Abstract
Removal of apoptotic cells is an essential process for normal development and tissue maintenance. Importantly, apoptotic cells stimulate their phagocytosis by macrophages while actively suppressing inflammatory responses. Growth arrest specific gene 6 (Gas6) is involved in this process, bridging phosphatidylserine residues on the surface of apoptotic cells to the Axl/Mer family of tyrosine kinases which stimulate phagocytosis. Animals with mutations or loss of these receptors exhibit phenotypes reflective of impaired phagocytosis and a hyperactive immune response. We report that Gas6 induces phagocytosis in microglia through a novel non-classical phagocytic mechanism. Gas6 stimulates a type-II-related phagocytic response, but requires Vav phosphorylation and Rac activation, distinguishing it from the classical type II mechanism. Importantly, Gas6 suppressed lipopolysaccharide-induced expression of the inflammatory molecules IL-1β and iNOS. Gas6 inhibited iNOS expression through suppression of promoter activity. The present data provide direct evidence for the role of Gas6 receptors in mediating an anti-inflammatory response to ligands found on apoptotic cells with the simultaneous stimulation of phagocytosis. These data provide a mechanistic explanation for the phenotype observed in animals lacking Axl/Mer receptors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microglia, the brain’s tissue macrophage (Streit et al. 2004), continuously surveys the brain for pathogens, foreign material, and apoptotic cells (Nimmerjahn et al. 2005). The capacity of these cells to efficiently remove apoptotic cells by phagocytosis is essential for normal development, tissue homeostasis, and responses to injury or disease (Fadok and Chimini 2001). An important adaptive characteristic of apoptosis is that cells are removed from the organism by macrophages through a highly specialized process in which the apoptotic cell does not elicit an immune response (Fadok et al. 1998; Voll et al. 1997). This is in contrast to the proinflammatory activation of these cells upon exposure to necrotic cells or induction of opsonic phagocytosis. Apoptotic cells are recognized by macrophages and microglia as a consequence of the presence of phosphatidylserine (PS) on their surface (Ravichandran 2003; Savill 1997). PS is normally found on the inner leaflet of the plasma membrane bilayer, but is translocated to the outer leaflet upon induction of apoptosis (Bratton et al. 1997). Immune effector cells detect PS on apoptotic cells directly through receptors like the PS receptor (Fadok and Henson 2003) or indirectly through adapter proteins such as the product of the growth arrest-specific 6 (Gas6) gene (Ishimoto et al. 2000). Gas6 is a secreted protein related to the anti-coagulant protein S (Schneider et al. 1988). Gas6 binds to PS and then associates with the Axl/Mer subfamily of receptor tyrosine kinases, thus, acting as a molecular bridge between the phagocyte and its target (Ishimoto et al. 2000). The binding of Gas6 to the Axl/Mer receptors stimulates the phagocytic machinery and uptake of the apoptotic cell (Ishimoto et al. 2000; Wu et al. 2006).
The Axl/Mer receptor subfamily is comprised of the related receptors Axl, Mer, and Tyro3 that are type I receptor tyrosine kinases. These receptors are expressed by monocytes and other cells within this lineage, including microglia (Prieto et al. 2000). Gas6 is able to activate its receptors without being bound to PS (Nagata et al. 1996; Nakano et al. 1997; Stitt et al. 1995; Varnum et al. 1995). Animals that lack one member of the Axl/Mer/Tyro3 receptor family exhibit relatively mild phenotypes. The most prominent phenotype is observed in Mer knockouts, which exhibit delayed phagocytosis and clearance of apoptotic cells. The Royal College of Surgeons rat model of retinal degeneration has an inactivating mutation in Mer that results in progressive loss of vision and retinal dystrophy caused by impaired phagocytic clearance of rod outer segments by the associated retinal pigmented epithelial cells (D’Cruz et al. 2000). Triple Axl/Mer/Tyro3 receptor knockouts exhibit no obvious developmental defects within the first 3 weeks of life. However, because of defective clearance of apoptotic cells, these animals subsequently develop enlarged lymphoid organs and elevated activation of the immune system with the development of a severe autoimmune lupus-like disease (Lu et al. 1999). Similarly, transgenic mice expressing a defective Mer kinase domain were unable to phagocytose apoptotic cells, suggesting that intracellular signals from Mer are required to initiate engulfment signals (Scott et al. 2001).
The activation of Axl/Mer receptors is associated with suppression of immune responses (Lemke and Lu 2003). Indeed, the repression of the proinflammatory response is directly coupled to the recognition of apoptotic cells (Cvetanovic and Ucker 2004). The interaction of macrophages with PS suppresses inflammatory cytokine production and stimulates production of anti-inflammatory molecules, limiting local damage caused by release or discharge of injurious or proinflammatory contents of apoptotic cell (Henson et al. 2001). It has been argued that these receptors, when activated, initiate inhibitory signals that lower the activation state of the macrophage to an initial immune response and convert macrophages from a classical to an alternative activation pattern (Pittoni and Valesini 2002; Scott et al. 2001). However, there is little direct evidence to support this view. The molecular mechanisms underlying this phenomenon remain obscure (Fadok et al. 1998).
To better understand the molecular basis of the clearance of apoptotic cells in the central nervous system (CNS), we investigated the ability of Gas6 to stimulate microglial phagocytosis and to simultaneously suppress inflammatory responses. We report that Gas6 induces microglial phagocytosis through a non-classical mechanism. The Gas6-stimulated phagocytosis is morphologically and pharmacologically similar to a type II phagocytic response; however, it is reliant upon Vav-mediated Rac activation, distinguishing it from a classical type II phagocytic response. Furthermore, we directly demonstrated that Gas6 stimulation is able to elicit an immunorepressive effect on microglia cells, acting to reduce lipopolysaccharide (LPS)-induced iNOS and IL-1β expression. Understanding the mechanisms through which these cells selectively regulate their inflammatory status may provide insight into new therapeutic approaches to inflammatory brain diseases.
Materials and methods
Materials
Gas6 was provided by Amgen, Inc. (Thousand Oaks, CA, USA) or purchased from R&D Systems (Minneapolis, MN, USA). LPS was obtained from List Biological Laboratories (Campbell, CA, USA). Zymosan was purchased from Sigma (St. Louis, MO, USA) and opsonized with complement by incubating with mouse serum for 30 min at 37°C followed by ten washes with 0.85% NaCl before resuspension in Phosphate-buffered saline (PBS). DiO and DiD were purchased from Invitrogen (Carlsbad, CA, USA). Immune IgG was prepared from goat anti-rabbit IgG (Sigma) and rabbit anti-mouse IgG from Cappel (Aurora, OH, USA) in a 5:1 ratio as described previously (Koenigsknecht and Landreth 2004). Nocodazole was purchased from Sigma, piceatannol and LY294002 from Alexis Biochemicals (San Diego, CA, USA). Blebbistatin was from Calbiochem (La Jolla, CA, USA). Anti-Mer antibody was purchased from R&D Systems. Anti-Axl (M20), anti-iNOS (Sc7271), anti-Vav (C14), and anti-ERK2 (Sc-154) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies to IL-1β (3ZD) were obtained from the National Cancer Institute (Bethesda, MD, USA). The anti-phosphotyrosine antibody, 4G10, and the anti-Rac (05-389) antibodies were from Upstate Biotechnology (Waltham, MA, USA). The anti-phosphotyrosine antibody, PY20, was from Transduction Laboratories (Greenland, NH, USA). PAK bound to glutathione-agarose beads (PAK02) was purchased from Cytoskeleton (Denver, CO, USA). The anti-mouse CD18 and anti-flotillin antibodies were purchased from BD Biosciences (San Jose, CA, USA).
Cell culture
The murine microglia cell line BV-2 was grown in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen) containing 2% fetal bovine serum (FBS) and gentamycin (25 ng/ml) in 5% CO2. Human THP1 monocytes were obtained from ATTC (Manassas, VA, USA) and grown in RPMI-1640 (Whitaker Bioproducts, Walkersville, MD, USA) containing 10% heat-inactivated FBS, 5 × 10−5 M β-mercaptoethanol, 5 mM HEPES, and 25 ng/ml gentamycin in an atmosphere of 5% CO2. Primary microglia were derived from postnatal days 1–3 brains of wild-type SV129 mice or from Vav−/− mice (Turner et al. 1997) provided by V.L.J. Tybulewiscz (National Institute for Medical Research, London, UK) and J. Rivera (NIH, Bethesda, MD, USA) as previously described (Combs et al. 2001; Wilkinson et al. 2006).
Evaluation of microglial phagocytosis
Microglial phagocytosis was measured by uptake of fluorescent microspheres as described previously (Koenigsknecht and Landreth 2004). Briefly, BV-2 cells or primary microglia were grown in serum-free DMEM, collected, and 1 × 106 cells were plated in 35-mm plates overnight. Gas6 was added (1–30 nM) for the indicated time periods (0–30 min). Typically, Gas6 (10 nM) was added for 30 min after the initiation of treatment with the indicated drugs. Microspheres (1 μm in diameter) were resuspended in PBS containing 1 mg/ml BSA, added and incubated for an additional 30 min. The cells were fixed with 2% paraformaldehyde, and the fraction of phagocytic cells was measured in three random fields. Statistical analysis was performed using a one-way analysis of variance (ANOVA) followed by post hoc Tukey–Kramer multiple comparison test to determine p values.
The phagocytosis of apoptotic cells was evaluated as described by Hirt and Leist (2003). Briefly, apoptosis of Jurkat cells was induced by addition of staurosporine (1 μM, 3 h). PS exposure was monitored by Alexa-488 conjugated annexin V according to the manufacturer’s instructions (Invitrogen). BV-2 cells were plated on poly-l-lysine-coated coverslips and grown in serum-free DMEM overnight. Gas6 (10 nM) was added for 30 min before initiation of phagocytosis. DiD-labeled (1:500) Jurkat cells were added into the DiO-labeled (1:200) BV-2 cell cultures and incubated for 0.5, 1, or 2 h at 37°C. Non-phagocytosed Jurkat cells were then removed by three washes with PBS. The cells were fixed with 4% paraformaldehyde, mounted with ProLong Gold anti-fade reagent (Invitrogen), and examined using a Zeiss LSM 510 Meta confocal microscope.
Flow cytometry
To measure phagocytosis efficiency, microglia were subjected to flow cytometry analysis. After co-incubation with the apoptotic Jurkat cells, the cultures were washed with PBS to remove unphagocytosed Jurkat cells. BV-2 cells were dislodged from the tissue culture plates by 5 mM ethylenediaminetetraacetic acid (EDTA) in PBS then fixed with 4% paraformaldehyde and resuspended in PBS. The cell suspension was then analyzed using a LSR II flow cytometer operated by FACSDiva software (BD Biosciences).
Phalloidin staining
BV-2 cells were grown in DMEM containing 2% FBS, collected, and plated on coverslips overnight. Cells were then incubated in the presence or absence of Gas6 for 30 min or before a 30-min stimulation with 1 mg/ml immune IgG or 1 mg/ml opsonized zymosan. Microspheres were then added for an additional 30 min. The cells were fixed in 2% paraformaldehyde and stained with AlexaFluor 488 phalloidin (6 IU diluted in 500 ml PBS) for 30 min.
Immunohistochemistry
Primary microglia or BV-2 cells were collected and plated on coverslips overnight. Glass coverslips were first blocked with 10% normal horse serum in PBS and subsequently incubated in the absence or presence of goat anti-Axl (1:10) or goat anti-Mer (1:5) at 4°C for 15 min followed by incubation with Alexa 488-conjugated donkey anti-goat-antibodies (1:1,000; Invitrogen). The coverslips were subsequently washed three times with double-distilled water, mounted with ProLong Gold antifade reagent with DAPI (Invitrogen), and examined using a Leica fluorescence microscope. Cells incubated in the absence of the primary antibodies did not exhibit any fluorescence (data not shown).
Western blots and immunoprecipitation
Lysates from BV-2 and THP1 cells were prepared by sonicating of the cells in lysis buffer (50 mM Tris–HCl pH 8, 120 mM NaCl, 5 mM EDTA, 0.5% (v/v) Igepal (NP-40), 160 mM phenylmethylsulfonyl fluoride) collected by centrifugation (15 min, 11,000×g, 4°C), and protein concentration was determined using the Bio-Rad Protein Assay (Biorad, Hercules, CA). Lysates (20–40 μg) were separated on a 9% (w/v) sodium dodecyl sulfate–polyacrylamide gel under reducing conditions and transferred to a PVDF membrane (Millipore, Bedford, MA, USA). Non-specific binding was blocked by incubation with 5% skim milk or with 3% BSA in TBST for 1 h. After incubation with the primary antibody overnight at 4°C (anti-Axl 1:100, anti-Mer 1:500, anti-iNOS 1:1,000, and anti-IL-1β 1:1,000), membranes were incubated with the secondary antibody (1:1,000). All immunoprecipitations were carried out as previously described (Wilkinson et al. 2006). Chemiluminescent signals (Pierce, Rockford, IL, USA) were visualized by exposure to Biomax X-ray film. Band intensities were quantified using NIH Image 1.62 software (Bethesda, MD, USA). Values statistically different from controls were calculated using a one-way ANOVA, and the Tukey–Kramer multiple comparisons test was used to determine p values.
Rac pull down
GTP-bound Rac was measured with an affinity-precipitation assay for the specific interaction of the Rac GTPase with its downstream effector, the p21-binding domain (PBD) of PAK coupled to GST (Li et al. 2002). The PBD-PAK is bound to glutathione-agarose beads and was used according to the manufacturer’s instructions (Cytoskeleton, Denver, CO, USA). BV-2 or THP-1 cells (5 × 106) were stimulated for 0–30 min in the presence of Gas6 (10 nM). After stimulation, cells were lysed with Mg2+ lysis buffer (25 mM Tris, pH 7.5, 5 mM MgCl2, 0.15 M NaCl, 1% Igepal (NP-40), 5% sucrose) according to the manufacturer’s instructions. A GST-fusion protein containing the PBD of PAK bound to glutathione-agarose beads was added to 1 mg/ml cell lysate and incubated with rocking for 1 h at 4°C. Samples were resolved by 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with an anti-Rac antibody (1:1,000). Aliquots of the cell lysates (40 μg/lane) were run as protein loading controls. The protein was detected by enhanced chemiluminescence, and band intensities were quantified using NIH Image 1.62 software. Values statistically different from controls were calculated using a one-way ANOVA, and the Tukey–Kramer multiple comparisons test was used to determine p values.
Subcellular fractionation
After stimulation by Gas6 (10 nM, 30 min), cells were lysed by incubation in relaxation buffer (100 mM KCl, 3 mM NaCl, 3.5 mM MgCl2, 1.25 mM EGTA, and 10 mM PIPES, pH 7.3) on ice for 15 min followed by 10 s of sonication. Cell debris was cleared by centrifugation at 3,000×g for 5 min at 4°C. The supernatant was then centrifuged for 1 h at 110,000×g at 4°C in a Beckman Coulter SW50.1 rotor. The resulting supernatant was removed and saved as the cytosolic fraction; the membrane pellet was then resuspended in relaxation buffer. Both fractions were resolved with SDS-PAGE (10%) and immunoblotted with anti-Axl (1:200) or anti-Mer (1:500) antibodies. Flotillin (1:1,000) was used as a marker for the membrane fraction.
Transfection and reporter assays
BV-2 cells (5 × 105 cells/well) were plated into 24-well plates 1 day before transfection. A murine iNOS promoter/luciferase construct (1 μg), kindly provided by Dr. N. Bhat (Bhat et al. 2002), along with 1 μg of control pRL-TK DNA (Promega) was transiently transfected into BV-2 cells using Lipofectamine 2000 (Invitrogen) according to the instructions provided by the manufacturer. One day after transfection, the cells were pre-incubated with 10 nM Gas6 for 30 min, followed by addition of vehicle or LPS (200 μg/ml), and incubated for an additional 12 h. Cells were then washed, lysed, followed by measurement of luciferase activity using a dual-luciferase assay kit (Promega). The luciferase activity was normalized with the expression of control pRL-TK and expressed as arbitrary units. Statistical analysis was performed using Student’s t test to determine p values.
Results
Murine microglia and primary microglia express the Gas6 receptors Axl and Mer
Microglial expression of Axl and Mer has not been previously demonstrated. Axl and Mer protein can be detected on the surface of the immortalized murine microglia cell line BV-2 and primary murine microglia (Fig. 1a–d). We further confirmed the expression of these receptors by subcellular fractionation which demonstrated that Axl and Mer were localized to the membrane fraction of BV-2 cells (Fig. 1e).
Axl and Mer protein are expressed on the plasma membrane of BV-2 and primary microglia. a–d Immunohistochemistry of Axl and Mer demonstrated their presence in primary murine microglia (a, b) and BV-2 cells (c, d). Bar, 20 μm. e Western blot analyses of membrane and cytosolic fractions isolated from BV-2 cells demonstrated that Axl and Mer were localized to membrane (mem) fractions. The data are representative of three independent experiments
Gas6-stimulates microglial phagocytosis
We evaluated the effect of Gas6 on microglial phagocytosis. Exposure of BV-2 microglia to Gas6 significantly increased phagocytosis, as monitored by the uptake of 1-μm fluorescent microspheres (Fig. 2). Gas6 was an efficient stimulant of microglial phagocytosis and was maximally induced at a concentration of Gas6 as low as 10 nM (Fig. 2e). The cellular response to 10 nM Gas6 was significantly greater than to 5 nM Gas6 (p < 0.05), but was not different from the response to higher concentrations. Microsphere uptake was time-dependent and was maximal after 30 min (Fig. 2f). A similar Gas6-stimulated phagocytic response was observed in primary murine microglia (Fig. 2g).
Gas6 stimulates phagocytosis in BV-2 and primary microglia. BV-2 cells were stimulated with vehicle (a, c) or 10 nM Gas6 (b, d) for 30 min followed by incubation with fluorescent microspheres (red). Cells were visualized by intracellular phalloidin staining (green). DAPI staining (blue) was used to identify nuclei. To assess the dosage dependence and kinetics of Gas6-stimulated phagocytosis, BV-2 cells were treated with the indicated concentrations of Gas6 (e) or treated with 10 nM Gas6 over a period of 0–30 min (f). Primary microglia from 129SV mice were stimulated with 10 nM Gas6 for 30 min (g). Gas6-stimulated phagocytosis was quantified by counting the phagocytic uptake of fluorescent microspheres. The data are representative of pooled results from six independent experiments. Bar, 50 μm. ***p < 0.0001; **p < 0.001; *p < 0.05 compared with control. In e, the response to Gas6 at 10 nM and higher concentrations were not significantly different from one another when analyzed by Student’s t test
We evaluated the phagocytosis of apoptotic cells and tested whether this process could be enhanced by provision of exogenous Gas6. We found that BV-2 microglia efficiently phagocytosed apoptotic Jurkat cells (Fig. 3). Inspection of the co-cultures 30 min after addition of apoptotic Jurkat cells demonstrated that some cells had been phagocytosed by the microglia, whereas adjacent microglia were observed to bind and then envelop the apoptotic cells. We quantitated apoptotic cell phagocytosis by FACS analysis under conditions that remove adherent cells. We found that the apoptotic cells were very effective in stimulating the phagocytosis of apoptotic cells and that addition of Gas6 treatment could not further stimulate this process (Fig. 3). Control experiments using PC-12 cells, which have no phagocytic ability, showed no internalization of Jurkat cells (data not shown). Non-apoptotic Jurkat cells, consistent with the absence of annexin V staining in these cells, were not efficiently taken up by the microglia (data not shown).
Phagocytic uptake of apoptotic Jurkat cells by BV-2 microglia. BV-2 cells were pre-incubated with vehicle or 10 nM Gas6 for 30 min. Apoptotic Jurkat cells were then added to the BV-2 cultures and incubated for an additional 0.5, 1, or 2 h. Representative fluorescence micrograph demonstrated phagocytosis of apoptotic Jurkat cells by BV-2 cells, visualized by DiD (red) and DiO (green), respectively. In the micrograph, Jurkat cells were either totally phagocytosed into a BV-2 cell (arrowhead) or undergoing engulfment by BV-2 cells (arrows). The phagocytosis of apoptotic Jurkat cells by BV-2 microglia was evaluated by FACS analysis. The fractions of BV-2 cells containing apoptotic cells are shown. The percentage of phagocytic cells was not different between Gas6 treated and non-treated groups. The data are reflective of pooled results from three independent studies
Characterization of the Gas6-stimulated phagocytic response
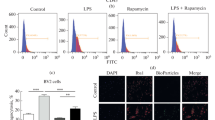
There are two classically recognized forms of phagocytosis. Type I is an “active” process typically elicited by immune IgG through FcRs and requires the action of the small G proteins Rac and cdc42. Morphologically, it is characterized by the extension of membrane processes that engulf the target (Caron and Hall 1998). In contrast, type II phagocytosis is stimulated by engagement of complement receptor 3 (CR3; CD11b/CD18) and is a “passive” process employing RhoA, with the phagocytic target “sinking” into the cell. Cells undergoing type II phagocytosis have a more rounded and flattened shape. Gas6 treatment of BV-2 microglia resulted in the formation of numerous phagosomes that filled the cytoplasm and was accompanied by expansion of the cell and acquisition of a “pancake” morphology (Fig. 4b). This distinctive morphology is clearly a type-II-related phagocytic response (Fig. 4d) and was similar to that elicited by the CR3 ligand mouse complement-opsonized zymosan (mOZ, Fig. 4c).
Gas6 stimulates a non-classical form of phagocytosis. a–d BV-2 cells were stimulated with vehicle (a), Gas6 (b; 10 nM), mouse complement-opsonized zymosan (c; mOZ 1 mg/ml), or immune IgG (d; 1 mg/ml) for 30 min before an additional 30-min incubation with fluorescent microspheres (red). Cellular morphology was visualized by intracellular actin staining (phalloidin; green). e–f BV-2 cells were pretreated with nocodazole (e; 5 mg/ml), blebbistatin (e; 100 μM), piceantanol (f; 10 μM), or LY294002 (f; 10 μM) for 30 min. The cells were then simulated with Gas6 (10 nM), and phagocytosis was evaluated by quantifying the phagocytic uptake of fluorescent microspheres 30 min later. Treatment of BV-2 cells with nocodazole, blebbistatin, piceantanol, or LY294002 without Gas6 stimulation serve as controls. The data represent the pooled outcome of three independent experiments. Bar, 50 μm. **p < 0.001 compared with control
To establish the cellular mechanisms through which Gas6 stimulates phagocytosis in microglia, we tested whether microspheres were taken up by classical phagocytic mechanisms through examination of functional requirements for cytoskeletal elements in the process of phagocytosis. In type II phagocytosis, microtubules have been shown to be essential for CR3-mediated phagocytosis, but not for the type I response (Newman et al. 1991). By using nocodazole to inhibit microtubule dynamics, we found that Gas6-stimulated phagocytosis was inhibited (Fig. 4e). Moreover, blebbistatin, a specific inhibitor of myosin II, a critical player in type II phagocytosis (Olazabal et al. 2002; Straight et al. 2003), also inhibited Gas6-stimulated phagocytosis (Fig. 4e). These findings indicate that Gas6-stimulated phagocytosis is reliant on microtubules and myosin II and is therefore related to a type II phagocytic response. On the other hand, type I phagocytosis, but not type II, is reliant upon the action of the tyrosine kinase Syk (Greenberg et al. 1993). The Gas6-stimulated phagocytosis was not inhibited by piceatannol, a specific inhibitor of Syk (Fig. 4f). We found that inhibition of phosphatidylinositol 3-kinase (PI3K) with LY294002 resulted in inhibition of Gas6-mediated phagocytosis. This result is consistent with the common and essential role for PI3K signaling pathway in the phagocytic process (Aderem and Underhill 1999; Greenberg 1995, 1999) and provides a negative control for these studies. Collectively, the results indicated that the phagocytic response stimulated by Gas6 is related to classical type II phagocytosis.
Gas6-stimulated phosphorylation of Vav is correlated with Rac activation
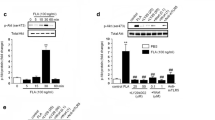
Vav proteins play a pivotal role in many aspects of cellular signaling, most prominently in cells of the immune system where they couple cell surface receptors to various effectors (Turner and Billadeau 2002). Vav proteins function, in part, as guanine-nucleotide exchange factors for the Rho family of GTPases. Previous work reported that Vav1 physically interacted with Mer and was tyrosine-phosphorylated after receptor activation in monocytes/macrophages (Mahajan and Earp 2003). We tested whether Gas6 engagement of microglia led to Vav1 phosphorylation. BV-2 microglia responded to 10 nM Gas6 treatment by transient phosphorylation of Vav1, which was maximal at 5 min and subsequently declined (Fig. 5a). We verified this response using THP-1 monocytes which exhibit lower basal levels of Vav activation owing to their growth in suspension and diminished integrin-based signaling, facilitating the examination of the Gas6-dependent signaling mechanisms. Gas6 stimulation of THP-1 cells resulted in a similar rapid, but transient, Vav phosphorylation (Fig. 5a, b). We assessed the functional role of Vav in Gas6-mediated signaling on the induction of the phagocytic response using primary microglia from Vav−/− mice. The microglia from wild-type and Vav null mice were stimulated with Gas6 (10 nM), and the phagocytic response was monitored. Gas6-stimulated phagocytosis was not observed in Vav−/− primary microglia (Fig. 5c), demonstrating an essential role of Vav in Gas6-mediated phagocytosis.
Gas6 stimulate Vav phosphorylation in microglia. a BV-2 cells and THP-1 cells were treated with Gas6 (10 nM) for the indicated periods of time. Vav phosphorylation was evaluated by immunoprecipitation with an anti-Vav antibody followed by Western blot analysis using the anti-phosphotyrosine antibody 4G10. Blots were then stripped and reprobed with an anti-Vav antibody as a protein-loading control. b Analysis of band intensity of Western blots of phosphorylated Vav normalized to Vav protein levels and expressed as relative ratio. The data which represent the results of five independent experiments (THP-1) revealed a significant upregulation of Vav phosphorylation at 5 and 10 min after Gas6 stimulation. c Primary microglia from wild-type or vav−/− mice were incubated with vehicle or Gas6 (10 nM) for 30 min, and phagocytosis was quantified by counting the uptake of fluorescent microspheres. The data are pooled from six independent experiments. ***p < 0.0001; *p < 0.01 compared with control
In type I phagocytosis, Vav acts to regulate Rac, a Rho family GTPase, activation by acting as a guanine nucleotide exchange factor (Bustelo 2001), but does not have a role in the type II phagocytic response. Gas6 treatment of BV-2 microglia or THP-1 cells resulted in the rapid activation of Rac (Fig. 6). We monitored the activation of RhoA in parallel, but failed to detect Gas6-stimulated changes in its activation state (data not shown). These results demonstrate that Gas6 induced the phosphorylation of Vav and Rac GTP loading in microglia. Collectively, these data demonstrate that Gas6 elicits a non-classical phagocytic response that morphologically resembles a type II response but employs intracellular signaling elements that have previously been linked to a type I phagocytic process.
Gas6 stimulates Rac activation. Activated Rac levels were measured in THP-1 cells after Gas6 stimulation. a Cells stimulated with Gas6 (10 nM) for the indicated periods were subject to a Rac affinity precipitation assay that captures only GTP-bound, activated, Rac. Precipitants were analyzed by immunoblot analysis using an anti-Rac antibody. Cell lysate samples (40 μg/lane) were run in parallel and immunoblotted with an anti-Rac antibody as a loading control. b Analysis of the relative density ratio of Rac-GTP loading from pooled data of four independent experiments revealed a significant induction of GTP-bound Rac after Gas6 stimulation in THP1 cells at 15 and 20 min. ***p < 0.0001; **p < 0.001 compared with control
Gas6 suppresses the inflammatory response in microglia
Clearance of apoptotic cells by phagocytes typically proceeds without triggering a proinflammatory response (Fadok et al. 1998; Takahashi et al. 2005; Voll et al. 1997). LPS is a bacteria membrane constituent that induces the proinflammatory activation of phagocytes. Microglial activation by LPS is accompanied by the induction of iNOS with the subsequent production of nitric oxide (Combs et al. 2001; Weldon et al. 1998) as well as the induction of proinflammatory cytokines, such as IL-1β (Lee et al. 1993). We tested whether Gas6 was able to suppress the expression of iNOS and IL-1β upon LPS stimulation. BV-2 microglia were treated with LPS alone or in combination with 10 nM Gas6 for 6, 12, or 24 h. In BV-2 microglia, LPS robustly induced either iNOS or IL-1β after 12 h of treatment (Fig. 7a–c). The induction of both proteins reached a peak at 12 h and declined afterwards. However, co-incubation with Gas6 dramatically inhibited iNOS expression. Gas6, acting alone, had no significant effect on iNOS and IL-1β protein levels. As the iNOS expression is principally regulated at the transcriptional level, the inhibitory effect of Gas6 on iNOS gene transcription was tested using an iNOS reporter assay. We found that Gas6 inhibited LPS-induced iNOS reporter activity as measured by luciferase fluorescence (Fig. 7d). These data provide the first direct evidence that Gas6 suppresses proinflammatory gene expression and suggests that this effect underlies Gas6-mediated anti-inflammatory action.
Gas6 exhibits anti-inflammatory actions by reducing IL-1β and iNOS gene expression and promoter activity. BV-2 cells were treated with LPS (1 μg/ml) alone or in combination with Gas6 (10 nM). a iNOS and IL-1β protein levels were detected by Western blot with the appropriate antibodies after 6, 12, and 24 h of stimulation. Blots were stripped and reprobed with anti-Erk2 antibody as a protein-loading control. Densitometry of three independent experiments showed a significant reduction of iNOS (b) as well as IL-1β (c) protein expression in cells stimulated with LPS in combination with Gas6 compared to LPS stimulation alone for 12 h. Gas6 itself did not alter iNOS or IL-1β protein expression compared to control cells. d BV-2 cells were transiently co-transfected with iNOS luciferase reporter with pRL-TK as a control. Cells were then treated with LPS (1 μg/ml) alone or in combination with Gas6 (10 nM) for 12 h, and the luciferase activity was quantified. iNOS luciferase activities were normalized to the constitutively expressed pRL-TK activity and presented as a ratio of the experimental sets treated with LPS alone. These data are representative of pooled results from three independent studies. **p < 0.001 compared with control
Discussion
Our primary interest has been the mechanisms by which macrophages and microglia selectively regulate their responses to apoptotic cells (Cvetanovic and Ucker 2004; Fadok et al. 1998; Wu et al. 2006). We report that microglia express Axl and Mer and that stimulation of these receptors by Gas6 results in the stimulation of phagocytosis. These findings are consistent with the responses of other myeloid lineage cells (Ishimoto et al. 2000). This response is of considerable biological importance, as evidenced by the accumulation of apoptotic cells within a number of organ systems of animals lacking these receptors individually or in combination (Fadok and Chimini 2001; Hall et al. 2005; Lu and Lemke 2001; Wu et al. 2006). Significantly, we have shown that Gas6 induces a non-classical form of phagocytosis which, morphologically, is similar to type II, but uses signaling elements typically associated with type I phagocytosis. Specifically, we have clearly shown that the Gas6-stimulated phagocytic response is dependent upon Vav phosphorylation and activation of its downstream effector, Rac. Vav1 was previously identified as a Mer-binding partner, and Mer activation resulted in tyrosine phosphorylation of Vav1 and release from Mer in monocytes/macrophages (Mahajan and Earp 2003). Notably, type II phagocytosis is associated with activation of RhoA (Caron and Hall 1998); however, we did not observe Rho-A activation after Gas6 stimulation. These results indicated that the Gas6-induced phagocytosis does not conform to either of the classical types of phagocytosis and represents a novel phagocytic response. These findings are of interest in view of newly recognized diversity in phagocytic mechanisms. Isberg et al. (2000) have recently identified a novel β1 integrin-dependent mechanism required for the phagocytosis of the bacteria Yersinia leading to Rac activation, actin reorganization, and phagosome formation. These findings suggest that macrophages and microglia employ diverse mechanisms to engage in a range of phagocytic responses that do not conform to its classical descriptions.
We have demonstrated that microglia efficiently phagocytose apoptotic cells. We did not observe that addition of Gas6 could enhance this process. This is likely to reflect that the apoptotic cells, acting alone, had maximally induced phagocytosis through engagement of Axl/Mer receptors or other PS receptors that stimulate this response. The induction of an apoptotic response in Jurkat cells requires the presence of serum; thus, newly exposed PS moieties may bind Gas6 before exposure to the microglia. Phagocytes possess functionally redundant mechanisms to ensure the removal of apoptotic cells, and it is possible that the apoptotic cells are taken up through Gas6-independent mechanisms. Indeed, a recent study showed that lack of Gas6 did not affect the phagocytosis of rod outer segments in Gas6 knockout mice (Hall et al. 2005).
In this paper, we provide the first evidence that Gas6 acts on microglia/macrophages to suppress the proinflammatory responses elicited by LPS. Analysis of animals lacking Axl/Mer receptors provides compelling evidence that they function physiologically to inhibit the immune response of macrophages (Lemke and Lu 2003). In Axl/Mer/Tyro3 triple knockout mice, the elevated expression of a number of inflammatory molecules (e.g., IL-2 receptor, Fas, IFN-γ, CD44) was observed, indicating constitutive activation of the immune system (Lemke and Lu 2003). Mer knockout mice displayed an enhanced sensitivity to endotoxic shock by LPS challenge. The hypersensitivity of the mice to endotoxin was associated with overproduction of TNF-α by macrophages (Camenisch et al. 1999). We have now directly demonstrated that Gas6 stimulation of Axl/Mer receptors results in the reduced expression of proinflammatory mediators by microglia in response to LPS. We found that Gas6 can inhibit iNOS promoter activity, indicating that this ligand acts to regulate proinflammatory gene expression at the transcriptional level (Fig. 8).
Mechanism of Gas6-induced phagocytosis and immunorepression. In microglia, Gas6 binding stimulates the activation of Axl/Mer and its subsequent phosphorylation of Vav. Activated Vav then stimulates the exchange of GDP for GTP on Rac and initiates cytoskeletal reorganization leading to phagocytosis. The activation of Axl/Mer also leads to transcriptional repression of proinflammatory cytokines, including iNOS and IL-1β
The present study has focused on the actions of Gas6 directed towards microglia. Gas6 is present at high levels in the brain throughout development and continues to be expressed in the adult (Prieto et al. 1999). It has been reported that Gas6 has an anti-apoptotic effect on neurons (Goruppi et al. 1996; Hasanbasic et al. 2004), especially on protecting neurons from amyloid-beta-induced apoptosis (Yagami et al. 2002). Furthermore, Gas6 may act as a neurotrophic factor for hippocampal (Funakoshi et al. 2002) and cortical (Yagami et al. 2003) neurons. The range of biologic actions mediated by Gas6 may be of therapeutic relevance in CNS diseases with an inflammatory component, including neurodegenerative conditions, stroke, or CNS trauma and makes it an attractive target for further investigations.
References
Aderem A, Underhill DM (1999) Mechanisms of phagocytosis in macrophages. Annu Rev Immunol 17:593–623
Bhat NR, Feinstein DL, Shen Q, Bhat AN (2002) p38 MAPK-mediated transcriptional activation of inducible nitric-oxide synthase in glial cells. Roles of nuclear factors, nuclear factor kappa B, cAMP response element-binding protein, CCAAT/enhancer-binding protein-beta, and activating transcription factor-2. J Biol Chem 277:29584–29592
Bratton DL, Fadok VA, Richter DA, Kailey JM, Guthrie LA, Henson PM (1997) Appearance of phosphatidylserine on apoptotic cells requires calcium-mediated nonspecific flip-flop and is enhanced by loss of the aminophospholipid translocase. J Biol Chem 272:26159–26165
Bustelo XR (2001) Vav proteins, adaptors and cell signaling. Oncogene 20:6372–6381
Camenisch TD, Koller BH, Earp HS, Matsushima GK (1999) A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. J Immunol 162:3498–3503
Caron E, Hall A (1998) Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282:1717–1721
Combs CK, Karlo JC, Kao SC, Landreth GE (2001) beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J Neurosci 21:1179–1188
Cvetanovic M, Ucker DS (2004) Innate immune discrimination of apoptotic cells: repression of proinflammatory macrophage transcription is coupled directly to specific recognition. J Immunol 172:880–889
D’Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, Vollrath D (2000) Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet 9:645–651
Fadok VA, Chimini G (2001) The phagocytosis of apoptotic cells. Semin Immunol 13:365–372
Fadok VA, Henson PM (2003) Apoptosis: giving phosphatidylserine recognition an assist-with a twist. Curr Biol 13:R655–R657
Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM (1998) Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 101:890–898
Funakoshi H, Yonemasu T, Nakano T, Matumoto K, Nakamura T (2002) Identification of Gas6, a putative ligand for Sky and Axl receptor tyrosine kinases, as a novel neurotrophic factor for hippocampal neurons. J Neurosci Res 68:150–160
Goruppi S, Ruaro E, Schneider C (1996) Gas6, the ligand of Axl tyrosine kinase receptor, has mitogenic and survival activities for serum starved NIH3T3 fibroblasts. Oncogene 12:471–480
Greenberg S (1995) Signal transduction of phagocytosis. Trends Cell Biol 5:93–99
Greenberg S (1999) Modular components of phagocytosis. J Leukoc Biol 66:712–717
Greenberg S, Chang P, Silverstein SC (1993) Tyrosine phosphorylation is required for Fc receptor-mediated phagocytosis in mouse macrophages. J Exp Med 177:529–534
Hall MO, Obin MS, Heeb MJ, Burgess BL, Abrams TA (2005) Both protein S and Gas6 stimulate outer segment phagocytosis by cultured rat retinal pigment epithelial cells. Exp Eye Res 81:581–591
Hasanbasic I, Cuerquis J, Varnum B, Blostein MD (2004) Intracellular signaling pathways involved in Gas6-Axl-mediated survival of endothelial cells. Am J Physiol Heart Circ Physiol 287:H1207–H1213
Henson PM, Bratton DL, Fadok VA (2001) Apoptotic cell removal. Curr Biol 11:R795–R805
Hirt UA, Leist M (2003) Rapid, noninflammatory and PS-dependent phagocytic clearance of necrotic cells. Cell Death Differ 10:1156–1164
Isberg RR, Hamburger Z, Dersch P (2000) Signaling and invasin-promoted uptake via integrin receptors. Microbes Infect 2:793–801
Ishimoto Y, Ohashi K, Mizuno K, Nakano T (2000) Promotion of the uptake of PS liposomes and apoptotic cells by a product of growth arrest-specific gene, gas6. J Biochem (Tokyo) 127:411–417
Koenigsknecht J, Landreth G (2004) Microglial phagocytosis of fibrillar beta-amyloid through a beta1 integrin-dependent mechanism. J Neurosci 24:9838–9846
Lee SC, Liu W, Dickson DW, Brosnan CF, Berman JW (1993) Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol 150:2659–2667
Lemke G, Lu Q (2003) Macrophage regulation by Tyro 3 family receptors. Curr Opin Immunol 15:31–36
Li S, Yamauchi A, Marchal CC, Molitoris JK, Quilliam LA, Dinauer MC (2002) Chemoattractant-stimulated Rac activation in wild-type and Rac2-deficient murine neutrophils: preferential activation of Rac2 and Rac2 gene dosage effect on neutrophil functions. J Immunol 169:5043–5051
Lu Q, Lemke G (2001) Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science 293:306–311
Lu Q, Gore M, Zhang Q, Camenisch T, Boast S, Casagranda F, Lai C, Skinner MK, Klein R, Matsushima GK, Earp HS, Goff SP, Lemke G (1999) Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature 398:723–728
Mahajan NP, Earp HS (2003) An SH2 domain-dependent, phosphotyrosine-independent interaction between Vav1 and the Mer receptor tyrosine kinase: a mechanism for localizing guanine nucleotide-exchange factor action. J Biol Chem 278:42596–42603
Nagata K, Ohashi K, Nakano T, Arita H, Zong C, Hanafusa H, Mizuno K (1996) Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem 271:30022–30027
Nakano T, Ishimoto Y, Kishino J, Umeda M, Inoue K, Nagata K, Ohashi K, Mizuno K, Arita H (1997) Cell adhesion to phosphatidylserine mediated by a product of growth arrest-specific gene 6. J Biol Chem 272:29411–29414
Newman SL, Mikus LK, Tucci MA (1991) Differential requirements for cellular cytoskeleton in human macrophage complement receptor- and Fc receptor-mediated phagocytosis. J Immunol 146:967–974
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308:1314–1318
Olazabal IM, Caron E, May RC, Schilling K, Knecht DA, Machesky LM (2002) Rho-kinase and myosin-II control phagocytic cup formation during CR, but not FcgammaR, phagocytosis. Curr Biol 12:1413–1418
Pittoni V, Valesini G (2002) The clearance of apoptotic cells: implications for autoimmunity. Autoimmun Rev 1:154–161
Prieto AL, Weber JL, Tracy S, Heeb MJ, Lai C (1999) Gas6, a ligand for the receptor protein-tyrosine kinase Tyro-3, is widely expressed in the central nervous system. Brain Res 816:646–661
Prieto AL, Weber JL, Lai C (2000) Expression of the receptor protein-tyrosine kinases Tyro-3, Axl, and mer in the developing rat central nervous system. J Comp Neurol 425:295–314
Ravichandran KS (2003) “Recruitment signals” from apoptotic cells: invitation to a quiet meal. Cell 113:817–820
Savill J (1997) Recognition and phagocytosis of cells undergoing apoptosis. Br Med Bull 53:491–508
Schneider C, King RM, Philipson L (1988) Genes specifically expressed at growth arrest of mammalian cells. Cell 54:787–793
Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK (2001) Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 411:207–211
Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, Mattsson K, Fisher J, Gies DR, Jones PF et al (1995) The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell 80:661–670
Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ (2003) Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science 299:1743–1747
Streit WJ, Mrak RE, Griffin WS (2004) Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation 1:14
Takahashi K, Rochford CD, Neumann H (2005) Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med 201:647–657
Turner M, Billadeau DD (2002) VAV proteins as signal integrators for multi-subunit immune-recognition receptors. Nat Rev Immunol 2:476–486
Turner M, Mee PJ, Walters AE, Quinn ME, Mellor AL, Zamoyska R, Tybulewicz VL (1997) A requirement for the Rho-family GTP exchange factor Vav in positive and negative selection of thymocytes. Immunity 7:451–460
Varnum BC, Young C, Elliott G, Garcia A, Bartley TD, Fridell YW, Hunt RW, Trail G, Clogston C, Toso RJ et al (1995) Axl receptor tyrosine kinase stimulated by the vitamin K-dependent protein encoded by growth-arrest-specific gene 6. Nature 373:623–626
Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I (1997) Immunosuppressive effects of apoptotic cells. Nature 390:350–351
Weldon DT, Rogers SD, Ghilardi JR, Finke MP, Cleary JP, O’Hare E, Esler WP, Maggio JE, Mantyh PW (1998) Fibrillar beta-amyloid induces microglial phagocytosis, expression of inducible nitric oxide synthase, and loss of a select population of neurons in the rat CNS in vivo. J Neurosci 18:2161–2173
Wilkinson B, Koenigsknecht-Talboo J, Grommes C, Lee CY, Landreth G (2006) Fibrillar beta-amyloid-stimulated intracellular signaling cascades require Vav for induction of respiratory burst and phagocytosis in monocytes and microglia. J Biol Chem 281:20842–20850
Wu Y, Tibrewal N, Birge RB (2006) Phosphatidylserine recognition by phagocytes: a view to a kill. Trends Cell Biol 16:189–197
Yagami T, Ueda K, Asakura K, Sakaeda T, Nakazato H, Kuroda T, Hata S, Sakaguchi G, Itoh N, Nakano T, Kambayashi Y, Tsuzuki H (2002) Gas6 rescues cortical neurons from amyloid beta protein-induced apoptosis. Neuropharmacology 43:1289–1296
Yagami T, Ueda K, Asakura K, Okamura N, Sakaeda T, Sakaguchi G, Itoh N, Hashimoto Y, Nakano T, Fujimoto M (2003) Effect of Gas6 on secretory phospholipase A(2)-IIA-induced apoptosis in cortical neurons. Brain Res 985:142–149
Acknowledgments
We would like to thank Dr. Hugh Perry for his constructive discussions leading to this work. We thank Dr. N. Bhat (Medical University of South Carolina) for providing us with the iNOS reporter construct. This work was supported by grants from the NIH (AG-20202 and AG-16740) and the Blanchette Hooker Rockefeller Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grommes, C., Lee, C.Y.D., Wilkinson, B.L. et al. Regulation of Microglial Phagocytosis and Inflammatory Gene Expression by Gas6 Acting on the Axl/Mer Family of Tyrosine Kinases. J Neuroimmune Pharmacol 3, 130–140 (2008). https://doi.org/10.1007/s11481-007-9090-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-007-9090-2