in the post-symptomatic stage. Some trends towards an increase were noted also for the levels of endocannabinoids, which in part correlate with a small reduction of FAAH. Some of these parameters were also analyzed in the cerebral cortex of TDP-43 transgenic mice, but we did not observe any significant change, in agreement with the absence of anomalies in cognitive tests. In conclusion, our data support the idea that the endocannabinoid signaling system, in particular the CB2 receptor, may serve for the development of a neuroprotective therapy in TDP-43-related disorders. We are presently engaged in pharmacological experiments to investigate this possibility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease produced by the damage of the upper and lower motor neurons leading to muscle denervation, atrophy and paralysis (Hardiman et al. 2011). The damage of these neurons occurs by the combination of several cytotoxic events including, among others, excitotoxicity, chronic inflammation, oxidative stress and protein aggregation (Foran and Trotti 2009; Ferraiuolo et al. 2011; Renton et al. 2014), although the pathogenesis of ALS is still pending of complete identification. The disease may be sporadic (the most abundant cases; see Al-Chalabi and Hardiman 2013) or familial, associated with mutations in genes encoding for superoxide dismutase-1 (SOD-1), TAR-DNA binding protein-43 (TDP-43) or FUS (fused in sarcoma) protein, as well as the more recent CCGGGG hexanucleotide expansion in the C9orf72 gene (Hardiman et al. 2011; Renton et al. 2014). In familial cases, which account for only 5 % of all ALS cases, depending on the mutated gene, ALS can be accompanied by features of frontotemporal lobar dementia (FTD), which supports the idea that, rather than being one disorder, ALS belongs to a spectrum of disorders having motor but also cognitive deficits (Cruts et al. 2013). One of these dual genes is TARDBP encoding TDP-43, which is involved in pre-mRNA splicing, transport and/or stability (Buratti and Baralle 2010; Lagier-Tourenne et al. 2010). Mutations in TDP-43 represent a new type of proteinopathy characterized by the accumulation of TDP-43 in the cytosol in the form of protein aggregates (Janssens and Van Broeckhoven 2013). Transgenic mice for TDP-43 have been recently developed (reviewed in Tsao et al. 2012) and they represent crucial tools for the study of ALS and also FTD, alternative to the classic mutant SOD-1 mice generated in the 90’s (Ripps et al. 1995).
Despite the efforts aimed at developing novel therapies for symptoms and/or disease progression in ALS, this disorder still lacks an effective treatment, with the antiexcitotoxic agent riluzole (Rilutek®) as the only approved medicine (Habib and Mitsumoto 2011). Recent preclinical studies suggest that several cannabinoids, e.g., Δ9-tetrahydrocannabinol (Raman et al. 2004), cannabinol (Weydt et al. 2005), WIN55,212-2 (Bilsland et al. 2006), and the selective CB2 agonist AM1241 (Kim et al. 2006; Shoemaker et al. 2007), may be beneficial as neuroprotectant agents in ALS. We recently investigated the phytocannabinoid-based medicine Sativex® and found a strong preservation of spinal motor neurons, although the translation of this effect to the neurological status and the survival of animals was poor (Moreno-Martet et al. 2014), thus indicating: (i) that neuron-muscle joint may be still affected despite the neuron preservation; and (ii) the need to investigate additional phytocannabinoid combinations. The efficacy of these cannabinoid treatments possibly relates to the changes in certain elements of the endocannabinoid signalling, which may serve as pharmacological targets for the investigated cannabinoids. For example, the efficacy shown by compounds that target the CB2 receptor (Kim et al. 2006; Shoemaker et al. 2007) correlates with the fact that this receptor has been found to be overexpressed in microglial cells in ALS (Yiangou et al. 2006; Shoemaker et al. 2007). Similarly, the efficacy of FAAH inhibition/inactivation (Bilsland et al. 2006) agrees with the elevated levels of endocannabinoids found in the spinal cord in ALS (Witting et al. 2004; Bilsland et al. 2006).
The major problem with the studies that so far have investigated the neuroprotective potential of cannabinoids in ALS is that they were conducted exclusively in the transgenic mouse model bearing mutations in SOD-1 (Ripps et al. 1995), the first gene that was identified in relation with the disease (Rosen et al. 1993). There is no evidence of similar studies in additional ALS models whose development has been initiated after the discovery of the new ALS-related genes, in particular the TDP-43 transgenic mice mentioned above, which provides the advantage to serve to investigate also the cognitive deficits associated with ALS or with the ALS/FTD spectrum of diseases (Robberecht and Philips 2013). The present study represents the first attempt to investigate the endocannabinoid system in the TDP-43 transgenic mouse model. In all experiments, we used these mice and their wild-type controls at two ages: (i) an early symptomatic stage (70–80 days of age), and (ii) a post-symptomatic stage (100–110 days of age). It was not possible to work with older animals as, during the course of our experiments, a couple of studies (Guo et al. 2012; Esmaeili et al. 2013) demonstrated that TDP-43 transgenic mice used in our study (Wegorzewska et al. 2009) develop intestinal obstruction after the appearance of ALS symptoms, and that these intestinal problems cause premature death at the age of 120 days after birth in males, and later in females (Esmaeili et al. 2013), thus making difficult to work with older animals. We used animals at 70–80 and 100–110 days of age to investigate the damage of motor neurons in the spinal cord in relation with possible changes in endocannabinoid ligands, receptors and enzymes. Given the relevance of TDP-43 transgenic mice for FTD too, we also performed some biochemical and behavioral analysis in relation with cognitive processes and cortical structures.
Materials and Methods
Animals, Treatments and Sampling
All experiments were conducted with Prp-hTDP-43(A315T) transgenic and non-transgenic littermate sibling mice bred in our animal facilities from initial breeders purchased to Jackson Laboratories (Bar Harbor, ME, USA) and subjected to genotyping for identifying the presence or absence of the transgene containing the TDP-43 mutation (Wegorzawska et al. 2009). All animals were housed in a room with controlled photoperiod (08:00–20:00 light) and temperature (22 ± 1 °C) with free access to standard food and water. All experiments were conducted according to local and European rules (directive 2010/63/EU) and approved by the “Comité de Experimentación Animal” of our university (ref. CEA-UCM 56/2012). In a first experiment, we used non-transgenic and Prp-hTDP-43(A315T) transgenic male mice for a longitudinal study aimed at characterizing the appearance of the pathological phenotype at behavioral and histological levels. To this end, we analyzed these mice in the rotarod test at two different disease stages: (i) early symptomatic stage that included animals of 70-80 days after birth, an age at which, according to previous studies (Wegorzewska et al. 2009), motor deficits in mutant mice were not still evident, but that, in our hands, already proved a worsened rotarod performance; and (ii) post-symptomatic stage that included animals at the age of 100–110 days after birth. Possible cognitive deficits were also investigated but only at the post-symptomatic stage. Immediately after the last behavioral recording, animals were transcardially perfused with saline followed by fresh 4 % paraformaldehyde prepared in 0.1 M phosphate buffered-saline (PBS), pH 7.4, and their spinal cord were collected and post-fixed for two days at 4 °C, then immersed in 30 % of sucrose solution for another two days, and finally stored at −80 °C for Nissl staining and immunohistochemical analysis. In a second experiment, Prp-hTDP-43(A315T) transgenic male and female mice and their corresponding wild-type animals were euthanized at the early symptomatic phase and at the post-symptomatic stage and their spinal cords and brains (to dissect the cerebral cortex) were rapidly removed, frozen in 2-methylbutane cooled in dry ice, and stored at −80 °C for subsequent biochemical analysis of endocannabinoid receptors and enzymes (qRT-PCR and western blotting), as well as of endocannabinoid levels. In the two experiments, we examined the intestinal tract of euthanized animals to exclude those cases in which a premature intestinal lesion might have influenced our results. We did not find any case working with ages younger than 110 days after birth, according to the data published by Esmaeili et al. (2013). In all experiments, at least 5–8 animals were used per experimental group.
Behavioral Recording
TDP-43 transgenic and wild-type mice were evaluated for possible motor weakness using the rotarod test, using a LE8200 device (Panlab, Barcelona, Spain). After a period of acclimation and training (first session: 0 r.p.m. for 30s; second and third sessions: 4 r.p.m. for 60 s, with periods of 10 min between sessions) conducted 30 min before, animals were tested with an acceleration from 4 to 40 r.p.m. over a period of 300 s. Mice were tested for 3 consecutive trials with a rest period of approximately 15 min between trials and the mean of the 3 trials was calculated. Mice were also evaluated for possible cognitive deficits using the Water Morris test following a previously-published procedure (Patil et al. 2009).
Real Time qRT-PCR Analysis
Total RNA was extracted from spinal cord samples using SurePrep™ RNA/Protein Purification kit (Fisher BioReagents, Fair Lawn, NJ, USA). The total amount of RNA extracted was quantitated by spectrometry at 260 nm and its purity was evaluated by the ratio between the absorbance values at 260 and 280 nm, whereas its integrity was confirmed in agarose geles. To prevent genomic DNA contamination, DNA was removed and single-stranded complementary DNA was synthesized from 1 μg of total RNA using a commercial kit (Rneasy Mini Quantitect Reverse Transcription, Qiagen, Izasa, Madrid, Spain). The reaction mixture was kept frozen at −20 °C until enzymatic amplification. Quantitative real-time PCR assays were performed using TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA, U.S.A.) to quantify mRNA levels for CB1 receptor (ref. Mm00432621_s1), CB2 receptor (ref. Mm00438286_m1), FAAH (ref. Mm00515684_m1), MAGL (ref. Mm00449274_m1), DAGL (ref. Mm00813830_m1) and NAPE-PLD (ref. Mm00724596_m1) using GAPDH expression (ref. Mm99999915_g1) as an endogenous control gene for normalization. The PCR assay was performed using the 7300 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) and the threshold cycle (Ct) was calculated by the instrument’s software (7300 Fast System, Applied Biosystems, Foster City, CA, USA). Expression levels were calculated using the 2-ΔΔCt method, but, for presentation, data were transformed to the percentage over the mean obtained in the wild-type group for each parameter
Western Blot Analysis
We employed the Stain-Free technology which has recently demonstrated to be a more reliable, more robust, and more sensitive normalization tool for Western blot analysis when compared to traditional housekeeping protein normalization (Gürtler et al. 2013). Purified protein fractions were isolated using SurePrep™ RNA/Protein Purification kit (Fisher BioReagents, Fair Lawn, NJ, USA). Subsequently, 10–15 μg of protein were boiled for 5 min in Laemmli SDS loading buffer (10 % glycerol, 5 % SDS, 5 % β-mercaptoethanol, 0.01 % bromophenol blue and 125 mM TRIS-HCl pH 6.8) and loaded on TGX Stain-Free™ FastCast™ Acrylamide kit (12 % gradient; Bio-Rad Laboratories, Hercules, CA, USA), and then transferred to a PVDF membrane using Trans-Blot® Turbo™ Blotting System (Bio-Rad Laboratories, Hercules, CA, USA). Membranes were incubated overnight at 4 °C with the following antibodies: (i) rabbit anti-FAAH antibody (1:200; Cayman Chemical, Ann Abor, MI, USA), or (ii) rabbit anti-CB2 antibody (1:200; Cayman Chemical, Ann Abor MI, USA), followed by a second incubation during 2 h at room temperature with an ECL™ Anti-Rabbit IgG, Horseradish Peroxidase-linked whole antibody (1:5000; Ge Healthcare UK Limited, Buckinghamshire, UK). Reactive bands were detected by chemiluminescence with the Amersham™ ECL™ Prime Western Blotting Detection Reagent (Healthcare UK Limited, Buckinghamshire, UK). Images were analyzed on a ChemiDoc station with Quantityone software (Bio-Rad Laboratories, Madrid, Spain). Data were calculated as the ratio between the optical densities of the specific protein band and the total protein bands transferred to a PVDF membrane, and they were normalized as % over the control group for presentation. Representative blots are presented in Supplementary Fig. S1)
Analysis of Endocannabinoid Levels
Tissues were homogenized in 5 vol of chloroform/methanol/Tris–HCl 50 mM (2:1:1) containing 10 pmol of d8-anandamide, d4-palmitoylethanolamide (PEA), d4-oleylethanolamide (OEA) and d5-2-AG. Deuterated standards were synthesized from d8-arachidonic acid and ethanolamine or glycerol, or from d4-ethanolamine and palmitic or oleic acid, as described (Devane et al. 1992; Bisogno et al. 1997, respectively). Homogenates were centrifuged at 13,000 g for 16 min (4 °C), the aqueous phase plus debris were collected and extracted again twice with 1 vol of chloroform. The organic phases from the three extractions were pooled and the organic solvents evaporated in a rotating evaporator. Lyophilized extracts were resuspended in chloroform/methanol 99:1 by vol. The solutions were then purified by open bed chromatography on silica as described (Bisogno et al. 1997). Fractions eluted with chloroform/methanol 9:1 by vol. (containing anandamide, 2-AG, OEA and PEA) were collected and the excess solvent evaporated with a rotating evaporator, and aliquots analyzed by isotope dilution-liquid chromatography/atmospheric pressure chemical ionisation/mass spectrometry (LC-APCI–MS) carried out under conditions described previously (Marsicano et al. 2002) and allowing the separations of 2-AG, anandamide, OEA and PEA. MS detection was carried out in the selected ion monitoring mode using m/z values of 356 and 348 (molecular ion +1 for deuterated and undeuterated anandamide), 384.35 and 379.35 (molecular ion +1 for deuterated and undeuterated 2-AG), 304 and 300 (molecular ion +1 for deuterated and undeuterated PEA), and 330 and 326 (molecular ion +1 for deuterated and undeuterated OEA). The amounts of endocannabinoids and related N-acylethanolamines were expressed as pmol/mg or g of tissue.
Histological Procedures
Tissue Slicing
Fixed spinal cords were sliced with a cryostat at the lumbar level (L4-L6) to obtain coronal sections (20 μm thick) that were collected on gelatin-coated slides. Sections were used for procedures of Nissl-staining, immunohistochemistry and immunofluorescence.
Nissl Staining
Slices were used for Nissl staining using cresyl violet, as previously described (Alvarez et al. 2008), which permitted to determine the effects of particular treatments on cell number. Particle analysis from ImageJ software (U.S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/, 1997–2012) was used to count large motor neurons (>400 μm2) in the anterior horn (different sections per mouse).
Immunohistochemistry
Slices were preincubated for 20 min in 0.1 M PBS with 0.1 % Triton X-100, pH 7.4, and subjected to endogenous peroxidase blockade by 1 h incubation at room temperature in peroxidase blocking solution (Dako Cytomation, Glostrup, Denmark). Then, they were incubated in 0.1 M PBS with 0.01 % Triton X-100, pH 7.4, with one of the following primary antibodies: (i) polyclonal anti-rabbit Iba-1 antibody (Wako Chemicals, Richmond, VI, USA) used at 1/1000; (ii) polyclonal anti-rabbit GFAP antibody (Dako Cytomation, Glostrup, Denmark) used at 1/1000; (iii) polyclonal anti-rabbit CB1 receptor antibody (Frontier Institute, Hokkaido, Japan) used at 1/500; (iv) polyclonal anti-goat CB2 receptor antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) used at 1/100; (v) polyclonal anti-goat FAAH antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) used at 1/400; and (vi) polyclonal anti-rabbit MAGL antibody (Frontier Institute, Hokkaido, Japan) used at 1/50. Incubation was prolonged overnight at 4 °C, then sections were washed in 0.1 M PBS and incubated for 2 h at room temperature with the appropriate biotin-conjugated anti-goat or anti-rabbit (1:200; Vector Laboratories, Burlingame, CA, USA) secondary antibodies. Vectastain® Elite ABC kit (Vector Laboratories, Burlingame, CA, USA) and a DAB substrate–chromogen system (Dako Cytomation, Glostrup, Denmark) were used to obtain a visible reaction product. Negative control sections were obtained using the same protocol with omission of the primary antibody. All sections for each immunohistochemical procedure were processed at the same time and under the same conditions. A Nikon Eclipse 90i microscope and a Nikon DXM 1200 F camera were used for slide observation and photography.
Immunofluorescence
Slices were used for double-labelling studies after preincubation for 1 h with Tris-buffered saline with 1 % Triton X-100 (pH 7.5). Then, sections were sequentially incubated overnight at 4 °C with a polyclonal anti-Iba-1 (1:2000; Wako Chemicals, Richmond, VI, USA), followed by washing in Tris-buffered saline and a new incubation (at 37 °C for 2 h) with an Alexa 488 anti-rabbit antibody conjugate made in donkey (1:200; Biolegend, San Diego, CA, USA), rendering green fluorescence for anti-Iba-1. Sections were then washed again and incubated overnight at 4 °C with a polyclonal anti-CB2 receptor (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA). This was followed by washing in Tris-buffered saline and a further incubation (at room temperature for 2 h) with a biotin-conjugated anti-goat (1:200; Vector Laboratories, Burlingame, CA, USA) secondary antibody, followed by a new washing and an incubation (at 37 °C for 2 h) with red streptavidin (Vector Laboratories, Burlingame, CA, USA) rendering red fluorescence for anti-CB2 receptor. Sections were counter-stained with nuclear stain TOPRO-3-iodide (Molecular Probes, Eugene, OR, USA) to visualize cell nuclei. A SP5 Leica confocal microscopy was used for slide observation and photography.
Statistics
Data were assessed by unpaired Student’s t-test or two-way ANOVA followed by the Student-Newman-Keuls test or the Bonferroni test, as required.
Results
Characterization of TDP-43 Transgenic Mice
TDP-43 transgenic male mice already showed a worsened rotarod performance compared to wild-type animals at the early symptomatic stage, with a reduction in the time on the rod of a relatively similar magnitude to that found in TDP-43 transgenic male mice at the post-symptomatic stage (genotype: F(1,22) = 18.29, p < 0.0005; time: F(1,22) = 8.357, p < 0.01; Fig. 1a). We assumed that the reduction found at this late stage was originated by a loss of motor neurons quantified in the spinal cord with Nissl staining (Figs. 1b and c). Microgliosis was also evident in the spinal cord of these TDP-43 transgenic male mice at the post-symptomatic stage (Fig. 1d), but we did not obtain any evidence of astrogliosis labeled with GFAP (Fig. 1e). We also evaluated possible cognitive deficits in TDP-43 transgenic male mice, using the Water Morris test, but were unable to find any alteration at the post-symptomatic stage (see Supplementary Figure S2).
a Rotarod performance of TDP-43 transgenic and wild-type male mice at early symptomatic (70–80 days after birth) and postsymptomatic (100–110 days after birth) stages. Values are means ± SEM for 6–8 animals per group. Data were assessed by two-way analysis of variance followed by the Bonferroni test (*p < 0.05, **p < 0.01 versus wild-type). b Number of motor neurons stained with Nissl (indicated by arrows in representative images shown in panel c magnification was 5×) in the spinal cord of TDP-43 transgenic and wild-type male mice at the postsymptomatic (100–110 days after birth) stage. Values are means ± SEM for 6–8 animals per group. Data were assessed by the unpaired Student’s t-test (***p < 0.005). d and e Representative DAB immunostainings for Iba-1 (magnification was 10×) and GFAP (magnification was 5×) in the spinal cord of TDP-43 transgenic and wild-type male mice at the postsymptomatic (100-110 days after birth) stage. Reactive microglial cells are indicated with arrowheads
Analysis of the Endocannabinoid System in TDP-43 Transgenic Mice
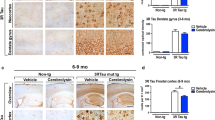
Next, we used TDP-43 transgenic male and female mice and their wild-type animals for recording the changes in different endocannabinoid genes, measured by qRT-PCR, at the two stages used in this study. We detected an important increase in mRNA levels for the CB2 receptor in the spinal cord of these animals at the post-symptomatic stage (Fig. 2), also evident at the early symptomatic stage (Fig. 2). These increases were found in both males and females (Fig. 2), and they were confirmed in post-symptomatic animals using Western blotting (Fig. 3a and representative blots in Supplementary Figure S1). We assumed that the up-regulation of CB2 receptors presumably occurs in reactive microglial cells. This was demonstrated in double-staining studies using antibodies against the CB2 receptor and the microglial marker Iba-1, which showed co-localization in the spinal cord (Fig. 4) that was confirmed by orthogonal reconstruction (data not shown). Some CB2 receptor-positive cells that were not positive for Iba-1 were also observed (Fig. 4). We hypothesize that these cells may be astrocytes, although this remains to be investigated. CB2 receptor immunostaining was significantly lower in the spinal cord of wild-type mice and the detection of co-localization with Iba-1 was almost negligible (Fig. 4). It is important to mention that, given that many of the available anti-CB2 antibodies lack the necessary specificity (reviewed in Atwood and Mackie 2010), we checked our antibody in a recently developed CB2-knockout mouse model in which the CB2 receptor protein is not truncated but completely absent (Vázquez et al. 2014). We found a complete disappearance of the CB2 receptor signal in these mice compared to wild-type and, in particular, TDP-43 transgenic mice (see Supplementary Figure S3), supporting that our CB2 receptor immunostaining was specific.
Gene expression for NAPE-PLD, DAGL, FAAH and MAGL enzymes and the CB1 and CB2 receptors measured by qRT-PCR in the spinal cord of male and female TDP-43 transgenic and wild-type mice at early symptomatic (70–80 days after birth) and postsymptomatic (100–110 days after birth) stages. Values correspond to the percentage over the mean obtained in the wild-type group for each parameter and are expressed as means ± SEM (n = 5 animals per group). Data were assessed by the unpaired Student’s t-test (*p < 0.05, **p < 0.01, ***p < 0.005 versus wild-type)
a and b Levels of CB2 receptors and FAAH measured by Western blotting in the spinal cord of male and female TDP-43 transgenic and wild-type mice at the postsymptomatic (100–110 days after birth) stage. Values correspond to the percentage over the mean obtained in the wild-type group for each parameter and are expressed as means ± SEM (n = 5 animals per group). Data were assessed by the unpaired Student’s t-test (*p < 0.05, **p < 0.01 versus wild-type). c, d and e Representative DAB immunostainings for CB1 receptors and FAAH and MAGL enzymes (magnification was 5×)
Double-immunofluorescence for CB2 receptors and Iba-1 (magnification was 40×), in the spinal cord of TDP-43 transgenic and wild-type male mice at the postsymptomatic (100-110 days after birth) stage. Cell nuclei were stained with TOPRO-3-iodide. Immunostainings were repeated in at least 3–5 animals per group. Cells positive for Iba-1 and CB2 receptors are indicated with arrowheads, whereas arrows indicate CB2 receptor-positive cells that were not labelled with Iba-1
Another important observation was a small reduction of FAAH gene expression seen in the spinal cord of TDP-43 transgenic males at the early symptomatic stage and of females at the post-symptomatic stage (Fig. 2). The changes in females were paralleled by a reduction in FAAH levels measured by Western blotting (Fig. 3b and representative blots in Supplementary Figure S1). We also measured FAAH levels by Western blotting in the spinal cord of post-symptomatic TDP-43 transgenic males but we did not find any difference compared to wild-type animals (Fig. 3b), in agreement with the lack of changes in FAAH gene expression (Fig. 2) and immunostaining (Fig. 3d) at the post-symptomatic stage. Given the changes in FAAH, we next measured the levels of endocannabinoids anandamide and 2-AG in the spinal cord of TDP-43 transgenic males and females at the two stages. Our data revealed that levels of anandamide showed a trend towards an increase in TDP-43 transgenic females (genotype: F(1,14) = 3.636, p = 0.077) and a statistically significant increase in TDP-43 transgenic males (genotype: F(1,16) = 7.294, p < 0.05), although the post-hoc test did not reveal specific differences between groups (Fig. 5). Similar trends were noted for 2-AG levels in TDP-43 transgenic males (genotype: F(1,16) = 10.55, p < 0.01), where the difference at the early symptomatic stage reached statistical significance (Fig. 5), but this was not the case for females (Fig. 5). In general, no significant changes were seen for other endocannabinoid-related lipids such as PEA and OEA (Table 1).
Levels of anandamide and 2-AG in the spinal cord of male and female TDP-43 transgenic and wild-type mice at early symptomatic (70–80 days after birth) and postsymptomatic (100–110 days after birth) stages. Values are expressed as means ± SEM (n = 5 animals per group). Data were assessed by the two-way analysis of variance (genotype x time) followed by the Bonferroni test (*p < 0.05 versus wild-type)
No changes were observed in other endocannabinoid elements, as revealed the data of gene expression for CB1 receptors and NAPE-PLD, DAGL and MAGL enzymes (Fig. 2), as well as those corresponding to CB1 receptor and MAGL immunolabellings, which were not different between TDP-43 transgenic and wild-type mice (Figs. 3c and e). Some of these parameters (gene expression for CB1 and CB2 receptors and for NAPE-PLD, DAGL, FAAH and MAGL enzymes, as well as endocannabinoid levels) were also analyzed in the cerebral cortex of TDP-43 transgenic male and female mice, but we did not obtain any significant change (Figs. 6 and 7), in agreement with the absence of cognitive effects in specific behavioral tests (see Supplementary Figure S2) and the lack of changes in a cognition-related marker as BDNF (data not shown). The only noteworthy effect was a certain trend towards an increase observed in CB2 receptor gene expression in post-symptomatic male mice (Fig. 6) and in 2-AG levels in TDP-43 transgenic females (genotype: F(1,15) = 10.45, p < 0.01; Fig. 7).
Gene expression for NAPE-PLD, DAGL, FAAH and MAGL enzymes and the CB1 and CB2 receptors measured by qRT-PCR in the cerebral cortex of male and female TDP-43 transgenic and wild-type mice at early symptomatic (70–80 days after birth) and postsymptomatic (100–110 days after birth) stages. Values correspond to the percentage over the mean obtained in the wild-type group for each parameter and are expressed as means ± SEM (n = 5 animals per group). Data were assessed by the unpaired Student’s t-test
Levels of anandamide and 2-AG in the cerebral cortex of male and female TDP-43 transgenic and wild-type mice at early symptomatic (70–80 days after birth) and postsymptomatic (100–110 days after birth) stages. Values are expressed as means ± SEM (n = 5 animals per group). Data were assessed by the two-way analysis of variance (genotype x time) followed by the Bonferroni test
Discussion
The relationship between the endocannabinoid system and ALS was revealed more than 10 years ago and has raised the possibility that endocannabinoid malfunctioning may contribute to pathogenesis, as well as that specific targets within this signalling system may be used to develop novel therapies for this disease, with activity on both specific symptoms, e.g., cramps, and disease progression (reviewed in de Lago et al. 2015). The issue has been even investigated at the clinical level with a few small clinical trials (Weber et al. 2010) and some studies using human samples (Yiangou et al. 2006). Most of the information, however, has been collected from preclinical studies and obtained in the only experimental model that has been available for long time, the mutant SOD-1 mouse, which has been extensively used for demonstrating the neuroprotective potential of some phytocannabinoids, e.g., Δ9-tetrahydrocannabinol (Raman et al. 2004), cannabinol (Weydt et al. 2005); phytocannabinoid combinations, e.g., Sativex®-like combination (Moreno-Martet et al. 2014); synthetic cannabinoids, e.g., the non-selective agonist WIN55,212-2 (Bilsland et al. 2006), the selective CB2 agonist AM1241 (Kim et al. 2006; Shoemaker et al. 2007); and FAAH inhibition (Bilsland et al. 2006). The recent discovery of new ALS-related genes has allowed the development of additional experimental models for the study of this disorder, and also for the study of FTD, which shares with ALS some of these mutated genes leading to the assumption that both disorders belong to an ALS-FTD spectrum of diseases (Cruts et al. 2013). One of these ALS/FTD-related genes encodes for the TDP-43 protein, for which transgenic mouse models have been recently developed (reviewed in Tsao et al. 2012) and provide novel aspects in relation with ALS pathogenesis, concerned in particular with possible alterations in RNA metabolism (Buratti and Baralle 2010; Lagier-Tourenne et al. 2010).
In the present study, we have used for the first time TDP-43 transgenic mice, which show motor anomalies (worsened rotarod performance) indicative of ALS, to investigate the changes in endocannabinoid signalling system in the spinal cord and the motor cortex. This information may be important for the adequate design of efficacious cannabinoid-based neuroprotective therapies for ALS. The most important observation that we made was in the spinal cord of these mice, in which we found the up-regulation of CB2 receptors (measured by qRT-PCR, Western blotting and immunostaining), described in other neurodegenerative and neuroinflammatory disorders (Fernández-Ruiz et al. 2007, 2010), including patients with ALS (Yiangou et al. 2006) and mutant SOD-1 mice (Shoemaker et al. 2007; Moreno-Martet et al. 2014). We show that this response takes place in reactive microglial cells, as revealed double-staining studies. This does not exclude that the up-regulation may also occur in other cell substrates, e.g., activated astrocytes, given that we also found CB2 receptor-positive cells that were not labelled with the microglial marker Iba-1, and that such a scenario has been already described in other disorders, e.g., Huntington’s disease (Sagredo et al., 2009). However, the presence of elevated CB2 receptor immunostaining in reactive microglia in the spinal cord is certainly evident in our study, as in other disorders, representing the first time that such response is found in an experimental model of a TDP-43-related proteinopathy. An additional interesting observation is that the elevation in gene expression for the CB2 receptor in TDP-43 transgenic mice occurred in both genders and appears to be already evident in early symptomatic stages in these transgenic animals, supporting the possibility that it is involved in the pathogenesis, possibly as an endogenous protective response.
Other endocannabinoid elements, e.g., CB1 receptors, synthesizing and hydrolyzing enzymes, were also investigated. However, they were in general not affected, with the only exception of FAAH enzyme, which experienced small reductions in both males (only gene expression) and females (both gene expression and protein levels), although the stage at which this reduction was found differs between genders (early symptomatic stage in males, and post-symptomatic stage in females). A reduction in FAAH frequently correlates with increased levels of endocannabinoids, in particular anandamide, and it is interesting that, although only as trends towards an increase, the analysis of endocannabinoids in the spinal cord of TDP-43 transgenic mice supported this correlation. For example, we found higher levels of anandamide and 2-AG in TDP-43 transgenic males at both stages, although the differences only reached statistical significance for 2-AG in the early symptomatic stage. This correlated with a reduced expression of FAAH gene also in males and at this stage. The elevation is in agreement with similar data found in mutant SOD-1 mice (Witting et al. 2004; Bilsland et al. 2006). The response in TDP-43 transgenic females followed the same trend, although only for anandamide at the two stages. This coincides with the reduction in gene expression and protein levels for FAAH found at the post-symptomatic stage in TDP-43 transgenic females.
We have also investigated whether similar responses, e.g., up-regulation of CB2 receptors, FAAH inactivation, changes in endocannabinoid levels, may also occur in cortical structures, and whether cognitive deficits are also evident in these mice. As mentioned above, mutations of TDP-43 are also related to the development of cognitive deficits in ALS and, additionally, they are representative of FTD, an ALS-related disorder within the so-called ALS/FTD spectrum (Janssens and Van Broeckhoven 2013). However, our data revealed the complete absence of cognitive deficits, as measured in the Water Morris test. This was in agreement with the data of endocannabinoid elements in the cerebral cortex of TDP-43 transgenic and wild-type mice.
In conclusion, our data support the idea that the endocannabinoid signaling system, in particular the CB2 receptor, which becomes up-regulated in reactive microglial cells in the spinal cord, may serve for the development of a neuroprotective therapy in TDP-43-related disorders. In addition, the inhibition of FAAH may also be of interest for such purpose, given the frequently lower levels found for this enzyme in the spinal cord of TDP-43 transgenic mice compared to wild-type animals. Even, we do not discard that targeting the CB1 receptor may have also therapeutic value, despite the levels of these receptors were not altered in TDP-43 transgenic mice. It is important to note that activation of CB1 receptors does not provide benefits in other neurodegenerative disorders due to the frequent loss of these receptors caused by neuronal death, a fact that does not occur in TDP-43 transgenic mice. We are presently investigating this issue in a series of pharmacological studies with different cannabinoid compounds in TDP-43 transgenic mice. Preliminary data show a significant recovery in the rotarod performance of TDP-43 transgenic mice after the treatment with the non-selective agonist WIN55,212-2 (data not shown), which will require further confirmation at the biochemical and histopathological levels.
References
Al-Chalabi A, Hardiman O (2013) The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol 9:617–628
Alvarez FJ, Lafuente H, Rey-Santano MC et al (2008) Neuroprotective effects of the nonpsychoactive cannabinoid cannabidiol in hypoxic-ischemic newborn piglets. Pediatr Res 64:653–658
Atwood BK, Mackie K (2010) CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol 160:467–479
Bilsland LG, Dick JR, Pryce G et al (2006) Increasing cannabinoid levels by pharmacological and genetic manipulation delay disease progression in SOD1 mice. FASEB J 20:1003–1005
Bisogno T, Sepe N, Melck D et al (1997) Biosynthesis, release and degradation of the novel endogenous cannabimimetic metabolite 2-arachidonoylglycerol in mouse neuroblastoma cells. Biochem J 322:671–677
Buratti E, Baralle FE (2010) The multiple roles of TDP-43 in pre-mRNA processing and gene expression regulation. RNA Biol 7:420–429
Cruts M, Gijselinck I, Van Langenhove T, van der Zee J, Van Broeckhoven C (2013) Current insights into the C9orf72 repeat expansion diseases of the FTLD/ALS spectrum. Trends Neurosci 36:450–459
de Lago E, Moreno-Martet M, Espejo-Porras F, Fernández-Ruiz J (2015) Endocannabinoids and amyotrophic lateral sclerosis. In: Fattore L (ed) Cannabinoids in neurologic and mental disease. Elsevier, The Netherlands, pp 99–124
Devane WA, Hanus L, Breuer A et al (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258:1946–1949
Esmaeili MA, Panahi M, Yadav S, Hennings L, Kiaei M (2013) Premature death of TDP-43 (A315T) transgenic mice due to gastrointestinal complications prior to development of full neurological symptoms of amyotrophic lateral sclerosis. Int J Exp Pathol 94:56–64
Fernández-Ruiz J, Romero J, Velasco G et al (2007) Cannabinoid CB2 receptor: a new target for controlling neural cell survival? Trends Pharmacol Sci 28:39–45
Fernández-Ruiz J, García C, Sagredo O, Gómez-Ruiz M, de Lago E (2010) The endocannabinoid system as a target for the treatment of neuronal damage. Expert Opin Ther Targets 14:387–404
Ferraiuolo L, Kirby J, Grierson AJ, Sendtner M, Shaw PJ (2011) Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat Rev Neurol 7:616–630
Foran E, Trotti D (2009) Glutamate transporters and the excitotoxic path to motor neuron degeneration in amyotrophic lateral sclerosis. Antioxid Redox Signal 11:1587–1602
Guo Y, Wang Q, Zhang K, An T, Shi P, Li Z, Duan W, Li C (2012) HO-1 induction in motor cortex and intestinal dysfunction in TDP-43 A315T transgenic mice. Brain Res 1460:88–95
Gürtler A, Kunz N, Gomolka M et al (2013) Stain-Free technology as a normalization tool in Western blot analysis. Anal Biochem 433:105–111
Habib AA, Mitsumoto H (2011) Emerging drugs for amyotrophic lateral sclerosis. Expert Opin Emerg Drugs 16:537–558
Hardiman O, van den Berg LH, Kiernan MC (2011) Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat Rev Neurol 7:639–649
Janssens J, Van Broeckhoven C (2013) Pathological mechanisms underlying TDP-43 driven neurodegeneration in FTLD-ALS spectrum disorders. Hum Mol Genet 22:R77–R87
Kim K, Moore DH, Makriyannis A, Abood ME (2006) AM1241, a cannabinoid CB2 receptor selective compound, delays disease progression in a mouse model of amyotrophic lateral sclerosis. Eur J Pharmacol 542:100–105
Lagier-Tourenne C, Polymenidou M, Cleveland DW (2010) TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration. Hum Mol Genet 19:R46–R64
Marsicano G, Wotjak CT, Azad SC et al (2002) The endogenous cannabinoid system controls extinction of aversive memories. Nature 418:530–534
Moreno-Martet M, Espejo-Porras F, Fernández-Ruiz J, de Lago E (2014) Changes in endocannabinoid receptors and enzymes in the spinal cord of SOD1(G93A) transgenic mice and evaluation of a Sativex®-like combination of phytocannabinoids: interest for future therapies in amyotrophic lateral sclerosis. CNS Neurosci Ther 20:809–815
Patil SS, Sunyer B, Höger H, Lubec G (2009) Evaluation of spatial memory of C57BL/6 J and CD1 mice in the Barnes maze, the Multiple T-maze and in the Morris water maze. Behav Brain Res 198:58–68
Raman C, McAllister SD, Rizvi G et al (2004) Amyotrophic lateral sclerosis: delayed disease progression in mice by treatment with a cannabinoid. Amyotroph Lateral Scler Other Motor Neuron Disord 5:33–39
Renton AE, Chiò A, Traynor BJ (2014) State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci 17:17–23
Ripps ME, Huntley GW, Hof PR, Morrison JH, Gordon JW (1995) Transgenic mice expressing an altered murine superoxide dismutase gene provide an animal model of amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A 92:689–693
Robberecht W, Philips T (2013) The changing scene of amyotrophic lateral sclerosis. Nat Rev Neurosci 14:248–264
Rosen DR, Siddique T, Patterson D et al (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362:59–62
Shoemaker JL, Seely KA, Reed RL et al (2007) The CB2 cannabinoid agonist AM-1241 prolongs survival in a transgenic mouse model of amyotrophic lateral sclerosis when initiated at symptom onset. J Neurochem 101:87–98
Tsao W, Jeong YH, Lin S, Ling J, Price DL, Chiang PM, Wong PC (2012) Rodent models of TDP-43: recent advances. Brain Res 1462:26–39
Vázquez C, García MC, Tolón RM et al (2014) New mouse model for the study of CB2 cannabinoid receptors. The 24th Annual Symposium on the Cannabinoids. International Cannabinoid Research Society, Research Triangle Park, NC, USA, page 260
Weber M, Goldman B, Truniger S (2010) Tetrahydrocannabinol (THC) for cramps in amyotrophic lateral sclerosis: a randomised, double-blind crossover trial. J Neurol Neurosurg Psychiatry 81:1135–1140
Wegorzewska I, Bell S, Cairns NJ, Miller TM, Baloh RH (2009) TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci U S A 106:18809–18814
Weydt P, Hong S, Witting A et al (2005) Cannabinol delays symptom onset in SOD1 (G93A) transgenic mice without affecting survival. Amyotroph Lateral Scler Other Motor Neuron Disord 6:182–184
Witting A, Weydt P, Hong S et al (2004) Endocannabinoids accumulate in spinal cord of SOD1 G93A transgenic mice. J Neurochem 89:1555–1557
Yiangou Y, Facer P, Durrenberger P et al (2006) COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol 6:12
Acknowledgements
This work has been supported by grants from CIBERNED (CB06/05/0089), MINECO (SAF2012/39173), CAM (S2011/BMD-2308), Alzheimer’s Association USA and GW Pharmaceuticals Ltd. These agencies had no further role in study design, the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication. Francisco Espejo-Porras is a predoctoral fellow supported by the MINECO (FPI Programme). Authors are indebted to Yolanda García-Movellán for administrative assistance.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Eva de Lago and Javier Fernández-Ruiz shared the senior authorship of this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
Representative blots for CB2 receptors and FAAH, always corresponding to tissues from females. Bottom panels are the total protein bands transferred to a PVCF membrane that were used as loading controls. (PPTX 3947 kb)
Figure S2
Behavioral recording in the Water Morris test of TDP-43 transgenic and wild-type male mice at the postsymptomatic (100-110 days after birth) stage. Values are means ± SEM for 6-8 animals per group. Data were assessed by the unpaired Student’s t-test. (PPTX 73 kb)
Figure S3
Immunofluorescence for CB2 receptors (magnification was 40x) showing a complete loss of immunostaining in the spinal cord of CB2 receptor-knockout mice when compared with the signal found in wild-type mice and, in particular, TDP-43 transgenic mice at the postsymptomatic (100-110 days after birth) stage. Immunostainings were repeated in at least 3 animals per group. (PPTX 241 kb)
Rights and permissions
About this article
Cite this article
Espejo-Porras, F., Piscitelli, F., Verde, R. et al. Changes in the endocannabinoid signaling system in CNS structures of TDP-43 transgenic mice: relevance for a neuroprotective therapy in TDP-43-related disorders. J Neuroimmune Pharmacol 10, 233–244 (2015). https://doi.org/10.1007/s11481-015-9602-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-015-9602-4