Abstract
Human herpesvirus 6 (HHV-6) infects and establishes latency in the central nervous system (CNS). Reactivation of latent HHV-6 has been associated with neurologic diseases including epilepsy and multiple sclerosis (MS). In vivo, HHV-6 has been localized to astrocytes and can infect human astrocytes in vitro, suggesting that this virus may have a tropism for glial cells and may affect glial cell function. An essential role of astrocytes in the CNS is active maintenance of the excitatory neurotransmitter glutamate. Dysregulation of glutamate has been implicated as a potential mechanism of disease in both epilepsy and MS. Both disorders have demonstrated elevated glutamate in CSF and may be associated with dysregulation of glutamate signaling, uptake, and metabolism. This study demonstrates dysregulation of glutamate uptake in human astrocytes infected with both variants of HHV-6, A and B, with differential effects of HHV-6 in acute and persistently infected cells. Whereas astrocytes acutely infected with HHV-6 demonstrated increased glutamate uptake, cells persistently infected with HHV-6A and HHV-6B demonstrated impaired glutamate uptake. Functional dysregulation of glutamate uptake was associated with early increases in mRNA and protein expression of the glial glutamate transporter EAAT-2 followed by a sustained decrease in mRNA expression in astrocytes infected with both HHV-6A and HHV-6B. Dysregulated glutamate uptake and transporter expression suggests a mechanism for dysregulation of glutamate levels in vivo and a potential mechanism for virus-associated neurologic disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human herpesvirus 6 (HHV-6) is a ubiquitous β-herpesvirus originally discovered in patients with lymphoproliferative disorders (Salahuddin et al. 1986). Exposure to HHV-6 normally occurs within the first 2 years of life, after which the virus establishes life-long latency. One site of HHV-6 latency is the central nervous system (CNS), and reactivation of the virus can occur after bone marrow transplant, particularly in association with immunosuppression (Fotheringham et al. 2007a). HHV-6 has been detected in brain material from patients with neurological disorders including multiple sclerosis (MS) and epilepsy (Cermelli et al. 2003; Challoner et al. 1995; Donati et al. 2003; Goodman et al. 2003). Although HHV-6 DNA can be found at low frequency in normal brains (Chan et al. 2001; Cuomo et al. 2001; Opsahl and Kennedy 2005) and may be a commensal pathogen in the CNS, reactivation associated with these disorders may contribute to disease pathology.
HHV-6 exists as two distinct variants, HHV-6A and HHV-6B, which differ in nucleotide sequence, cellular tropism, antigenicity, and etiology (De Bolle et al. 2005a). HHV-6B is the causative agent of the childhood febrile illness exanthem subitum (roseola) (Yamanishi et al. 1988). Although both variants of HHV-6 have been detected in human brain, MS has most often been associated with variant A (Akhyani et al. 2000; Alvarez-Lafuente et al. 2004, 2002; Fogdell-Hahn et al. 2005), whereas a subset of patients with mesial temporal lobe epilepsy (MTLE) have demonstrated exclusive detection of HHV-6B (Donati et al. 2003; Fotheringham et al. 2007b). A common mechanism that links these divergent diseases with HHV-6 is the observation that HHV-6 demonstrates tropism for astrocytes. Both HHV-6A and HHV-6B can infect primary astrocytes and glioma cells in vitro, although the two variants demonstrate different growth characteristics (Ahlqvist et al. 2005; Akhyani et al. 2006; De Bolle et al. 2005b; Donati et al. 2005; He et al. 1996; Yao et al. 2006). Expression of viral antigen in MS, MTLE, and post-bone marrow transplant brain can be detected in reactive astrocytes (Challoner et al. 1995; Donati et al. 2003; Fotheringham et al. 2007a; Fotheringham et al. 2007b; Goodman et al. 2003), and primary isolates of HHV-6 have recently been propogated in astrocytes cultured from freshly resected MTLE tissue (Fotheringham et al. 2007b). Infection of astrocytes with a large DNA virus like HHV-6 that codes for approximately 100 genes of known and unknown function (De Bolle et al. 2005a) raises the hypothesis that HHV-6 could encode multiple viral proteins that may affect the function of infected glial cells and thereby contribute to disease pathology.
Long thought to be strictly the scaffold or support cells of the brain, astrocytes are now known to be actively involved in intercellular communication and important regulators of brain function (Araque et al. 1999; Danbolt 2001; Matute et al. 2006). The active interaction between astrocytes and neurons regulates synaptic transmission, and alterations in this cell–cell relationship may contribute to CNS dysfunction. Astrocytes are crucial for maintenance of extracellular glutamate levels, the main excitatory neurotransmitter in the brain (Danbolt 2001). Active uptake of glutamate, intracellular metabolism, and release of glutamate and glutamine by astrocytes serve to preserve low extracellular glutamate levels, thereby protecting healthy cells from excitotoxicity (Danbolt 2001). Maintenance of extracellular glutamate is mediated primarily by the high-affinity, sodium-dependent glial glutamate transporters EAAT-1 and EAAT-2 (Danbolt 2001). EAAT-2 is the most abundant transporter expressed on astrocytes and is thought to mediate the majority of glutamate uptake in the CNS (Tanaka et al. 1997). A role for astrocytes and regulation of glutamate has been implicated in the generation of seizure (Cavus et al. 2005; de Lanerolle and Lee 2005; During and Spencer 1993; Tian et al. 2005). In addition, mutation or alternative splicing of glutamate transporters has been suggested to dysregulate glutamate uptake in epilepsy and promote the generation of seizures (Hoogland et al. 2004; Jen et al. 2005).
Regulation of glutamate, the major excitatory neurotransmitter in the brain, is associated with proper CNS function and is a primary function of astrocytes. Consequently, in this study, we examined the infection of primary human astrocytes with HHV-6 variants A and B and determined functional changes in glutamate uptake and changes in glutamate transporter expression. Infection of astrocytes with HHV-6A and HHV-6B induced dysregulation of glutamate uptake at acute and chronic time points and was associated with decreased expression of the glutamate transporter EAAT-2.
Materials and methods
Cell culture and viral infections
Fresh human brain tissue was obtained from NIH and Children’s National Medical Center (Washington, DC) under protocols approved by the respective institutional review boards. Tissue was kept on ice in Hibernate A media (Brain Bits, Springfield, IL, USA) containing 2% B27 supplement, 0.5 mM glutamine, 1% penicillin/streptomycin, and 1:1,000 fungizone until processing. Tissue was dissociated manually with a razor blade and enzyme digested in Earl’s balanced salt solution containing 0.1% DNase and 20 U/ml papain at 37°C for 1 h. Dissociated tissue was centrifuged at 1,500 rpm for 10 min and mechanically dissociated using a sterile glass pipette. After passing through a 40-μm filter, the single cell suspension was separated on a percoll gradient by centrifugation at 15,000 rpm for 30 min. The glial cell layer was removed, washed, and plated on poly-l-lysine coated tissue culture flasks in Dulbecco’s modified Eagle’s medium/F12 supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, and 0.1% gentimycin. Fresh media was added daily until cell debris was completely removed. Purity of adult astrocyte cultures was confirmed by immunofluorescence staining for glial fibrillary acidic protein (GFAP) and cells were infected in six-well plates with HHV-6 at a density of 2.5 × 104 cells/ml.
Primary human astrocytes and U251 astroglioma cells were infected with both variants of HHV-6, A and B. Briefly, cell cultures were incubated for 3 h with cell-free supernatants from T cells infected with HHV-6A (U1102 strain) or 6B (Z29 strain) (inoculum 109 viral copies/106 cells), as described previously (Ahlqvist et al. 2005), or with cell-free supernatants first passed through a 0.1-μm filter. Cells were washed three times with sterile phosphate-buffered saline (PBS) and fresh growth media was added. Infections were monitored for cytopathic effect and postinfection for viral load with quantitative polymerase chain reaction (PCR) and for viral RNA by reverse transcriptase (RT) PCR. As demonstrated in previous studies, infection with HHV-6A induced cytopathic effect early during infection (Ahlqvist et al. 2005; Donati et al. 2005), and both HHV-6A- and HHV-6B-infected cells could be maintained and propagated in culture for 60 days (Ahlqvist et al. 2005).
Quantitative TaqMan PCR
DNA was extracted using the QIamp viral RNA kit for cell-free supernatants (Qiagen, Valencia, CA, USA) and the DNeasy kit (Qiagen) for cell pellets according to manufacturer’s instructions. DNA from samples and controls was amplified using TaqMan technology on an ABI PRISM 7700 Sequence Detector System (Perkin Elmer, Waltham, MA, USA). Specific primers and probes for HHV6-A and HHV6-B and plasmids have been described (Nitsche et al. 2001). Results from cell lysates were normalized to β-actin.
Immunofluorescence
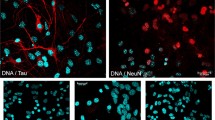
Astrocytes were grown on four-well chamber slides and fixed at −20°C for 10 min in acetone/methanol (1:1). Slides were blocked for 10 min at room temperature with 3% BSA prepared in PBS. Primary antibody dilutions were prepared in PBS (1:100 mouse anti-gp116/54/64 (Advanced Biotechnologies, Columbia, MD, USA), 1:100 rabbit anti-GFAP (DAKO, Carpinteria, CA, USA), 1:40 mouse anti-HHV-6A immediate early 2 (IE2), a generous gift from Dr. Louis Flamand, and incubated on slides for 30 min at 37°C or for 1 h at room temperature. Slides were washed three times in PBS and incubated with secondary antibody [1:100 anti-rabbit IgG1 FITC, 1:100 anti-mouse IgG1 rhodamine, or 1:1,000 anti-mouse IgG2b rhodamine (Molecular Probes, Eugene, OR, USA)] prepared in PBS for 30 min at 37°C or for 1 h at room temperature. Slides were mounted with mounting media containing DAPI and were visualized using a fluorescence microscope (Carl Zeiss Microimaging, Thornwood, NY, USA) at 20× magnification.
Glutamate uptake
Astrocyte cultures were washed three times in warm Kreb’s buffer (119 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 25 mM NaHCO3, 2.5 mM CaCl3, 1.0 mM MgCl2, 5.5 mM glucose, pH 7.2) and then incubated for 15 min with 2.5 μM glutamate (0.5 μCi/well) in Kreb’s buffer at 37°C. Glutamate uptake was terminated by addition of ice-cold Kreb’s buffer, and excess extracellular glutamate was removed by washing cells three times in ice-cold Kreb’s. Cells were lysed overnight at room temperature in 0.25 mM NaOH containing 0.1% Triton ×100. Cell lysates were harvested and radioactivity was determined using a Beta counter. Protein concentration was determined using the detergent compatible protein assay from BioRad (Hercules, CA, USA) according to the manufacturer’s instructions.
RT-PCR and TaqMan
RNA for RT-PCR and TaqMan was isolated from cell pellets using the RNeasy Plus kit as per manufacturer’s instructions (Qiagen). The RNeasy Plus kit includes a spin step to remove genomic DNA, eliminating the need for DNase treatment of isolated RNA. RNA was reverse-transcribed using the First Strand Superscript III cDNA synthesis kit (Invitrogen, Carlsbad, CA, USA), as per manufacturer’s instructions, using random hexamer primers and 8 μl of purified RNA. Two microliters of cDNA was PCR amplified for EAAT-1, EAAT-2, U12, U16/17, or actin (Table 1) using the Taq PCR master mix kit from Qiagen. RT-PCR products were run on 1.5% agarose gels and visualized with ethidium bromide staining.
Two microliters of cDNA from samples and controls was amplified for EAAT-2 expression using TaqMan technology on an ABI PRISM 7700 Sequence Detector System (Perkin Elmer). Specific primers and probes for EAAT-2 were custom synthesized by Synthegen, Houston, TX, USA (sense, CTCCTCATTCTGACAGCCGTG; antisense, CCCACAACATTGACTGAAGTTCTC; FAM/TAMRA probe, TGTCCAGCAGCCAGTCCACAGCCA). Results from cell lysates were normalized to expression of HPRT.
Results
HHV-6A actively infects primary human astrocytes
To characterize the infection of HHV-6A and HHV-6B in primary human astrocytes, viral loads in cell lysates and supernatant were quantitated by variant-specific real-time PCR at an early time point representative of acute infection (e.g., day 5) and a later time point representative of a chronic infection (e.g., day 25). In primary human astrocytes, viral load in cell lysates from HHV-6A-infected cells increased from 1.19 × 108 copies/cell at day 5 to 4.87 × 108 copies/cell at day 25, suggesting active HHV-6A replication. This observation is consistent with a previous report demonstrating active infection and viral mRNA expression in primary human astrocytes infected with HHV-6A (Yao et al. 2006). Viral load in supernatant decreased from 4.55 × 105 copies/ml at day 5 to undetectable levels at day 25 (Fig. 1a), indicating that, by day 25 postinfection, viral particles were not being released from HHV-6A-infected cells. In contrast to astrocytes infected with HHV-6A, primary astrocytes infected with HHV-6B demonstrated significantly lower viral loads in cell lysate and supernatant that decreased over time, suggesting HHV-6B-infection of primary astrocytes was nonproductive (Fig. 1a). This observation is supported by HHV-6B infection of an in vitro glioma U251 cell line, which demonstrated HHV-6B mRNA transcripts at days 1–4 that were no longer detectable by day 6 (Fig. 2a).
HHV-6 infects and dysregulates glutamate uptake in primary human astrocytes. Primary human astrocytes were mock-infected or were infected with HHV-6A (U1102) and HHV-6B (Z29) for 5 days. a Viral load in primary human astrocytes infected with HHV-6A or HHV-6B was measured by variant-specific TaqMan PCR 5 and 25 days postinfection. Viral load was quantitated as viral copies per 106 cells in cell lysates or as viral copies per milliliter in cell culture supernatant. b Primary human astrocytes were infected with mock, HHV-6A, or HHV-6B, and uptake of 3H-glutamate was determined during early (day 5) and late (days 25–44) infection. Glutamate uptake is expressed as percent change compared to mock (n = 3). c Semiquantitative expression of EAAT-2 was determined by RT-PCR in HHV-6A- and HHV-6B-infected cells at day 5. EAAT-2 and actin PCR product sizes are indicated by black arrows. d EAAT-2 expression was confirmed by quantitative real-time PCR. U251 glioma cells served as positive controls for both assays, the T lymphocyte cell lines JJahn and SupT1 served as negative controls.
HHV-6 infects and dysregulates glutamate uptake in U251 glioma cells. a mRNA expression for the HHV-6 immediate/early genes U12 and U16/17 was measured by RT-PCR in U251 glioma cells infected with filtered (6A-F, 6B-F) and nonfiltered HHV-6A and HHV-6B inocula at 4 days postinfection. Band sizes for actin, U12, and U16/17 PCR products are noted in Table 1. b Expression of HHV-6 viral protein in U251 glioma cells was determined 5 days postinfection with the HHV-6A variant specific antibody towards IE2 (red) and the nonvariant specific antibody towards gp116/54/64 (red). HHV-6 viral protein staining was colocalized with the astrocyte specific marker GFAP (green). Images are taken using a 20× objective. c Glutamate uptake was determined in U251 glioma cells infected with HHV-6A and HHV-6B during early (day 5) and late (day 32) infection. Glutamate uptake is represented as percent change compared to mock infection (n = 4).
Glutamate uptake is dysregulated in primary human astrocytes infected with HHV-6
To determine whether infection of human astrocytes with HHV-6 was associated with functional changes in glutamate uptake, we measured uptake of 3H-glutamate during early (day 5) and late (day 25) infection. In primary human astrocytes, infected with HHV-6A and HHV-6B, glutamate uptake was higher compared to mock during the early stages of infection (106.9 and 94.2% increase compared to mock, respectively; Fig. 1b). At later stages of infection, however, glutamate uptake in both HHV-6A- and HHV-6B-infected cells was decreased compared to uptake in mock-infected cells (62.2 and 45.3% lower than mock, respectively; Fig. 1b), suggesting a differential effect on uptake in early vs. late HHV-6 infection.
Glutamate transporter expression is decreased in HHV-6 infected primary human astrocytes
To determine a mechanism by which HHV-6-induced dysregulation of glutamate uptake might occur, we measured mRNA expression of the glial glutamate transporters EAAT-1 and EAAT-2. As a positive control, U251 glioma cells expressed mRNA for both transporters, and also expressed the alternatively spliced variant of EAAT-2 (702-bp band), which was not expressed by uninfected (mock) primary astrocytes (Fig. 1c). JJahn T cells did not express mRNA for either transporter and served as a negative control (Fig. 1c,d). HHV-6A- and HHV-6B-infected U251 did not demonstrate any detectable change in EAAT-1 mRNA expression at day 5 postinfection (data not shown). Surprisingly, mRNA levels of EAAT-2 were decreased at day 5 postinfection in both HHV-6A and HHV-6B infected astrocytes at the same time point that glutamate uptake was increased (Fig. 1b). The observation that glutamate uptake and EAAT-2 expression was discordant suggests that HHV-6-induced increases in glutamate uptake may occur through a different mechanism than decreased EAAT-2. Decreased expression of EAAT-2 mRNA in primary human astrocytes at day 5 postinfection with HHV-6A and 6B was confirmed by quantitative real-time PCR demonstrating a 4.0- and 2.9-fold decrease, respectively (Fig. 1d).
HHV-6 actively infects U251 glioma cells
Because HHV-6A and HHV-6B infection of primary astrocytes had comparable effects on glutamate uptake even though HHV-6B infection appeared to be nonproductive, we investigated whether the effect on glutamate uptake was directly viral or caused by a virus-induced soluble factor (e.g., cytokines). Primary human astrocytes derived from a single brain are difficult to propogate in large quantities and grow slowly, and insufficient quantities are available to extensively investigate. As a result, we utilized the established model system of GFAP-positive U251 glioma cells that has been used to study HHV-6 (Akhyani et al. 2006; Yoshikawa et al. 2002) to confirm that the characteristics of HHV-6 infection and dysregulation of glutamate would mimic our observations in primary astrocytes.
To determine whether U251 astrocytes were actively infected with HHV-6, mRNA expression for the HHV-6 immediate/early genes U12 and U16/17 was measured by RT-PCR at days 1 and 4 postinfection. U251 astrocytes infected with HHV-6A and HHV-6B inocula expressed mRNA for U12 at days 1 and 4 (nonspliced transcript 327 bp; spliced transcript 202 bp), whereas only HHV-6A-infected cells expressed the spliced mRNA transcript for U16 (nonspliced transcript 240 bp; spliced transcript 147 bp) at days 1 and 4 (Fig. 2a). Expression of the spliced transcript suggests that U251 were actively infected with HHV-6A, consistent with our observation of active HHV-6A infection in primary human astrocytes. HHV-6B-infected cells expressed the nonspliced mRNA transcript for U16/17 at day 1 that was barely detectable by day 4, suggesting that U16/17 was expressed but not spliced. Expression of a nonspliced transcript is suggestive of a nonproductive infection. These results are consistent with the observation in primary human astrocytes that HHV-6A establishes an active infection, whereas infection with HHV-6B is nonproductive (Fig. 1a). Difference in viral gene expression in HHV-6A- and HHV-6B-infected glial cells suggests that infection with variant A may be more productive, as has been previously suggested (Ahlqvist et al. 2005; Donati et al. 2005; Yao et al. 2006). Although HHV-6B can infect U251 astrocytes and induces expression of at least one viral gene, viral replication may be incomplete.
Finally, we determined examined viral protein expression of the immediate/early protein IE2 and the late HHV-6 glycoprotein gp116/54/64. As shown in Fig. 2b, U251 cells infected with HHV-6A for 5 days demonstrated punctate staining for IE2 localized to the nucleus of cells that were visibly enlarged (Fig. 2b). U251 cells infected with HHV-6B, however, did not demonstrate any staining for gp116/54/64 or any morphological changes indicative of active infection, such as enlargement or syncytia formation (Fig. 2b). Expression of HHV-6 genes in infected U251 cells confirmed our finding in primary astrocytes that HHV-6A infection is more productive than HHV-6B infection.
Glutamate uptake is dysregulated in HHV-6 infected U251 glioma cells
When glutamate uptake was compared in HHV-6A- and HHV-6B-infected U251 glioma cells during early (e.g., day 4) and late infection (e.g., day 32), the results mirrored those observed for HHV-6-infected primary human astrocytes (Fig. 1b). As shown in Fig. 2c, early infection with HHV-6A, and to a lesser extent, HHV6-B, increased glutamate uptake compared to mock-infected cells. Both HHV-6A and HHV-6B infected U251 showed decreased glutamate uptake compared to mock-infected cells during late infection (e.g., day 32). These data demonstrate that HHV-6 infection of primary human astrocytes and U251 glioma cells leads to increased glutamate uptake early in infection and decreased glutamate uptake later in infection. Collectively, these results demonstrate that HHV-6-infected U251 glioma cells are a faithful representation of virus-infected primary human astrocytes.
HHV-6-induced dysregulation of glutamate uptake involves a viral-induced soluble factor
Because HHV-6A and HHV-6B infection of primary astrocytes and U251 cells dysregulated glutamate uptake even though both cell types demonstrated nonproductive infection with HHV-6B and productive infection with HHV-6A, we investigated whether the changes in glutamate uptake could be associated with a virus-induced soluble factor rather than a direct viral effect. HHV-6 virions are approximately 160 nm in diameter, and filtration of HHV-6 inocula through a 0.1-μm filter should eliminate infectious virus while allowing small, soluble factors to pass through. U251 glioma cells were infected with HHV-6A and HHV-6B that was filtered or nonfiltered, and viral load was determined over time. As expected, there was a significant decrease in viral load from days 1 to 6 in cells infected with both HHV-6A and HHV-6B filtered inocula, demonstrating that filtration eliminated a significant amount of infectious virus (Fig. 3a). To verify this observation, mRNA expression of the HHV-6 immediate/early genes U12 and U16/17 was determined in U251 cells infected with filtered inocula at days 1 and 4 postinfection. U251 cells infected with filtered HHV-6A or HHV-6B inocula did not express either viral gene, confirming that filtration of HHV-6 removed infectious virus (Fig. 2b).
HHV-6 Induced Dysregulation of Glutamate Uptake is Associated With a Virus-Induced Soluble Factor. U251 glioma cells were mock infected or were infected with filtered and nonfiltered HHV-6A (U1102) and HHV-6B (Z29) inocula. a Viral load was measured by variant-specific TaqMan PCR at 1, 4, and 6 days postinfection. Viral load was quantitated as viral copies per 106 cells. b Glutamate uptake determined at day 4 postinfection is represented as percent change compared to mock (representative experiment).
As HHV-6 infection of primary human astrocytes and U251 glioma cells increased glutamate uptake, we investigated whether this effect was directly viral or associated with a virus-induced soluble factor. As shown in Fig. 3b, infection of U251 astrocytes with HHV-6A and HHV-6B for 4 days demonstrated increased glutamate uptake (p < 0.001), again recapitulating the results observed with HHV-6-infected primary astrocytes (Fig. 1b). However, this increase in glutamate uptake was not affected by filtration of HHV-6 inocula (Fig. 3b; p < 0.001), suggesting that, during early infection, increased glutamate uptake was associated with an HHV-6-induced soluble factor and may not be directly related to an active virus infection.
HHV-6 infection of U251 glioma cells dysregulates glutamate transporter expression
To determine if HHV-6 infection of U251 glioma cells dysregulated EAAT-1 or EAAT-2 expression, glutamate transporter expression was measured in U251 infected with nonfiltered and filtered inocula at day 4 postinfection. RT-PCR determination of EAAT-1 mRNA demonstrated no detectable changes in HHV-6A- or HHV-6B-infected U251 glioma cells (data not shown). However, real-time PCR determination of EAAT-2 mRNA demonstrated decreased expression following infection with nonfiltered HHV-6A or HHV-6B inocula (Fig. 4a; p < 0.05), confirmed by RT-PCR (data not shown), consistent with the observation in primary human astrocytes (Fig. 1c,d). Filtration of HHV-6A inocula significantly reversed the decrease in EAAT-2 mRNA expression (Fig. 4a; p < 0.05), suggesting that the effect of HHV-6A on EAAT-2 expression at day 4 was directly related to virus infection and not due to a virus-induced soluble factor. By contrast, filtration of HHV-6B did not reverse the decrease in EAAT-2 mRNA expression, suggesting that, unlike the observation with HHV-6A, the HHV-6B-induced decrease in EAAT-2 mRNA is associated with a soluble factor (Fig. 4a). The dysregulation of glutamate uptake and transporter expression (Figs. 3b and 4a) by an HHV-6B-induced soluble factor is consistent with the observation that HHV-6B is associated with a nonproductive infection in primary astrocytes and U251 cells (Figs. 1a and 2a,b).
HHV-6 induces an early increase in EAAT-2 expression. U251 glioma cells were mock infected or infected for 4 days with filtered and nonfiltered HHV-6A (U1102) and HHV-6B (Z29) inocula. a EAAT-2 expression was determined by quantitative real-time PCR. b EAAT-2 expression was determined by quantitative real-time PCR at days 1, 2, 3, and 7 postinfection. Data are expressed as percent difference from levels of EAAT-2 expression in mock-infected cells.
Because infection of primary astrocytes and U251 glioma cells with HHV-6 increased glutamate uptake during early infection, at a time when mRNA levels for EAAT-2 were low, we investigated whether increased glutamate uptake was related to EAAT-2 expression. As shown in Fig. 4b, EAAT-2 mRNA expression was increased at day 2 in both HHV-6A- and HHV-6B-infected U251 cells compared to mock and would be consistent with the increase in glutamate uptake observed in HHV-6-infected primary astrocytes (Fig. 1b) and U251 cells (Fig. 2c) at days 4–5. The increase in mRNA levels at days 1 and 2 is likely associated with a virus-induced soluble factor because HHV-6A viral antigen expression, indicating full viral replication, is not detectable until day 5 (Fig. 2b) and infection with HHV-6B is nonproductive. By day 3, EAAT-2 mRNA levels begin to decrease following both HHV-6A and HHV-6B infection (Fig. 4b). This decrease in EAAT-2 transcript by day 3 is consistent with the decreases previously detected in primary astrocytes and U251 cells during early infection (Figs. 1c,d and 4a). The sustained decrease in EAAT-2 mRNA first detectable on day 3 may translate to the consistent decrease in glutamate uptake during late infection. The temporal relationship of mRNA for EAAT-2 and glutamate uptake in HHV-6-infected astrocytes is schematically represented in Fig. 5. In this model, the early (days 1–2) increase in EAAT-2 and uptake for both HHV-6A- and HHV-6B-infected astrocytes is associated with a soluble factor because the effect was not reversed by filtration of the inocula. Later in infection (e.g., day 30), the observed decrease in glutamate uptake and EAAT-2 expression appears to be directly related to virus for HHV-6A-infected cells because this effect was reversed by filtration. As we have demonstrated that HHV-6B is a nonproductive infection in glial cells, the decrease in glutamate uptake and EAAT-2 expression later in infection may be associated with an HHV-6B-induced soluble factor or with low levels of viral DNA that have been shown to persist throughout the culture (Ahlqvist et al. 2005).
Schematic model of HHV-6-induced dysregulation of glutamate uptake and glutamate transporter expression. Changes in EAAT-2 mRNA expression are represented by the blue line, and changes in glutamate uptake are represented by the red line. Changes are displayed graphically over time. Mechanisms for HHV-6-induced changes in EAAT-2 or glutamate uptake are indicated as factor (soluble factor effect) or virus (direct viral effect). Only the mechanisms for day 30 are proposed.
Discussion
HHV-6 infection in the CNS has been associated with neurologic disorders including epilepsy, MS, and encephalitis (Cermelli et al. 2003; Challoner et al. 1995; Donati et al. 2003; Fotheringham et al. 2007a; Goodman et al. 2003). Although active viral infection has been localized to astrocytes in resected tissue from patients with MTLE and in MS lesions (Challoner et al. 1995; Donati et al. 2003; Goodman et al. 2003), an unanswered question is how HHV-6 infection of astrocytes may contribute to disease pathology. In this study, we show that HHV-6A and HHV-6B exhibit different infection characteristics in human astrocytes, and that both variants induce dysregulation of glutamate uptake and expression of glutamate transporters.
Active HHV-6 infection has been detected in astrocytes in vivo and in vitro (Akhyani et al. 2006; De Bolle et al. 2005b; Donati et al. 2003, 2005; Fotheringham et al. 2007a; Goodman et al. 2003; He et al. 1996; Yao et al. 2006). While HHV-6A and HHV-6B both infected human astrocytes, expression of only the nonspliced mRNA for immediate/early U16/17 and lack of detection of late viral protein suggests that HHV-6B replication was incomplete. Several published studies have demonstrated limited viral mRNA detection in HHV-6B-infected astrocytes compared to viral mRNA expressed following HHV-6A infection (De Bolle et al. 2005b; Donati et al. 2005). Similarly, our study found expression of the immediate/early gene U12 mRNA but expression only of the nonspliced U16/17 at day 1, and viral mRNA expression was lost by 4–6 days postinfection. These data, in combination with other published studies, suggest that HHV-6B can infect but does not establish a productive, active infection in astrocytes. Active infection of astrocytes with HHV-6A, however, is demonstrated by detection of spliced viral mRNA for U12 and U16/17, as well as protein expression of IE2. Both HHV-6A- and HHV-6B-infected cells had undetectable levels of viral mRNA by days 14 and 6, respectively, but stable levels of cellular viral DNA for up to 4–6 weeks postinfection, suggesting that both variants establish persistent, but low-level, infection.
Although the characteristics of early infection were different for HHV-6A and HHV-6B, particularly with respect to mRNA expression and cytopathic effect, each infection demonstrated consistently high levels of glutamate uptake compared to mock-infected cells. After removing infectious virus from our inocula through a 0.1-μm filter, the HHV-6A- and HHV-6B-induced increase in glutamate uptake was preserved, suggesting that a virus-induced soluble factor may be influencing uptake rather than being a direct effect of the virus in astrocytes. This suggests that an HHV-6-induced factor may trigger increased glutamate uptake. Glutamate uptake can be affected by proinflammatory cytokines including TNF-α, and HHV-6 can induce expression of proinflammatory genes (Mayne et al. 2001), suggesting a cytokine or a viral chemokine may cause the effect we report on glutamate.
During the chronic phase of infection, both HHV-6A and HHV-6B-infected astrocytes demonstrated an impaired ability to uptake glutamate, and this functional change was associated with decreased expression of the glial glutamate transporter EAAT-2. The early, acute phase of infection was associated with high glutamate uptake in astrocytes infected with either HHV-6A or HHV-6B. mRNA for EAAT-2 was increased at days 1 and 2 and decreased by day 3. The decrease in EAAT-2 mRNA expression at day 3 is sustained through the chronic phase of infection and may correlate with impaired glutamate uptake at later time points. The increase in EAAT-2 mRNA expression at days 1 and 2 likely translates to an increase in protein expression and in glutamate uptake that was detected at day 4. Whether the early increase in message and protein for EAAT-2 results from a soluble factor (may occur for 6B) or is directly viral (may occur for 6A), it correlates with the early expression of viral RNA and may be an early response of the astrocyte to HHV-6 replication. The long-term decrease in EAAT-2 expression correlates with a sustained decrease in glutamate uptake first detected at day 5 that is maintained throughout late infection and may be associated with low levels of viral DNA.
Glutamate dysregulation has been proposed as a disease mechanism in epilepsy and MS (Binder and Steinhauser 2006; Bolton and Paul 2006; Groom et al. 2003; Matute et al. 2006), and both disorders have been associated with HHV-6 infection of astrocytes (Cermelli et al. 2003; Challoner et al. 1995; Donati et al. 2003; Goodman et al. 2003). Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor blockers have demonstrated reduction of neurologic disability in rodent models of experimental autoimmune encephalomyelitis (EAE), and these studies have suggested AMPA-mediated excitotoxicity of oligodendrocytes as a mechanism of disease (Pitt et al. 2000; Smith et al. 2000; Werner et al. 2000). In humans, high levels of CSF glutamate have been associated with exacerbation in relapsing–remitting MS, and decreased glutamate transporter expression has been shown on oligodendrocytes surrounding MS lesions (Pitt et al. 2003; Sarchielli et al. 2003). Based on animal and human studies of glutamate in EAE and MS, mechanisms have been proposed whereby glutamate levels in these disorders are not properly controlled. Dysregulation of glutamate can trigger glutamate-induced excitotoxic death of oligodendrocytes, which may lead to the development of MS lesions. Here, we suggest that HHV-6 infection of astrocytes in MS lesions (Cermelli et al. 2003) may likewise lead to impaired glutamate uptake through downregulated transporters, high glutamate levels, and excitotoxicity.
In studies of epilepsy, glutamate has long been considered a major contributor to disease pathology. High levels of glutamate are detected following seizure (Cavus et al. 2005; de Lanerolle and Lee 2005; During and Spencer 1993), expression of alternatively spliced glutamate transporters is increased (Hoogland et al. 2004), expression of glutamate-metabolizing enzymes is dysregulated in patients with epilepsy (de Lanerolle and Lee 2005; Eid et al. 2004; van der Hel et al. 2005), and injection of the glutamate receptor agonist kainic acid induces seizures in rodents (Leite et al. 2002). High levels of extracellular glutamate resulting from impaired glutamate uptake by astrocytes may induce seizures by causing depolarization and hyperexcitability of neurons. HHV-6 DNA has been detected in resected tissue from patients with MTLE, and active HHV-6 infection has been localized to astrocytes in primary cell cultures isolated from these patients (Donati et al. 2003). A recent study of primary astrocytes isolated from patients with MTLE demonstrated the presence of HHV-6 viral antigen and mRNA, indicating active infection, and low levels of glutamate transporter mRNA, suggesting HHV-6 may be associated with downregulated transporter expression in vivo (Fotheringham et al. 2007b).
Although HHV-6 infection in the CNS has been associated with neurologic disorders, it is unknown how this virus may contribute to disease pathology. Dysregulated glutamate uptake and transporter expression in HHV-6-infected primary astrocytes constitutes the first report of this virus altering cell function and proposes an intriguing mechanism for the contribution of HHV-6 to the pathology of neurologic diseases like MS and epilepsy.
References
Ahlqvist J, Fotheringham J, Akhyani N, Yao K, Fogdell-Hahn A, Jacobson S (2005) Differential tropism of human herpesvirus 6 (HHV-6) variants and induction of latency by HHV-6A in oligodendrocytes. J Neurovirol 11:384–394
Akhyani N, Berti R, Brennan MB, Soldan SS, Eaton JM, McFarland HF, Jacobson S (2000) Tissue distribution and variant characterization of human herpesvirus (HHV)-6: increased prevalence of HHV-6A in patients with multiple sclerosis. J Infect Dis 182:1321–1325
Akhyani N, Fotheringham J, Yao K, Rashti F, Jacobson S (2006) Efficacy of antiviral compounds in human herpesvirus-6-infected glial cells. J Neurovirol 12:284–293
Alvarez-Lafuente R, Martin-Estefania C, de Las Heras V, Castrillo C, Picazo JJ, Varela de Seijas E, Gonzalez RA (2002) Active human herpesvirus 6 infection in patients with multiple sclerosis. Arch Neurol 59:929–933
Alvarez-Lafuente R, De las Heras V, Bartolome M, Picazo JJ, Arroyo R (2004) Relapsing-remitting multiple sclerosis and human herpesvirus 6 active infection. Arch Neurol 61:1523–1527
Araque A, Sanzgiri RP, Parpura V, Haydon PG (1999) Astrocyte-induced modulation of synaptic transmission. Can J Physiol Pharmacol 77:699–706
Binder DK, Steinhauser C (2006) Functional changes in astroglial cells in epilepsy. Glia 54:358–368
Bolton C, Paul C (2006) Glutamate receptors in neuroinflammatory demyelinating disease. Mediators Inflamm 2006:93684
Cavus I, Kasoff WS, Cassaday MP, Jacob R, Gueorguieva R, Sherwin RS, Krystal JH, Spencer DD, Abi-Saab WM (2005) Extracellular metabolites in the cortex and hippocampus of epileptic patients. Ann Neurol 57:226–235
Cermelli C, Berti R, Soldan SS, Mayne M, D’Ambrosia JM, Ludwin SK, Jacobson S (2003) High frequency of human herpesvirus 6 DNA in multiple sclerosis plaques isolated by laser microdissection. J Infect Dis 187:1377–1387
Challoner PB, Smith KT, Parker JD, MacLeod DL, Coulter SN, Rose TM, Schultz ER, Bennett JL, Garber RL, Chang M et al (1995) Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc Natl Acad Sci U S A 92:7440–7444
Chan PK, Ng HK, Hui M, Cheng AF (2001) Prevalence and distribution of human herpesvirus 6 variants A and B in adult human brain. J Med Virol 64:42–46
Cuomo L, Trivedi P, Cardillo MR, Gagliardi FM, Vecchione A, Caruso R, Calogero A, Frati L, Faggioni A, Ragona G (2001) Human herpesvirus 6 infection in neoplastic and normal brain tissue. J Med Virol 63:45–51
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65:1–105
De Bolle L, Naesens L, De Clercq E (2005a) Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev 18:217–245
De Bolle L, Van Loon J, De Clercq E, Naesens L (2005b) Quantitative analysis of human herpesvirus 6 cell tropism. J Med Virol 75:76–85
de Lanerolle NC, Lee TS (2005) New facets of the neuropathology and molecular profile of human temporal lobe epilepsy. Epilepsy Behav 7:190–203
Donati D, Akhyani N, Fogdell-Hahn A, Cermelli C, Cassiani-Ingoni R, Vortmeyer A, Heiss JD, Cogen P, Gaillard WD, Sato S, Theodore WH, Jacobson S (2003) Detection of human herpesvirus-6 in mesial temporal lobe epilepsy surgical brain resections. Neurology 61:1405–1411
Donati D, Martinelli E, Cassiani-Ingoni R, Ahlqvist J, Hou J, Major EO, Jacobson S (2005) Variant-specific tropism of human herpesvirus 6 in human astrocytes. J Virol 79:9439–9448
During MJ, Spencer DD (1993) Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet 341:1607–1610
Eid T, Thomas MJ, Spencer DD, Runden-Pran E, Lai JC, Malthankar GV, Kim JH, Danbolt NC, Ottersen OP, de Lanerolle NC (2004) Loss of glutamine synthetase in the human epileptogenic hippocampus: possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet 363:28–37
Fogdell-Hahn A, Soldan SS, Shue S, Akhyani N, Refai H, Ahlqvist J, Jacobson S (2005) Co-purification of soluble membrane cofactor protein (CD46) and human herpesvirus 6 variant A genome in serum from multiple sclerosis patients. Virus Res 110:57–63
Fotheringham J, Akhyani N, Vortmeyer A, Donati D, Williams E, Oh U, Bishop M, Barrett J, Gea-Banacloche J, Jacobson S (2007a) Detection of active human herpesvirus-6 infection in the brain: correlation with polymerase chain reaction detection in cerebrospinal fluid. J Infect Dis 195:450–454
Fotheringham J, Donati D, Akhyani N, Fogdell-Hahn A, Vortmeyer A, Heiss JD, Williams E, Weinstein S, Bruce DA, Gaillard WD, Sato S, Theodore WH, Jacobson S (2007b) Association of human herpesvirus-6B with mesial temporal lobe epilepsy. PLoS Med 4:e180
Goodman AD, Mock DJ, Powers JM, Baker JV, Blumberg BM (2003) Human herpesvirus 6 genome and antigen in acute multiple sclerosis lesions. J Infect Dis 187:1365–1376
Groom AJ, Smith T, Turski L (2003) Multiple sclerosis and glutamate. Ann N Y Acad Sci 993:229–275, discussion 287–288
He J, McCarthy M, Zhou Y, Chandran B, Wood C (1996) Infection of primary human fetal astrocytes by human herpesvirus 6. J Virol 70:1296–1300
Hoogland G, van Oort RJ, Proper EA, Jansen GH, van Rijen PC, van Veelen CW, van Nieuwenhuizen O, Troost D, de Graan PN (2004) Alternative splicing of glutamate transporter EAAT2 RNA in neocortex and hippocampus of temporal lobe epilepsy patients. Epilepsy Res 59:75–82
Jen JC, Wan J, Palos TP, Howard BD, Baloh RW (2005) Mutation in the glutamate transporter EAAT1 causes episodic ataxia, hemiplegia, and seizures. Neurology 65:529–534
Leite JP, Garcia-Cairasco N, Cavalheiro EA (2002) New insights from the use of pilocarpine and kainate models. Epilepsy Res 50:93–103
Matute C, Domercq M, Sanchez-Gomez MV (2006) Glutamate-mediated glial injury: mechanisms and clinical importance. Glia 53:212–224
Mayne M, Cheadle C, Soldan SS, Cermelli C, Yamano Y, Akhyani N, Nagel JE, Taub DD, Becker KG, Jacobson S (2001) Gene expression profile of herpesvirus-infected T cells obtained using immunomicroarrays: induction of proinflammatory mechanisms. J Virol 75(23):11641–11650 (December)
Nitsche A, Muller CW, Radonic A, Landt O, Ellerbrok H, Pauli G, Siegert W (2001) Human herpesvirus 6A DNA is detected frequently in plasma but rarely in peripheral blood leukocytes of patients after bone marrow transplantation. J Infect Dis 183:130–133
Opsahl ML, Kennedy PG (2005) Early and late HHV-6 gene transcripts in multiple sclerosis lesions and normal appearing white matter. Brain 128:516–527
Pitt D, Werner P, Raine CS (2000) Glutamate excitotoxicity in a model of multiple sclerosis. Nat Med 6:67–70
Pitt D, Nagelmeier IE, Wilson HC, Raine CS (2003) Glutamate uptake by oligodendrocytes: implications for excitotoxicity in multiple sclerosis. Neurology 61:1113–1120
Salahuddin SZ, Ablashi DV, Markham PD, Josephs SF, Sturzenegger S, Kaplan M, Halligan G, Biberfeld P, Wong-Staal F, Kramarsky B et al (1986) Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science 234:596–601
Sarchielli P, Greco L, Floridi A, Floridi A, Gallai V (2003) Excitatory amino acids and multiple sclerosis: evidence from cerebrospinal fluid. Arch Neurol 60:1082–1088
Smith T, Groom A, Zhu B, Turski L (2000) Autoimmune encephalomyelitis ameliorated by AMPA antagonists. Nat Med 6:62–66
Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K (1997) Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 276:1699–1702
Tian GF, Azmi H, Takano T, Xu Q, Peng W, Lin J, Oberheim N, Lou N, Wang X, Zielke HR, Kang J, Nedergaard M (2005) An astrocytic basis of epilepsy. Nat Med 11:973–981
van der Hel WS, Notenboom RG, Bos IW, van Rijen PC, van Veelen CW, de Graan PN (2005) Reduced glutamine synthetase in hippocampal areas with neuron loss in temporal lobe epilepsy. Neurology 64:326–333
Werner P, Pitt D, Raine CS (2000) Glutamate excitotoxicity—a mechanism for axonal damage and oligodendrocyte death in multiple sclerosis? J Neural Transm Suppl (60):375–385
Yamanishi K, Okuno T, Shiraki K, Takahashi M, Kondo T, Asano Y, Kurata T (1988) Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet 1:1065–1067
Yao K, Mandel M, Akyani N, Maynard K, Sengamalay N, Fotheringham J, Ghedin E, Kashanchi F, Jacobson S (2006) Differential HHV-6A gene expression in T cells and primary human astrocytes based on multi-virus array analysis. Glia 53:789–798
Yoshikawa T, Asano Y, Akimoto S, Ozaki T, Iwasaki T, Kurata T, Goshima F, Nishiyama Y (2002) Latent infection of human herpesvirus 6 in astrocytoma cell line and alteration of cytokine synthesis. J Med Virol 66:497–505
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke. JF was supported by the MS Society of Canada.
Rights and permissions
About this article
Cite this article
Fotheringham, J., Williams, E.L., Akhyani, N. et al. Human Herpesvirus 6 (HHV-6) Induces Dysregulation of Glutamate Uptake and Transporter Expression in Astrocytes. J Neuroimmune Pharmacol 3, 105–116 (2008). https://doi.org/10.1007/s11481-007-9084-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-007-9084-0