Abstract
Fibroblast growth factor-2 (FGF2), also known as basic FGF, is a multi-functional growth factor. One of the 22-member FGF family, it signals through receptor tyrosine kinases encoding FGFR1-4. FGF2 activates FGFRs in cooperation with heparin or heparin sulfate proteoglycan to induce its pleiotropic effects in different tissues and organs, which include potent angiogenic effects and important roles in the differentiation and function of the central nervous system (CNS). FGF2 is crucial to development of the CNS, which explains its importance in adult neurogenesis. During development, high levels of FGF2 are detected from neurulation onwards. Moreover, developmental expression of FGF2 and its receptors is temporally and spatially regulated, concurring with development of specific brain regions including the hippocampus and substantia nigra pars compacta. In adult neurogenesis, FGF2 has been implicated based on its expression and regulation of neural stem and progenitor cells in the neurogenic niches, the subventricular zone (SVZ) and the subgranular zone (SGZ) of the hippocampal dentate gyrus. FGFR1 signaling also modulates inflammatory signaling through the surface glycoprotein CD200, which regulates microglial activation. Because of its importance in adult neurogenesis and neuroinflammation, manipulation of FGF2/FGFR1 signaling has been a focus of therapeutic development for neurodegenerative disorders, such as Alzheimer’s disease, multiple sclerosis, Parkinson’s disease and traumatic brain injury. Novel strategies include intranasal administration of FGF2, administration of an NCAM-derived FGFR1 agonist, and chitosan-based nanoparticles for the delivery of FGF2 in pre-clinical animal models. In this review, we highlight current research towards therapeutic interventions targeting FGF2/FGFR1 in neurodegenerative disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction: background on FGF2
Fibroblast growth factors (FGFs) are a superfamily of proteins, most of which bind heparin and extracellular heparin sulfate proteoglycans (HSPGs) and have a homologous central core of 140 amino acids (Burgess & Maciag 1989). FGF2 has pleiotropic effects in different tissues and organs, including potent angiogenic effects and an important role in the differentiation and function of the central nervous system (CNS). In human, five different polypeptides can be formed from the same FGF2 gene via five different mRNA translation initiation sites (Florkiewicz & Sommer 1989; Arnaud et al. 1999). In mouse, FGF2 mRNA generates two 22/21 kDa high molecular weight (HMW) isoforms and one 18 kDa low molecular weight (LMW) isoform. Signaling of FGF2 occurs through the high-affinity tyrosine kinase receptors FGFR1-4 (Jaye et al. 1992), however for the purposes of this review we will focus on its main receptor in the CNS, FGFR1. FGF2 is an established neurogenic factor for proliferation and differentiation of multipotent neural stem cells both during development and in the adult mouse brain(Tao et al. 1996; Rai et al. 2007; Werner et al. 2011).
FGF2 and neurogenesis
FGF2/FGFR expression in development and in the adult
FGF2 is central to development of the CNS, which explains its importance in adult neurogenesis. During development, high levels of FGF2 are detected from neurulation onwards(Murphy et al. 1994). Moreover, expression of FGF2 and its receptors is temporally and spatially regulated during development, concurring with neurogenesis in specific brain regions (Powell et al. 1991). For example, in the inferior colliculus and occipital cortex of the postnatal rat brain, expression of FGF2 and FGFR2 increased over the first month, with no increase in FGFR1 mRNA. In contrast, in the cerebellum, FGF2 and FGFR1 mRNA expression was highest at postnatal day 1, but FGFR2 expression showed little change with age (el-Husseini et al. 1994). This regional and temporal specificity of FGF2 and FGFR expression may contribute to its regulation in the development of specific neuronal populations within different regions, such as dopaminergic neurons in the nigrostriatal pathway in mice (Baron et al. 2012).
In the adult CNS, FGF2 is expressed in the neurogenic niches (the subventricular zone of the lateral ventricles–SVZ; and the subgranular zone of the hippocampal dentate gyrus–SGZ) and has been implicated in the control of adult neurogenesis based on changes in proliferation and differentiation of adult neural stem and progenitor cells (Rai et al. 2007; Werner et al. 2011). In the SVZ, FGF2 was found to be highly expressed by glial fibrillary acidic protein (GFAP)–positive cells, but not by nestin–positive immature neurons, suggesting a pro–proliferative role of astrocytes and GFAP—positive radial glia or neural stem cells in this area (Newman et al. 2000). Indeed, astrocytic FGF2 has been implicated in enhanced neurogenesis in the SGZ following acute stress (Kirby et al. 2013). One of the FGF2 receptors, FGFR1, which is also highly expressed in neural stem cells (NSCs) in the SVZ and SGZ, is essential for hippocampal NSC proliferation. Ohkubo et al. (2004) showed that genetic deletion of FGFR1 in cells of the radial glial lineage, which are the progenitors of the majority of pyramidal neurons of the mouse dentate gyrus, resulted in dramatically reduced proliferation during embryonic development, accompanied by reduced hippocampal volume and impaired growth during postnatal development (Ohkubo et al. 2004). In the adult rat CNS, FGFR1 and FGFR4 are predominantly expressed in neurons, whereas FGFR2 and FGFR3 are more highly expressed in oligodendrocytes and astrocytes, respectively (Asai et al. 1993) (Miyake et al. 1996). It was found more than a decade ago that FGF2 administration to neuronal progenitor cells isolated from the septum and striatum of adult rats induces their proliferation in vitro, evidence that in the brain, neurogenic capacity is due not only to intrinsic properties of NPCs, but to regional differences in regulatory signals and receptor expression with FGF2/FGFR signaling being central to this capacity (Palmer et al. 1995).
FGF2 and proliferation
FGF2 regulates NSC propagation both in vitro and in vivo. In vitro, it has been found to stimulate efficient neural stem cell proliferation and can also induce proliferation of adult mouse stem cells by maintaining these progenitor in the cell cycle and preventing them from further differentiation (Gritti et al. 1996).
Several studies have shown the importance of FGF2 in NSC proliferation in vivo. After subcutaneous injection of FGF2, there was a 30 % increase in proliferating granule cell precursors in the external granule layer of the newborn rat cerebellum, and a significant increase in DNA synthesis in the SVZ and hippocampus, but not in the basal pons or cerebral cortex, confirming the regional specificity of FGF2 signaling in cell proliferation (Tao et al. 1996). Furthermore, treatment with neutralizing antibodies to FGF2 reduced proliferation of cerebellar and hippocampal precursor cells in postnatal day 1 (P1) rats (Tao et al. 1997), and chronic infusion of FGF2 into the lateral ventricle of middle-aged rats led to increased DCX-positive neuronal precursors (Rai et al. 2007). However, although these studies show that pharmacological addition or blockade of FGF2 alters proliferation, several studies of FGF2−/− mice show no deficit in overall NSC proliferation (Dono et al. 1998) (Werner et al. 2011), suggesting that FGF2 is a critical neurogenic factor, but that its neurogenic role can be compensated.
FGF2 and differentiation
In contrast to studies describing FGF2 as a proliferative factor, multiple groups have shown that FGF2 deficiency does not cause defects in neuronal precursor proliferation during development and in adult neurogenesis. Dono et al. reported that FGF2-deficient mice are viable and show normal neuronal progenitor proliferation during development, but that a fraction of these progenitors fail to colonize their target layers in the cerebral cortex, implying that FGF2 controls fate, migration and differentiation rather than proliferation of neuronal progenitors during development (Dono et al. 1998). The authors concluded that although FGF2 had been shown as an essential proliferative factor in vitro, FGF2 in fact functions as a migratory signal for neural progenitors, or alternatively its deficiency is functionally compensated by other FGFs in vivo. Werner et al. (2011) demonstrated that FGF2 (−/−) mice had no deficits in overall proliferation of stem cells in the adult dentate gyrus as measured by total BrdU incorporation, but did show impairments in differentiation of progenitors assigned to the neuronal lineage, being double-positive for BrdU and doublecortin (DCX; a marker for immature neurons) or BrdU and neuronal nuclei (NeuN; a marker for mature neurons)(Werner et al. 2011). In this study, exogenous FGF2 was unable to rescue the impairment, suggesting that LMW FGF2 is ineffective and HMW FGF2 isoforms may function as differentiation factors. Further study is needed since this was only tested in ex vivo hippocampal slices and not in vivo.
Role of isoform–specific FGF2 signaling in neurogenesis
FGF2 mRNA generates several isoforms, which have been suggested to mediate differential signaling in neurogenesis. In human, five different polypeptides can be formed from the same FGF2 gene via five different mRNA translation initiation sites (Florkiewicz & Sommer 1989) (Arnaud et al. 1999). In mouse, FGF2 mRNA generates two 22/21 kDa high molecular weight (HMW) isoforms and one 18 kDa low molecular weight (LMW) isoform. Translation of the 18 kDa LMW isoform is initiated at an internal AUG codon, while translation of the HMW isoforms are initiated at CUG codons (86, 319, 346, 361) that are 5’ to the AUG site and thus contain the entire 18 kDa amino acid sequence plus N-terminal extensions of different lengths. All isoforms contain a carboxyl-terminal bipartite nuclear localization signal (NLS). The HMW isoforms (34, 24, 22.5 and 22 kDa) also contain an amino-terminal Glutamic acid—Arginine (GR) repeat domain that acts as an NLS (Delrieu 2000). The CUG-initiated HMW isoforms are predominantly localized in the nucleus and function in an autocrine or intracrine manner (Delrieu 2000), while the 18 kDa AUG-initiated isoform is predominantly localized in the cytoplasm but can be secreted, and binds to plasma membrane receptors including FGFR (Touriol et al. 2003). Intriguingly, FGF2 does not have a secretion signal peptide, but is secreted by an atypical ER/golgi-independent protein secretion pathway(Schafer et al. 2004). Although the pathway or pathways by which it is secreted remain unclear, studies have shown that FGF2 membrane translocation is unidirectional and requires integral and peripheral membrane proteins (Schafer et al. 2004), including the Na+/K+−ATPase(Florkiewicz et al. 1998) and extracellular membrane-proximal HSPGs (Zehe et al. 2006).
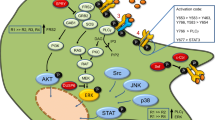
Differential isoform expression is highly regulated in both human and mouse, and LMW and HMW isoforms are thought to play unique roles in the cell. In vitro, differential isoform expression is translationally regulated by stress and cell density in non-transformed cells, but is constitutively active via the IRES in transformed cells (Touriol et al. 2003). HMW FGF2 is known to interact with nuclear Fibroblast Growth Factor Receptor-1 (nFGFR1), which acts as a developmental gene regulator via interactions with HMW FGF2, CREB-binding protein (CBP), and Ribosomal S6 Kinase-1 (RSK1), forming a transcription regulator complex which has been shown to regulate cell differentiation(Fang et al. 2005). CBP induces gene expression associated with neuronal development and functions (Stachowiak et al. 2003). Indeed, CBP is critical in both short and long-term memory formation and regulates the gene expression of calcium-calmodulin-dependent kinases (CaMKs) and excitatory glutamate receptors: N-methyl-D-aspartate (NMDA) and 2-amino-3-(5-methyl-3-oxo-1,2- oxazol-4-yl) propanoic acid (AMPA) receptors (Chen et al. 2010; Korzus et al. 2004). Our preliminary study indicates that several neurogenic genes, such as PROX1 and SEMA5A, are highly expressed in the hippocampus of AAV2/1-FGF2 infected vs. AAV2/1-GFP infected APP + PS1 mice, which provides a potential mechanism of enhanced neurogenesis (Fig. 1).
Potential mechanism of FGF2-induced neurogenesis. a Above: Heat map of differentially expressed genes derived from Affymetrix Genechip analysis of APP + PS1 mice infected with AAV2/1-FGF2 or AAV2/1-GFP vs. wildtype (WT) uninfected control. Several neurogenesis-related functions, such as neuronal differentiation, apoptosis, synaptic plasticity and nervous system development are among the most highly differentially regulated gene clusters. Below: 5 most significantly differentially regulated genes, as measured by one-way ANOVA and Tukey post hoc. PKP2, plakophilin 2; CPNE7, copine VII; PROX1, prospero-related homeobox 1; RAS1, RAS, dexamethasone-induced 1; SEMA5A, semaphorin-5A. b Scheme of potential mechanism of FGF2-induced neurogenesis: binding of high-molecular weight (HMW) FGF2 to nuclear FGFR1 leads to induction of neurogenic genes, such as PROX1 and SEMA5A, which are highly expressed in hippocampus of AAV2/1-FGF2 infected vs. AAV2/1-GFP infected APP + PS1 mice (see A). HMW FGF2 also binds to anti-apoptotic molecules apoptosis inhibitor 5 (Api5) and survival motor neuron 1 (SMN) to potentiate cell survival
Furthermore, we have found that AAV-FGF2- infected neural stem cells mainly express HMW FGF2 in the nucleus and initiate neuronal differentiation in vitro, which is apparently a different mode of action from the mitogenic effect of LMW FGF2. In support of this idea, our laboratory recently found that silencing of endogenous CBP suppresses neuronal differentiation of neural stem cells expressing either wildtype (WT) or HMW FGF2. Interestingly, HMW FGF2 also binds to anti-apoptotic molecules Api5 and SMN and potentiates cell survival, suggesting further functional diversity as a neurotrophic factor (See Fig. 1b)(Krejci et al. 2007; Claus et al. 2003).
LMW FGF2, on the other hand, is a classic mitogenic factor, and induces transformation of normal or non-tumorigenic cells into tumor cells (Quarto et al. 1991). The differential functions of HMW and LMW isoforms are cell-type specific. In one study, HMW FGF2 was found to inhibit rat C6 glioma cell proliferation. Expression of HMW FGF2 in C6 glioma cells showed a cell-cycle arrest at G2M, suggesting a regulatory role of HMW FGF2 in entry into mitosis (Lemiere et al. 2008). However, in another study by the same group, HMW or LMW FGF2 stimulated cell proliferation in the human U251MG glioma cell line, an effect believed to be mediated by the nuclear forms of FGFR1 (Joy et al. 1997). On the other hand, some cell types such as rat pheochromocytoma (PC12) and Schwann cell precursors show differentiation after over-expression of HMW FGF2 (Grothe et al. 1998). These studies suggest that dependent on the cell type, HMW FGF2 can function as either a proliferation or differentiation factor, which may reflect the fine balance of different HMW FGF2- interacting molecules and their effect on signal transduction and gene expression.
FGF2 and synaptic plasticity
FGF2 has also been shown to modulate synaptic plasticity and axonal branching in vitro and in vivo. FGF2 applied to rat cerebral cortical neurons enhances neurite outgrowth(Morrison et al. 1986) and axonal branching in hippocampal neurons (Aoyagi et al. 1994). Application of FGF2 enhanced LTP in rat hippocampal slices (Terlau & Seifert 1990), and intracerebroventricular (ICV) injection of EGF and FGF2 promoted LTP generation in synapses between perforant path and dentate granule cells of anesthetized rats (Ishiyama et al. 1991). Although the exact mechanism of FGF2’s synaptic effects is not fully understood, the dual action of FGF2 as a neurotrophic factor and inducer of synaptic plasticity is highly significant for development of therapies, as it has the potential to aid in the incorporation of new cells into a strengthened network.
FGF2 and neurodegeneration: therapeutic innovations
FGF2 in animal models of Alzheimer’s disease
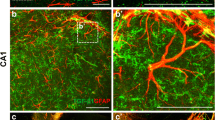
In line with its role in neurogenesis and synaptic enhancement, FGF2 has proven to be highly efficient at the regeneration of neurons in multiple experimental animal models, including those of optic nerve injury and excitotoxic cell death (Sapieha et al. 2003). Several groups have accordingly shown the potential use of FGF2 as a therapeutic for neurodegenerative conditions such as Alzheimer’s disease (AD) and Parkinson’s disease (PD). Our lab has found that adeno-associated virus serotype 2/1 hybrid (AAV2/1)-mediated gene expression of FGF2 in AD transgenic mouse models (APP + PS1 and J20) significantly restores spatial learning, hippocampal CA1 long-term potentiation (LTP), and neurogenesis in the SGZ when administered both pre- and post-symptomatically (Kiyota et al. 2011). Interestingly, in addition to its neurogenic properties, FGF2 appears to have an anti-inflammatory and amyloidosis- decreasing effect: AAV2/1-FGF2–injected APP + PS1 mice showed a reduction in total Aβ and plaque load accompanied by enhanced microgliosis around plaque regions. Furthermore, FGF2 treatment of primary cultured microglia enhanced Aβ phagocytosis, and AAV2/1-FGF2 infection of primary cultured neurons reduced Aβ production, suggesting that FGF2 has potent effects not only on neurons but on microglial phagocytosis.
FGF2 as a therapeutic in other neurodegenerative conditions
Parkinson’s disease (PD) is a common neurodegenerative movement disorder involving degeneration of dopaminergic neurons in the substantia nigra pars compacta, leading to progressive motor dysfunction and cognitive impairment. FGF2 regulates dopaminergic neuron and nigrostriatal pathway development in vivo, the main pathway affected in humans in PD (Baron et al. 2012). It has been shown that reactive astroglia display increased FGF2 immunoreactivity following dopaminergic cell degeneration induced by 6-hydroxydopamine (6-OHDA) lesions in the rat striatum, implying a possible mechanism of dopaminergic neuron repair (Silva et al. 2009). Further evidence of the importance of FGF2 in dopaminergic neuron viability was shown by the finding that FGF2 enhances neuronal survivability and protects from 6-OHDA-induced cell death in the substantia nigra of PD mouse models (Timmer et al. 2007). In this study, both FGF2(−/−) mice and FGF2-overexpressing mice showed enlarged substantia nigra volumes, suggesting a critical developmental role of FGF2 in the establishment of the correct size and neuronal number in the substantia nigra. On the other hand, FGF2(−/−) mice showed significantly decreased survivability of dopaminergic neurons following 6-OHDA lesions to the substantia nigra as compared to FGF2-overexpressing mice and wildtype controls, suggesting an overcompensation of the absence of FGF2 during development, but a lack of these compensation mechanisms after lesion (Timmer et al. 2007).
FGF2 has also been studied as a therapeutic for brain repair after traumatic brain injury (TBI). Rats treated with ICV FGF2 immediately following TBI showed enhanced neurogenesis in the SVZ and SGZ at 1 and 4-weeks post TBI, increased numbers of surviving neurons, and improved cognitive function as compared to controls (Sun et al. 2009). In another study, after mice were given TBI according to the CHI model, which recapitulates focal TBI resulting in isolated frontoparietal cortical injury, mice treated with continuous infusion of FGF2 to the lateral ventricles showed improved motor function, increased astrocytic proliferation and number of blood vessels in the perilesional cortex vs. control, and increased neuronal proliferation when FGF2 was given in combination with vascular endothelial growth factor (VEGF)(Thau-Zuchman et al. 2012).
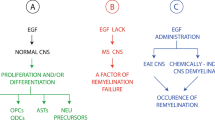
In Experimental Autoimmune Encephalomyelitis (EAE), a mouse model of multiple sclerosis (MS), FGF2 has been identified as a neuroprotective factor in preventing disease and in treating EAE. In one study, intrathecal injection of a recombinant herpes simplex virus (HSV) type-1 vector carrying the human FGF2 gene significantly reverted pathological features of EAE in mice, including a decrease in the number of myelinotoxic cells (T-cells and macrophages) in the CNS parenchyma and leptomeningeal space and an increase in the number of oligodendrocyte precursors and myelin-forming oligodendrocytes in areas of demyelination and axonal loss (Ruffini et al. 2001).
In a recent study, more severe EAE was induced in FGF2(−/−) mice vs. FGF2(+/+) mice, specifically measured by increased infiltration of macrophages/microglia and CD8+ T-cells, increased nerve fiber degeneration, and decreased remyelination of axons, suggesting a protective role of FGF2 (Rottlaender et al. 2011). Additionally, spinal cord FGF2 mRNA levels were found to peak at the initial stage of remyelination in EAE, implying that FGF2 is involved in positive regulation of oligodendrocyte myelin production (Messersmith et al. 2000). FGF2 levels are increased in the cerebrospinal fluid of MS patients, especially those in clinical relapse, possibly signifying a protective function of FGF2 (Sarchielli et al. 2008). Moreover, in postmortem MS human brain tissue, FGF2 is expressed in microglia and macrophages in active lesions (lesions exhibiting signs of remyelination), and oligodendrocyte precursor cells recruited to these lesions express FGFR1(Clemente et al. 2011). Consistent with this positive role of FGF signaling in myelination, transgenic mice lacking FGFR1 and FGF2 expression in oligodendrocyte lineage cells show no difference in proliferation or differentiation, but severe CNS hypomyelination (Furusho et al. 2012). These studies are in contrast to findings suggesting that FGF2 is inhibitory to oligodendrocyte myelin production. In an animal study, increased levels of FGF2 led to brain demyelination in adult rats, while another study showed that FGF2 is inhibitory to oligodendrocyte progenitor differentiation (Zhou et al. 2006). Altogether, these opposing findings may suggest that an optimal level of FGF2 is necessary for remyelination in EAE and MS.
Novel methods for FGF2 delivery to the CNS
Intranasal administration
Because growth factors cannot readily cross the blood–brain barrier (BBB), investigation has been done into alternative methods of delivering FGF2 to the brain. Intranasal administration is particularly attractive because it delivers substances directly to the brain through the nasal epithelium, while eliminating the need for and potential risks of peripheral intravenous or intramuscular administration. Accordingly, intranasal administration of FGF2 led to increased neurogenesis in the SVZ of mouse brain (Jin et al. 2003), as well as improved spatial memory impairment and decreased hippocampal degeneration in AD rat models, although neurogenesis was not assessed in this study (Feng et al. 2012).
Chitosan-based microspheres for FGF2 delivery
Another method being used for growth factor delivery across the BBB is microspheres, small spheres containing pharmacological agents which can be enzymatically degraded upon entry into the CNS. Skop et al. (2013) recently developed microspheres made of chitosan, derived by alkaline deacetylation of chitin, a highly abundant natural polysaccharide (Skop et al. 2013). Because chitosan is anionic, it can interact with cationic GAGs such as heparin, which binds FGF2. Heparin is often used in combination with FGF2 in stem cell culturing protocols, as it is essential for its binding to FGFR1 and prevents thermal degradation of the growth factor (Sommer & Rifkin 1989). The group also used Genipin, a plant-derived crosslinking agent with potential anti-inflammatory properties, to crosslink heparin to chitosan (Nam et al. 2010).
Although the microspheres weren’t tested in vivo, they have many properties that may be beneficial for in vivo use. For example, the degradation rate of chitosan can be reduced by crosslinking the polymer, which may be useful for in vivo applications. Interestingly, Nam et al. (2010) found that in cultured rat brain microglial cells, Genipin inhibited lipopolysaccharide (LPS)-induced nitric oxide (NO) release, reduced the LPS-stimulated production of inflammatory molecules, and reduced NO release from microglia stimulated with interferon-gamma and amyloid-beta (Nam et al. 2010). This suggests that Genipin-containing microspheres may be especially promising for neurodegenerative diseases involving neuroinflammation. In vitro, the FGF2 chitosan microspheres were more efficient than standard culture conditions in sustaining growth and survival of a neural stem cell line (GFP + RG3.6), suggesting their potential for in vivo testing. The in vivo neurogenic effect of other types of microspheres has been demonstrated, including striatal and cortical transplantation of FGF2-releasing gelatin microspheres in combination with neural progenitor cells in a rat stroke model (Matsuse et al. 2011) as well as the angiogenic effect in phase I/II trials of hydrogel gelatin FGF2 microspheres implanted into human patients with limb ischemia (Marui et al. 2007).
NCAM mimetic peptide, Fibroblast growth loop (FGL), as an alternative to FGF2
An interesting alternative to FGF2 therapies is the neural cell adhesion molecule (NCAM) mimetic peptide Fibroblast Growth Loop (FGL). In the CNS, NCAM shows ubiquitous expression and is involved in neuronal processes including synaptic outgrowth and plasticity (Zhang et al. 2008). The NCAM extracellular domain participates in homophilic interactions as well as heterophilic interactions with other molecules such as FGFR1. The NCAM FGFR1 binding site is in the extracellular second fibronectin type III (F3) region of NCAM. The FGL peptide is a synthetic 15-amino acid peptide derived from and encompassing this NCAM FGFR binding site (Kiselyov et al. 2003). Homophilic NCAM-NCAM binding to FGFR leads to FGFR phosphorylation and subsequent activation of intracellular signal transduction pathways, including Ras-mitogen activated protein kinase (MAPK) and Phosphatidylinositol-3-kinase (PI3K)-Akt pathways (Kolkova et al. 2000), which are known to control several fundamental cellular processes including proliferation, differentiation, cytoskeleton reorganization, death, and survival (Pearson et al. 2001; Vivanco & Sawyers 2002). FGL crosses the BBB and exerts its effects by acting on FGFR1 on neurons, simulating FGFR-NCAM heterophilic interactions (Walmod et al. 2004).
FGL has been found to induce many neuroprotective effects similar to those induced by NCAM activation of FGFR, including in memory, synapse formation and anti-inflammatory effects in multiple disease models. In FGFR1-expressing rat dopaminergic, hippocampal and cerebellar granule neurons, FGL treatment induced neuronal differentiation and enhanced neurite outgrowth through direct activation of FGFR1, and this was dependent on MEK and PI3K activation similar to that induced by NCAM activation (Neiiendam et al. 2004). Rats showed memory improvement in water maze testing and fear conditioning paradigms immediately following ICV FGL administration, and in primary cultured rat hippocampal neurons, FGL promoted synapse formation and enhanced presynaptic function via FGFR1 activation (Cambon et al. 2004). Dallérac et al. (2011) then demonstrated facilitation of LTP induction and maintenance in awake, behaving rats for up to 24 h following ICV infusion of FGL, as well as altered neurogenesis associated with LTP (Dallerac et al. 2011).
It was recently shown that FGL mediates this enhanced synaptic transmission via delivery of excitatory glutamatergic AMPA receptors (AMPARs) to synapses, along with activity-dependent enhancement of NMDA-dependent LTP. It was also found that the synaptic delivery of AMPARs is dependent on activation of PKC, a downstream effector of FGFR1 and molecule involved in synaptic plasticity, leading to persistent CaMKII activation (Knafo et al. 2012). It is especially significant that FGL enhances LTP in an activity-dependent manner, because this confers specificity of enhancement to active synapses, rather than global enhancement, which could lead to epileptic seizures. Furthermore, PKC activation persisted for at least 24 h after FGL treatment, supporting the in vivo findings of long-term LTP enhancement.
The neuroprotective effects of FGL are beneficial in AD and PD animal models as well as in the aging brain. FGL administration has been shown to protect not only against cell death induced by 6-hydroxydopamine and Aβ in primary cultures of dopaminergic, hippocampal and cerebellar granule neurons (Neiiendam et al. 2004), but also against Aβ-induced inflammation and cognitive deficits in vivo (Klementiev et al. 2007). Altered synaptic morphology, progressive synapse loss and astro/microglial cell activation are hallmarks of the aging brain and are also key aspects of AD pathology (Conde & Streit 2006). FGL has shown anti-inflammatory effects in the aging brain, specifically by restoring imbalances in levels of insulin-like growth factor-1 (IGF1) and pro-inflammatory interferon-gamma (IFN-γ) in hippocampal neurons, as well as reducing age-related glial activation in vitro (Downer et al. 2009).
Neuroimmunomodulation of CD200 signaling by FGF2 signaling
Interestingly, FGL’s anti-inflammatory effects are associated with increased expression of the neuronal glycoprotein CD200 by astrocytes (Downer et al. 2009) and neurons (Cox et al. 2013) and have subsequently been found to be dependent on CD200 expression (Cox et al. 2013). CD200 is known to play a role in maintaining microglia in a non-inflammatory (“quiescent”) state, which has been suggested to protect synaptic function in the aging brain (Lyons et al. 2007). Its cognate receptor, CD200R, is expressed on cells of the myeloid lineage, which are mainly microglia in the brain (Barclay et al. 2002). Downer et al. (2010) showed that in vivo administration of FGL to aged rats for 3 weeks attenuated age-related increases in microglial activation and corresponding deficits in LTP, effects postulated to be due at least in part to the actions of CD200 (Downer et al. 2010). Importantly, CD200 expression is reduced in affected AD brain areas, and CD200(−/−) or CD200R(−/−) mice show enhanced inflammation in the brain (Lue et al. 2010). Thus, a pathway involving activation of FGFR leading to enhanced CD200 expression, which leads to subsequent activation of anti-inflammatory pathways via microglial CD200R activation, may be disturbed in AD patients due to CD200 decrease by Aβ, pathogen-associated molecular patterns (PAMPs), or damage-associated molecular patterns (DAMPs) recognized by microglia (See Fig. 2). FGF2, NCAM mimetic peptide, or other molecules activating the FGFR1 receptor could potentially be used pharmacologically to reverse this disturbance.
Scheme of neuroimmunomodulation of CD200 signaling by FGF2 signaling. Activation of FGFR leads to enhanced CD200 expression, which leads to subsequent activation of anti-inflammatory pathways via microglial CD200 receptor (CD200R) activation. This pathway may be disturbed in AD patients due to CD200 decrease by amyloid-β (Aβ), pathogen-associated molecular patterns (PAMPs), or damage-associated molecular patterns (DAMPs) recognized by microglia, leading to neuroinflammation-induced suppression of LTP, synaptic transmission and neuronal viability in AD brain
One caution with interpretation of the results of Downer et al. is that the authors did not systematically investigate microglial activation phenotype and any changes in phenotype with FGL treatment. They described observations of higher intracellular vacuole content, phagolysosomal and electron-dense material, which are attributed to greater phagocytic activity of microglia and an increased general ‘activation state.’ However, microglia may be protective to neurons when they are in an anti-inflammatory activation state, and continue to phagocytose apoptotic cells (Takahashi et al. 2005). The effects of microglia on neural stem cells are highly dependent on the type of microglial activation state, which is known to be inhibitory to hippocampal neurogenesis when microglia are classically activated (M1 skewing; pro-inflammatory) by LPS (Monje et al. 2003), but supportive to neuronal differentiation and survival when microglia are activated by anti-inflammatory cytokines such as IL-4 (M2a skewing; see Fig. 2) (Kiyota et al. 2012). Interestingly, Cox et al. (2013) recently demonstrated FGL-induced, CD200-dependent attenuation of LPS-induced changes in microglial pro-inflammatory activation (Cox et al. 2013), suggesting that the earlier findings of Downer et al. (2010) may correspond to attenuation of a pro-inflammatory phenotype of microglial activation.
FGL’s beneficial effects on age-related synaptic changes have been suggested to be associated with changes in CD200 expression. Treatment with FGL in rats attenuated age-related loss of synaptophysin immunoreactivity (ir) (a synaptic vesicle protein expressed mainly at synapses) within the hippocampal CA3 and hilus regions, and of CD200-ir in the CA3 region. FGL treatment also prevented age-related loss of astrocyte-synapse contacts and reduced the number of microglia-synapse contacts in the CA3 stratum radiatum, but had no effect on the average number of synapses in that region. This suggests that FGL may mediate its neuroprotective effects not only through neuron-neuron synaptic contacts but also by regulating altered glial-synaptic interactions associated with synaptic damage (Ojo et al. 2012a).
In contrast to its effects in the aging brain, subcutaneous injections of FGL to young (4-month old) rats resulted in reduced total volume of the dorsal hippocampus and an associated decrease in total pyramidal neuron numbers in CA1 and CA3. This suggests that the neuroprotective effects of FGL in aged animals may be dependent on glial activation status and its effects on synaptic viability, which are altered in aged animals (Ojo et al. 2012b). In line with this idea, subcutaneous FGL injection reduced microglial CD11b and MHCII immunoreactivity, as well as MHC-II-positive microglial cell density in the hippocampi of aged (22 months) but not young rats (Ojo et al. 2011). Of note, FGL treatment actually enhanced MHC-II-ir in the dentate gyrus/hilus region of young rats, although the explanation for this opposite effect is unclear. Interestingly, although FGL was found to decrease pyramidal cell numbers in young rats, in these animals it was also associated with increased DCX-ir neurons in the neurogenic SGZ of the dentate gyrus, suggesting that FGL enhances SGZ neurogenesis in young rats possibly via its actions on FGFR1 (Ojo et al. 2012b).
Because of its observed effects on inflammation as well as synapse viability and hippocampal neuron growth, FGL is an attractive potential therapy for AD and other age-related neurodegenerative diseases. Gene therapy using AAV-FGF2 is similar in these regards, however FGL can be administered systemically and crosses the BBB, whereas AAV must be injected directly into the CNS, excluding AAV9 (Dayton et al. 2012).
Despite these positive attributes, caution should be taken when assessing FGL’s potential for treating disease in humans. Although FGL did show benefits in Aβ-injected rats, to our knowledge it has not been tested in published AD mouse models. FGL showed no toxicity in rats, dogs or monkeys in preclinical studies, and an 8-day safety trial in 24 males of one intranasal FGL dose showed no serious adverse effects (Anand et al. 2007). In Europe, FGL is currently in clinical trials in AD patients, scheduled to end in 2014 (Development of a novel FGL therapy and translational tests for regenerative treatment of neurological disorders 2012). However, the human safety study was only 8 days long, which may not reveal any long-term adverse effects of the drug. Additionally, since FGL was shown to potentially enhance inflammation in the dentate gyrus (Ojo et al. 2011) and to reduce hippocampal cell volume and pyramidal cell number in young, healthy mice (Ojo et al. 2012b), in contrast to its effects on aged mice, FGL may differentially affect patients depending on age and/or disease severity.
Summary
A plethora of studies have demonstrated the crucial role of FGF2 in neurogenesis, both in proliferation and differentiation of stem cells during development and in the adult brain. FGF2/FGFR1 signaling holds great promise for therapeutic interventions for CNS diseases, due to its established effects not only on neurogenesis, but on synaptic formation, neuron-glia interactions, inflammation, and amyloidosis. Much progress has been made on the role of FGF2 in the CNS and on development of therapeutic interventions, and although these interventions have not yet been applied for treatment of human patients, the research devoted to advanced techniques such as gene therapy, microspheres, and novel BBB-permeable molecules are rapidly moving the field forward.
References
Anand R et al (2007) Tolerability, safety and pharmacokinetics of the FGLL peptide, a novel mimetic of neural cell adhesion molecule, following intranasal administration in healthy volunteers. Clin Pharmacokinet 46(4):351–8
Aoyagi A et al (1994) Characterization of basic fibroblast growth factor-mediated acceleration of axonal branching in cultured rat hippocampal neurons. Brain Res 661(1–2):117–26
Arnaud E et al (1999) A new 34-kilodalton isoform of human fibroblast growth factor 2 is cap dependently synthesized by using a non-AUG start codon and behaves as a survival factor. Mol Cell Biol 19(1):505–14
Asai T et al (1993) Differential expression of two members of FGF receptor gene family, FGFR-1 and FGFR-2 mRNA, in the adult rat central nervous system. Brain Res Mol Brain Res 17(1–2):174–8
Barclay AN et al (2002) CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol 23(6):285–90
Baron O, Ratzka A, Grothe C (2012) Fibroblast growth factor 2 regulates adequate nigrostriatal pathway formation in mice. J Comp Neurol 520(17):3949–61
Burgess WH, Maciag T (1989) The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem 58:575–606
Cambon K et al (2004) A synthetic neural cell adhesion molecule mimetic peptide promotes synaptogenesis, enhances presynaptic function, and facilitates memory consolidation. J Neurosci 24(17):4197–204
Chen G et al (2010) CREB binding protein is required for both short-term and long-term memory formation. J Neurosci 30(39):13066–77
Claus P et al (2003) Differential intranuclear localization of fibroblast growth factor-2 isoforms and specific interaction with the survival of motoneuron protein. J Biol Chem 278(1):479–85
Clemente D et al (2011) FGF-2 and Anosmin-1 are selectively expressed in different types of multiple sclerosis lesions. J Neurosci 31(42):14899–909
Conde JR, Streit WJ (2006) Microglia in the aging brain. J Neuropathol Exp Neurol 65(3):199–203
Cox FF et al (2013) The neural cell adhesion molecule-derived peptide, FGL, attenuates lipopolysaccharide-induced changes in glia in a CD200-dependent manner. Neuroscience 235:141–8
Dallerac G et al (2011) The neural cell adhesion molecule-derived peptide FGL facilitates long-term plasticity in the dentate gyrus in vivo. Learn Mem 18(5):306–13
Dayton RD, Wang DB, Klein RL (2012) The advent of AAV9 expands applications for brain and spinal cord gene delivery. Expert Opin Biol Ther 12(6):757–66
Delrieu I (2000) The high molecular weight isoforms of basic fibroblast growth factor (FGF-2): an insight into an intracrine mechanism. FEBS Lett 468(1):6–10
Development of a novel FGL therapy and translational tests for regenerative treatment of neurological disorders 2012 [cited 2013; Available from: http://cordis.europa.eu/projects/rcn/102442_en.html
Dono R et al (1998) Impaired cerebral cortex development and blood pressure regulation in FGF-2-deficient mice. EMBO J 17(15):4213–25
Downer EJ et al (2009) A synthetic NCAM-derived mimetic peptide, FGL, exerts anti-inflammatory properties via IGF-1 and interferon-gamma modulation. J Neurochem 109(5):1516–25
Downer EJ et al (2010) A novel anti-inflammatory role of NCAM-derived mimetic peptide, FGL. Neurobiol Aging 31(1):118–28
el-Husseini AE-D, Paterson JA, Shiu RP (1994) Basic fibroblast growth factor (bFGF) and two of its receptors, FGFR1 and FGFR2: gene expression in the rat brain during postnatal development as determined by quantitative RT-PCR. Mol Cell Endocrinol 104(2):191–200
Fang X et al (2005) Control of CREB-binding protein signaling by nuclear fibroblast growth factor receptor-1: a novel mechanism of gene regulation. J Biol Chem 280(31):28451–62
Feng C et al (2012) Enhancement of nose-to-brain delivery of basic fibroblast growth factor for improving rat memory impairments induced by co-injection of beta-amyloid and ibotenic acid into the bilateral hippocampus. Int J Pharm 423(2):226–34
Florkiewicz RZ, Sommer A (1989) Human basic fibroblast growth factor gene encodes four polypeptides: three initiate translation from non-AUG codons. Proc Natl Acad Sci U S A 86(11):3978–81
Florkiewicz RZ, Anchin J, Baird A (1998) The inhibition of fibroblast growth factor-2 export by cardenolides implies a novel function for the catalytic subunit of Na+, K + −ATPase. J Biol Chem 273(1):544–51
Furusho M et al (2012) Fibroblast growth factor receptor signaling in oligodendrocytes regulates myelin sheath thickness. J Neurosci 32(19):6631–41
Gritti A et al (1996) Multipotential stem cells from the adult mouse brain proliferate and self-renew in response to basic fibroblast growth factor. J Neurosci 16(3):1091–100
Grothe C et al (1998) Over-expression of the 18 kD and 21/23 kD fibroblast growth factor-2 isoforms in PC12 cells and Schwann cells results in altered cell morphology and growth. Brain Res Mol Brain Res 57(1):97–105
Ishiyama J, Saito H, Abe K (1991) Epidermal growth factor and basic fibroblast growth factor promote the generation of long-term potentiation in the dentate gyrus of anaesthetized rats. Neurosci Res 12(3):403–11
Jaye M, Schlessinger J, Dionne CA (1992) Fibroblast growth factor receptor tyrosine kinases: molecular analysis and signal transduction. Biochim Biophys Acta 1135(2):185–99
Jin K et al (2003) Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell 2(3):175–83
Joy A et al (1997) Nuclear accumulation of FGF-2 is associated with proliferation of human astrocytes and glioma cells. Oncogene 14(2):171–83
Kirby ED et al (2013) Acute stress enhances adult rat hippocampal neurogenesis and activation of newborn neurons via secreted astrocytic FGF2. Elife 2:e00362
Kiselyov VV et al (2003) Structural basis for a direct interaction between FGFR1 and NCAM and evidence for a regulatory role of ATP. Structure 11(6):691–701
Kiyota T et al (2011) FGF2 gene transfer restores hippocampal functions in mouse models of Alzheimer's disease and has therapeutic implications for neurocognitive disorders. Proc Natl Acad Sci U S A 108(49):E1339–48
Kiyota T et al (2012) AAV serotype 2/1-mediated gene delivery of anti-inflammatory interleukin-10 enhances neurogenesis and cognitive function in APP + PS1 mice. Gene Ther 19(7):724–33
Klementiev B et al (2007) A neural cell adhesion molecule-derived peptide reduces neuropathological signs and cognitive impairment induced by Abeta25-35. Neuroscience 145(1):209–24
Knafo S et al (2012) Facilitation of AMPA receptor synaptic delivery as a molecular mechanism for cognitive enhancement. PLoS Biol 10(2):e1001262
Kolkova K et al (2000) Neural cell adhesion molecule-stimulated neurite outgrowth depends on activation of protein kinase C and the Ras-mitogen-activated protein kinase pathway. J Neurosci 20(6):2238–46
Korzus E, Rosenfeld MG, Mayford M (2004) CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron 42(6):961–72
Krejci P et al (2007) The antiapoptotic protein Api5 and its partner, high molecular weight FGF2, are up-regulated in B cell chronic lymphoid leukemia. J Leukoc Biol 82(6):1363–4
Lemiere S et al (2008) Overexpression of high molecular weight FGF-2 forms inhibits glioma growth by acting on cell-cycle progression and protein translation. Exp Cell Res 314(20):3701–11
Lue LF et al (2010) Microglia activation and anti-inflammatory regulation in Alzheimer's disease. Mol Neurobiol 41(2–3):115–28
Lyons A et al (2007) CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: a role for IL-4. J Neurosci 27(31):8309–13
Marui A et al (2007) A novel approach to therapeutic angiogenesis for patients with critical limb ischemia by sustained release of basic fibroblast growth factor using biodegradable gelatin hydrogel: an initial report of the phase I-IIa study. Circ J 71(8):1181–6
Matsuse D et al (2011) Combined transplantation of bone marrow stromal cell-derived neural progenitor cells with a collagen sponge and basic fibroblast growth factor releasing microspheres enhances recovery after cerebral ischemia in rats. Tissue Eng Part A 17(15–16):1993–2004
Messersmith DJ et al (2000) Fibroblast growth factor 2 (FGF2) and FGF receptor expression in an experimental demyelinating disease with extensive remyelination. J Neurosci Res 62(2):241–56
Miyake A et al (1996) Rat oligodendrocytes and astrocytes preferentially express fibroblast growth factor receptor-2 and −3 mRNAs. J Neurosci Res 45(5):534–41
Monje ML, Toda H, Palmer TD (2003) Inflammatory blockade restores adult hippocampal neurogenesis. Science 302(5651):1760–5
Morrison RS et al (1986) Basic fibroblast growth factor supports the survival of cerebral cortical neurons in primary culture. Proc Natl Acad Sci U S A 83(19):7537–41
Murphy M et al (1994) FGF2 regulates proliferation of neural crest cells, with subsequent neuronal differentiation regulated by LIF or related factors. Development 120(12):3519–28
Nam KN et al (2010) Genipin inhibits the inflammatory response of rat brain microglial cells. Int Immunopharmacol 10(4):493–9
Neiiendam JL et al (2004) An NCAM-derived FGF-receptor agonist, the FGL-peptide, induces neurite outgrowth and neuronal survival in primary rat neurons. J Neurochem 91(4):920–35
Newman MP, Feron F, Mackay-Sim A (2000) Growth factor regulation of neurogenesis in adult olfactory epithelium. Neuroscience 99(2):343–50
Ohkubo Y et al (2004) Fibroblast growth factor receptor 1 is required for the proliferation of hippocampal progenitor cells and for hippocampal growth in mouse. J Neurosci 24(27):6057–69
Ojo B et al (2011) A neural cell adhesion molecule-derived peptide, FGL, attenuates glial cell activation in the aged hippocampus. Exp Neurol 232(2):318–28
Ojo B et al (2012a) Age-related changes in the hippocampus (loss of synaptophysin and glial-synaptic interaction) are modified by systemic treatment with an NCAM-derived peptide, FGL. Brain Behav Immun 26(5):778–88
Ojo, B., et al. (2012) An NCAM Mimetic, FGL, Alters Hippocampal Cellular Morphometry in Young Adult (4 Month-Old) Rats. Neurochem Res
Palmer TD, Ray J, Gage FH (1995) FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci 6(5):474–86
Pearson G et al (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22(2):153–83
Powell PP et al (1991) Temporal, differential and regional expression of mRNA for basic fibroblast growth factor in the developing and adult rat brain. Brain Res Mol Brain Res 11(1):71–7
Quarto N et al (1991) Selective expression of high molecular weight basic fibroblast growth factor confers a unique phenotype to NIH 3T3 cells. Cell Regul 2(9):699–708
Rai KS, Hattiangady B, Shetty AK (2007) Enhanced production and dendritic growth of new dentate granule cells in the middle-aged hippocampus following intracerebroventricular FGF-2 infusions. Eur J Neurosci 26(7):1765–79
Rottlaender A et al (2011) Neuroprotective role of fibroblast growth factor-2 in experimental autoimmune encephalomyelitis. Immunology 133(3):370–8
Ruffini F et al (2001) Fibroblast growth factor-II gene therapy reverts the clinical course and the pathological signs of chronic experimental autoimmune encephalomyelitis in C57BL/6 mice. Gene Ther 8(16):1207–13
Sapieha PS et al (2003) Fibroblast growth factor-2 gene delivery stimulates axon growth by adult retinal ganglion cells after acute optic nerve injury. Mol Cell Neurosci 24(3):656–72
Sarchielli P et al (2008) Fibroblast growth factor-2 levels are elevated in the cerebrospinal fluid of multiple sclerosis patients. Neurosci Lett 435(3):223–8
Schafer T et al (2004) Unconventional secretion of fibroblast growth factor 2 is mediated by direct translocation across the plasma membrane of mammalian cells. J Biol Chem 279(8):6244–51
Silva C, Fuxe K, Chadi G (2009) Involvement of astroglial fibroblast growth factor-2 and microglia in the nigral 6-OHDA parkinsonism and a possible role of glucocorticoid hormone on the glial mediated local trophism and wound repair. J Neural Transm Suppl 73:185–202
Skop NB et al (2013) Heparin crosslinked chitosan microspheres for the delivery of neural stem cells and growth factors for central nervous system repair. Acta Biomater
Sommer A, Rifkin DB (1989) Interaction of heparin with human basic fibroblast growth factor: protection of the angiogenic protein from proteolytic degradation by a glycosaminoglycan. J Cell Physiol 138(1):215–20
Stachowiak EK et al (2003) cAMP-induced differentiation of human neuronal progenitor cells is mediated by nuclear fibroblast growth factor receptor-1 (FGFR1). J Neurochem 84(6):1296–312
Sun D et al (2009) Basic fibroblast growth factor-enhanced neurogenesis contributes to cognitive recovery in rats following traumatic brain injury. Exp Neurol 216(1):56–65
Takahashi K, Rochford CD, Neumann H (2005) Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med 201(4):647–57
Tao Y, Black IB, DiCicco-Bloom E (1996) Neurogenesis in neonatal rat brain is regulated by peripheral injection of basic fibroblast growth factor (bFGF). J Comp Neurol 376(4):653–63
Tao Y, Black IB, DiCicco-Bloom E (1997) In vivo neurogenesis is inhibited by neutralizing antibodies to basic fibroblast growth factor. J Neurobiol 33(3):289–96
Terlau H, Seifert W (1990) Fibroblast Growth Factor Enhances Long-term Potentiation in the Hippocampal Slice. Eur J Neurosci 2(11):973–977
Thau-Zuchman O et al (2012) Combination of vascular endothelial and fibroblast growth factor 2 for induction of neurogenesis and angiogenesis after traumatic brain injury. J Mol Neurosci 47(1):166–72
Timmer M et al (2007) Fibroblast growth factor (FGF)-2 and FGF receptor 3 are required for the development of the substantia nigra, and FGF-2 plays a crucial role for the rescue of dopaminergic neurons after 6-hydroxydopamine lesion. J Neurosci 27(3):459–71
Touriol C et al (2003) Generation of protein isoform diversity by alternative initiation of translation at non-AUG codons. Biol Cell 95(3–4):169–78
Vivanco I, Sawyers CL (2002) The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2(7):489–501
Walmod PS et al (2004) Zippers make signals: NCAM-mediated molecular interactions and signal transduction. Neurochem Res 29(11):2015–35
Werner S, Unsicker K, von Bohlen und Halbach O (2011) Fibroblast growth factor-2 deficiency causes defects in adult hippocampal neurogenesis, which are not rescued by exogenous fibroblast growth factor-2. J Neurosci Res 89(10):1605–1617
Zehe C et al (2006) Cell-surface heparan sulfate proteoglycans are essential components of the unconventional export machinery of FGF-2. Proc Natl Acad Sci U S A 103(42):15479–84
Zhang Y et al (2008) Cell adhesion molecules of the immunoglobulin superfamily in axonal regeneration and neural repair. Restor Neurol Neurosci 26(2–3):81–96
Zhou YX et al (2006) Retroviral lineage analysis of fibroblast growth factor receptor signaling in FGF2 inhibition of oligodendrocyte progenitor differentiation. Glia 54(6):578–90
Acknowledgments
We would like to thank Dr. Tomomi Kiyota for the Affymetrix GeneChip analysis of the AAV-FGF2 and AAV-GFP-injected AD mouse model study. This work is supported in part by NIH Training Grant 5 T32 GM008541 (MEW) in association with the Biomolecular Pharmacology Training Program
Conflict of Interest Disclosure
The author have neither a financial nor a personal relationship that might bias this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Woodbury, M.E., Ikezu, T. Fibroblast Growth Factor-2 Signaling in Neurogenesis and Neurodegeneration. J Neuroimmune Pharmacol 9, 92–101 (2014). https://doi.org/10.1007/s11481-013-9501-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-013-9501-5