Abstract
Intranasal drug administration is a noninvasive method of bypassing the blood–brain barrier (BBB) to deliver neurotrophins and other therapeutic agents to the brain and spinal cord. This method allows drugs that do not cross the BBB to be delivered to the central nervous system (CNS) and eliminates the need for systemic delivery, thereby reducing unwanted systemic side effects. Delivery from the nose to the CNS occurs within minutes along both the olfactory and trigeminal neural pathways. Intranasal delivery occurs by an extracellular route and does not require that drugs bind to any receptor or undergo axonal transport. Intranasal delivery also targets the nasal associated lymphatic tissues (NALT) and deep cervical lymph nodes. In addition, intranasally administered therapeutics are observed at high levels in the blood vessel walls and perivascular spaces of the cerebrovasculature. Using this intranasal method in animal models, researchers have successfully reduced stroke damage, reversed Alzheimer’s neurodegeneration, reduced anxiety, improved memory, stimulated cerebral neurogenesis, and treated brain tumors. In humans, intranasal insulin has been shown to improve memory in normal adults and patients with Alzheimer’s disease. Intranasal delivery strategies that can be employed to treat and prevent NeuroAIDS include: (1) target antiretrovirals to reach HIV that harbors in the CNS; (2) target therapeutics to protect neurons in the CNS; (3) modulate the neuroimmune function of moncyte/macrophages by targeting the lymphatics, perivascular spaces of the cerebrovasculature, and the CNS; and (4) improve memory and cognitive function by targeting therapeutics to the CNS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While the blood–brain barrier (BBB) serves to protect the brain and spinal cord from a variety of pathogens and toxic substances, it also presents a significant barrier to treating disorders of the central nervous system (CNS). Most charged and/or large therapeutic agents are inhibited or prevented from entering the brain by the BBB (Frey 2002). This includes most proteins and peptides such as neurotrophic factors and anti-inflamatory cytokines, polynucleotides such as RNAi and therapeutic genes and a variety of other small and large therapeutic agents for treating neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, and stroke, and other disorders such as brain tumors. Therapeutic agents developed for treating HIV/AIDS and, more specifically, neuroAIDS are no exception. Even if a drug is capable of penetrating the BBB, a second line of defense, drug efflux transporters, protects the brain from invading substances. It is estimated that almost half of candidate drugs are substrates for the P-glycoprotein (P-gp) efflux pump. Most antiretrovirals have poor CNS penetration, in part due to the action of P-gp (Gimenez et al. 2004).
Because HIV harbors in the brain, it can be an important source of reinfection and relapse in addition to causing neurodegeneration in the CNS, leading to the neurological and psychiatric symptoms of neuroAIDS. These include dementia with neurocognitive impairment, motor deficits associated with neuropathy and depression. Gimenez et al. (2004) noted that a number of HIV drugs do achieve significant levels in the cerebrospinal fluid (CSF) of patients including nevirapine and efavirenz. Although some HIV treatments penetrate the BBB to reach the CSF, the levels in CSF are low and the concentrations in parenchymal brain tissue are likely to be even lower. Low levels of drug in the CSF do not assure effective delivery to the brain itself. In addition, how many drugs and which treatment regimens are needed in the brain to control replication and prevent the development of resistance remains to be determined. Furthermore, beyond attempting to control the virus itself, there remains a need to protect the CNS against neurodegeneration and neuroinflammation associated with neuroAIDS.
Because HIV-1 is highly localized within perivascular and infiltrated parenchymal blood-derived macrophages and microglia (Pereira and Nottet 2000; Koenig et al. 1986; Wiley et al. 1986; Vazeux et al. 1987), there is also a reason to target both the lymphatic system (to prevent immune activation of lymphocytes and monocytes) and the perivascular spaces of the cerebrovasculature (to prevent monocyte/marcrophage infiltration across the BBB and to block the release of proinflammatory cytokines) in addition to targeting the brain parenchyma to treat and prevent neuroAIDS and the neurodegeneration and neuroinflammation it entails.
Intranasal delivery—a new approach to targeting the CNS, lymphatics and cerebrovascular perivascular spaces
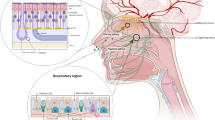
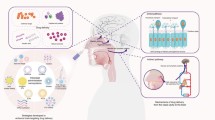
In 1989, Frey first developed a noninvasive, intranasal method of bypassing the BBB to deliver therapeutic agents to the CNS (Frey 1991). This method allows drugs that do not cross the BBB to be delivered to the CNS. It also directly targets drugs that do cross the BBB to the CNS, eliminating the need for systemic delivery and thereby reducing unwanted systemic side effects. Delivery from the nose to the CNS occurs within minutes along both the olfactory and trigeminal neural pathways. Delivery occurs by an extracellular route and does not require that the drugs bind to any receptor or undergo axonal transport. In addition to targeting the CNS, this intranasal delivery method also targets the nasal associated lymphatic tissues (NALT), the deep cervical lymph nodes and the perivascular spaces and blood vessel walls associated with the cerebrovasculature.
While intranasal delivery can reduce systemic exposure, this effect is dependent on the characteristics (size and charge) of the molecule being delivered. For example, charged molecules such as insulin reach the brain from the nasal cavity without altering blood levels of insulin or glucose (Born et al. 2002). However, formulation of such agents with permeation enhancers can increase delivery to the systemic circulation if this is desired. On the other hand, small, lipophilic molecules can readily enter the blood stream from the nasal mucosa and may subsequently reach the CNS by crossing the BBB.
Direct delivery of a wide variety of therapeutic agents to the CNS following intranasal administration, as well as the therapeutic benefit of intranasal drug treatments, has been demonstrated in mice, rats, primates, and humans by both our group and others (Thorne et al. 2001; Frey 2002; Born et al. 2002; Dhanda et al. 2005). From drug distribution studies, it appears that drug traveling along the olfactory neural pathway distributes into rostral brain structures including the olfactory bulb, anterior olfactory nucleus, frontal cortex, and hippocampus. In addition, drug traveling along the trigeminal nerve distributes into caudal brain structures including upper cervical spinal cord, midbrain, pons, and hypothalamus. This general distribution can be altered by the presence of CNS receptors that may bind to the therapeutic agent and it may vary between species. These drug distribution studies usually measure tissue concentration following perfusion to remove blood from the cerebrovasculature and subsequent fixation. Such measurements would include drug present in the parenchyma and their interstitial fluids.
There are many opportunities for the use of intranasal delivery to target therapeutics to the brain, lymphatics, perivascular spaces of the cerebrovasculature, and CNS for the treatment and prevention neuroAIDS.
Intranasal targeting of antiretrovirals to reach HIV that harbors in the CNS
The poor penetration of antiviral agents into the CNS may potentially be overcome by intranasal delivery to directly target the brain and reduce and/or eliminate HIV, thereby preventing neuroAIDS from ever developing. Intranasal delivery could be used to target any of the types of antivirals to the CNS including entry inhibitors, nucleoside reverse transcriptase inhibitors (NRTI), nonnucleoside reverse transcripase inhibitors (NNRTI), antisense antivirals, transcription inhibitors (TI), protease inhibitors (PI), and maturation inhibitors.
Intranasal administration of peptide T [d-ala-peptide T-amide (DAPTA)], a viral entry inhibitor, is currently being developed for treatment of cognitive impairment associated with HIV (Ruff et al. 2003). Peptide T prevents initial infection of cells expressing CCR5 receptors, including monocytes and microglia (Ruff et al. 2001). In addition to inhibiting viral entry, peptide T is an antagonist of free gp120 and has been reported to block its toxic effects. Peptide T has been shown to improve cognitive function in patients with HIV (Heseltine et al. 1998). More recently, antiviral and immunological benefits of intranasal peptide T have been reported including decreased viral levels in monocyte reservoirs, increased CD4, and increased antiviral cytotoxic T cells (Polianova et al. 2003).
A similar approach using intranasal delivery to target chemotherapeutics to the CNS has shown great promise for the treatment of brain tumors in preclinical studies (Shingaki et al. 1999; Wang et al. 2004, 2005; Hashizume et al. 2006; da Fonseca et al. 2006).
Intranasal targeting of therapeutics to protect neurons in the CNS
There is substantial evidence that intranasally administered growth factors are successfully targeted to the brain and are neuroprotective. Intranasally administered neuroprotective agents such as nerve growth factor (NGF), insulin-like growth factor-I (IGF-I), brain-derived neurotrophic factor (BDNF), and EPO may be beneficial for the treatment of neuroAIDS.
IGF-I, a 7,600-Da protein, has been intranasally delivered to the brain and spinal cord (Thorne et al. 2004). In addition, very high delivery of IGF-I was observed to the lymphatics (i.e., NALT and deep cervical lymph nodes) and walls of the cerebrovasculature (Thorne et al. 2004). In an animal model of stroke, intranasal IGF-I given up to 4 h after stroke markedly reduces infarct volume and improves neurological function (Liu et al. 2004). Intranasal erythropoietin, a 30,400-Da glycoprotein, also protects against focal cerebral ischemia (Yu et al. 2005).
Intranasal NGF, a 26,500-Da protein, bypasses the BBB and targets the CNS (Frey et al. 1997; Chen et al. 1998). Intranasal NGF, administered once every 2 days according to a procedure modified from Frey et al. (1997) was found to successfully protect against and even reverse neurodegeneration in a transgenic mouse model of Alzheimer’s disease (Capsoni et al. 2002). De Rosa et al. (2005) further reported that intranasal NGF rescues recognition memory deficits in this same Alzheimer’s disease mouse model, the AD11 mouse. Intranasal neurotrophins FGF-2 or heparin-binding EGF have also been shown to stimulate neurogenesis in adult mice (Jin et al. 2003). In addition, intranasal administration of antioxidants may also be neuroprotective and beneficial for the treatment of NeuroAIDS. For example, intranasal deferoxamine, a low-molecular-weight therapeutic agent, has been shown to precondition and protect the brain against stroke (Panter et al. 2004).
Intranasal treatments to modulate the neuroimmune function of moncyte/macrophages by targeting the lymphatics, perivascular spaces of the cerebrovasculature, and the CNS
Intranasal administration of therapeutics can target the lymphatics and perivascular spaces in addition to the CNS. Intranasal delivery to modulate immune function could be used in three ways for the treatment and/or prevention of neuroAIDS: (1) targeting of the lymphatic system to prevent the activation of lymphocytes and monocytes; (2) targeting of the perivascular spaces to prevent monocyte/macrophage infiltration across the BBB and to block the release of proinflammatory cytokines; (3) targeting of the brain parenchyma to block the neuroinflammation associated with neurodegeneration. Possible intranasal therapeutics include immunomodulatory and anti-inflammatory agents such as interferon beta-1b and GSK-3beta (up-regulated by HIV-1 neurotoxins) inhibitors (Dou et al. 2004).
Interferon beta-1b, a 20,000-Da anti-inflammatory protein, has been intranasally delivered to the brain and spinal cord, as well as lymphatics in rodents (Ross et al. 2004). In addition, studies in cynomolgus monkeys also demonstrate rapid intranasal delivery of interferon beta-1b to the CNS, cerebrovascular blood vessel walls and lymphatics (Thorne et al., unpublished observations). Intranasal delivery has also been used to noninvasively target gene therapy to the CNS (Draghia et al. 1995; Lemiale et al. 2003; Jerusalmi et al. 2003; Laing et al. 2006).
Intranasal delivery is useful for delivery of small as well as large molecules; low-molecular-weight therapeutics can also be targeted to the CNS, lymphatics, and perivascular spaces via intranasal delivery. The peptide hypocretin-1, a potential treatment for narcolepsy, is intranasally targeted to the brain and spinal cord (Hanson et al. 2004). Intranasal PT-141 has been successfully used in humans to treat erectile dysfunction by acting at melanocortin receptors in the hypothalamus, which are involved in both appetite and sexual response. Intranasal leptin reduces food consumption and body weight in animals (Schulz et al. 2004) and reduces appetite (Shimizu et al. 2005). Intranasal oxytocin has been found to increase trust in humans following direct delivery from the nose to the brain (Kosfeld et al. 2005).
Improve memory and cognitive function by intranasally targeting therapeutics to the CNS
Intranasal delivery of therapeutics may be used to treat neuroAIDS and its symptoms including memory loss and cognitive dysfunction after neurodegeneration has occurred. Possible therapeutics includes insulin, exendin, and NAP.
Intranasal insulin improves memory and mood in healthy adults (Benedict et al. 2004) and improves memory in patients with Alzheimer’s disease without altering blood levels of insulin or glucose (Reger et al. 2006). On a side note, intranasal insulin has also been shown to reduce body fat in normal, but not obese men (Hallschmid et al. 2004, 2006). Banks has demonstrated that intranasal exendin is directly delivered to the brain and improves memory, cognition, and neuronal survival (Banks et al. 2004; During et al. 2003). Finally, Gozes has used intranasal delivery to target NAP and ADNF to the brain to treat anxiety and neurodegeneration (Alcalay et al. 2004; Gozes et al. 2000).
Formulation of intranasal therapeutics
This intranasal method does not require any modification of the therapeutic agent and does not require that the drug be coupled to any carrier. The method can deliver a wide variety of therapeutic agents to the CNS, including both small molecules and macromolecules as described above. However, this delivery method is not a panacea. It works best with potent therapeutic agents that are active in the nanomolar range (Dhanda et al. 2005). Because delivery occurs along the olfactory and trigeminal neural pathways, high concentrations of intranasal therapeutics are routinely observed in the olfactory bulb and trigeminal nerves; thus candidate drugs should be screened for potential side effects in these sensory neurons. Furthermore, P-gp has been reported to operate in the nasal epithelium (Graff and Pollack 2003, 2005; Kandimalla and Donovan 2005a, b), although reasonably good intranasal delivery to the brain has been reported for P-gp substrates (Graff and Pollack 2003). Also, it is not yet known whether nasal congestion due to allergies or colds may interfere with intranasal delivery. Finally, small lipophilic molecules that rapidly enter the blood from the nasal mucosa may require special formulation to enhance delivery to the CNS while reducing systemic exposure.
Ease of intranasal delivery
Intranasal delivery is a noninvasive method of drug delivery, associated with little pain and preferable to other methods such as injections. Intranasal administration is convenient and patients can administer intranasal therapeutics at home. Directly targeting therapeutics to the brain and reducing systemic exposure would likely result in a decrease in unwanted side effects. Several nasal spray devices are on the market for intranasal delivery that can target drugs to the upper portion of the nasal cavity (Dhanda et al. 2005).
Conclusions
Intranasal delivery targets therapeutics to the CNS, lymphatics, and perivascular spaces of the cerebrovasculature. There are many potential strategies for the use of intranasal therapeutics for the treatment of neuroAIDS including targeting of antiretrovirals to reach HIV that harbors in the CNS, targeting of therapeutics to protect neurons in the CNS, targeting of neuroimmune modulators, and targeting therapeutics to improve memory and cognitive function.
References
Alcalay RN, Giladi E, Pick CG, Gozes I (2004) Intranasal administration of NAP, a neuroprotective peptide, decreases anxiety-like behavior in aging mice in the elevated plus maze. Neurosci Lett 361:128–131
Banks WA, During MJ, Niehoff ML (2004) Brain uptake of the glucagon-like peptide-1 antagonist exendin(9–39) after intranasal administration. J Pharmacol Exp Ther 309:469–475
Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, Kern W (2004) Intranasal insulin improves memory in humans. Psychoneuroendocrinology 29:1326–1334
Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL (2002) Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci 5:514–516
Capsoni S, Giannotta S, Cattaneo A (2002) Nerve growth factor and galantamine ameliorate early signs of neurodegeneration in anti-nerve growth factor mice. Proc Nat Acad Sci USA 99:12432–12437
Chen XQ, Fawcett JR, Rahman YE, Ala TA, Frey WH II (1998) Delivery of nerve growth factor to the brain via the olfactory pathway. J Alzheimer’s Dis 1:35–44
da Fonseca CO, Landeiro JA, Clark SS, Quirico-Santos T, da Costa Carvalho Mda G, Gattass CR (2006) Recent advances in the molecular genetics of malignant gliomas disclose targets for antitumor agent perillyl alcohol. Surg Neurol 65 S1:2–1:9
De Rosa R, Garcia AA, Braschi C, Capsoni S, Maffei L, Berardi N, Cattaneo A (2005) Intranasal administration of nerve growth factor (NGF) rescues recognition memory deficits in AD11 anti-NGF transgenic mice. Proc Natl Acad Sci USA 102:3811–3816
Dhanda DS, Frey WH 2nd, Leopold D, Kompella UB (2005) Approaches for drug deposition in the human olfactory epithelium. Drug Deliv Technol 5:64–72
Dou H, Kingsley JD, Mosley RL, Gelbard HA, Gendelman HE (2004) Neuroprotective strategies for HIV-1 associated dementia. Neurotox Res 6:503–521
Draghia R, Caillaud C, Manicom R, Pavirani A, Kahn A, Poenaru L (1995) Gene delivery into the central nervous system by nasal instillation in rats. Gene Ther 2:418–23
During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ, Haile CN (2003) Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nature Med 9:1173–1179
Frey WH 2nd (1991) Neurologic agents for nasal administration the brain. World Intellectual Property Organization. PCT priority date 5.12.89, WO 91/07947
Frey WH 2nd (2002) Bypassing the blood–brain barrier to delivery thereapeutic agents to the brain and spinal cord. Drug Deliv Technol (5):46–49
Frey WH 2nd, Liu, J, Chen, X, Thorne, RG, Fawcett, JR, Ala, TA, Rahman, Y-E (1997) Delivery of 125I-NGF to the brain via the olfactory route. Drug Deliv 4:87–92
Gimenez F, Fernandez C, Mabondzo A (2004) Transport of HIV protease inhibitors through the blood–brain barrier and interactions with the efflux proteins, P-glycoprotein and multidrug resistance proteins. J Acquir Immune Defic Syndr 36:649–58
Gozes I, Zamostiano R, Pinhasov A, Bassan M, Giladi E, Steingart RA, Brenneman DE (2000) A novel VIP responsive gene. Activity dependent neuroprotective protein. Ann NY Acad Sci 921:115–118
Graff CL, Pollack GM (2003) P-glycoprotein attenuates brain uptake of substrates after nasal instillation. Pharm Res 20:1225–1230
Graff CL, Pollack GM (2005) Functional evidence for P-glycoprotein at the nose–brain barrier. Pharm Res 22:86–93
Hallschmid M, Benedict C, Schultes B, Fehm HL, Born J, Kern W (2004) Intranasal insulin reduces body fat in men but not in women. Diabetes 53:3024–3029
Hallschmid M, Smolnik R, McGregor G, Born J, Fehm HL (2006) Overweight humans are resistant to the weight-reducing effects of melanocortin4–10. J Clin Endocrinol Metab 91:522–525
Hanson LR, Martinez PM, Taheri S, Kamsheh L, Mignot E, Frey WH 2nd (2004) Intranasal administration of hypocretin 1 (orexin A) bypasses the blood–brain barrier and targets the brain: a new strategy for the treatment of narcolepsy. Drug Delive Technol 4:65–71
Hashizume R, Ozawa T, Gryaznov SM, Santos RA, Lamborn KR, Frey WH 2nd, Deen DF (2006) Intranasal delivery of specific telomerase inhibitor GRN163 in human glioblastoma xenografts. American Association of Neurological Surgeons Abstract: 2006 Apr 24
Heseltine PN, Goodkin K, Atkinson JH, Vitello B, Rochon J, Heaton RK, Eaton EM, Wilkie FL, Sobel E, Brown SJ, Feaster D, Schneider L, Goldschmidts WL, Stover ES (1998) Randomized double-blind placebo-controlled trial of peptide T for HIV-associated cognitive impairment. Arch Neurol 55:41–51
Jerusalmi A, Morris-Downes MM, Sheahan BJ, Atkins GJ (2003) Effect of intranasal administration of Semliki Forest virus recombinant particles expressing reporter and cytokine genes on the progression of experimental autoimmune encephalomyelitis. Mol Ther 8:886
Jin K, Xie L, Childs J, Sun Y, Mao XO, Logvinova A, Greenberg DA (2003) Cerebral neurogenesis is induced by intranasal administration of growth factors. Ann Neurol 53:405–409
Kandimalla KK, Donovan MD (2005a) Localization and differential activity of P-glycoprotein in the bovine olfactory and nasal respiratory mucosae. Pharm Res 22:1121–1128
Kandimalla KK, Donovan MD (2005b) Carrier mediated transport of chlorpheniramine and chlorcyclizine across bovine olfactory mucosa: implications on nose-to-brain transport. J Pharm Sci 94:613–624
Koenig S, Gendelman HE, Orenstein JM, Dal Canto MC, Pezeshkpour GH, Yungbluth M, Janotta F, Aksamit A, Martin MA, Fauci AS (1986). Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science 233:1089–93
Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E (2005) Oxytocin increases trust in humans. Nature 435(7042):673–676
Laing JM, Gober MD, Golembewski EK, Thompson SM, Gyure KA, Yarowsky PJ, Aurelian L (2006) Intranasal administration of the growth-compromised HSV-2 vector DeltaRR prevents kainate-induced seizures and neuronal loss in rats and mice. Mol Ther 13(5):870–881
Lemiale F, Kong WP, Akyurek LM, Ling X, Huang Y, Chakrabarti BK, Eckhaus M, Nabel GJ (2003) Enhanced mucosal immunoglobulin A response of intranasal adenoviral vector human immunodeficiency virus vaccine and localization in the central nervous system. J Virol 77:10078–10087
Liu XF, Fawcett JR, Hanson LR, Frey WH 2nd (2004) The window of opportunity for treatment of focal cerebral ischemic damage with noninvasive intranasal insulin-like growth factor-I in rats. J Stroke Cerebrovasc Dis 13:16–23
Panter SS, Coppes VG, Ferrell CM, Chavez JC, Ratan RR, Frey WH 2nd (2004) Intranasal deferoxamine protects against subsequent stroke. Program No. 456.15.2004 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience
Pereira CF, Nottet HLSM (2000) The blood–brain barrier in HIV-associated Dementia. NeuroAids 3(2), online
Polianova MT, Ruscetti FW, Pert CB, Tractenberg RE, Leoung G, Strang S, Ruff MR (2003) Antiviral and immunological benefits in HIV patients receiving intranasal peptide T (DAPTA). Peptides 24:1093–1098
Reger MA, Watson GS, Frey WH 2nd, Baker LD, Cholerton B, Keeling ML, Belongia DA, Fishel MA, Plymate SR, Schellenberg GD, Cherrier MM, Craft S (2006) Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging 27:451–458
Ross TM, Martinez PM, Renner JC, Thorne RG, Hanson LR, Frey WH 2nd (2004) Intranasal administration of interferon beta bypasses the blood–brain barrier to target the central nervous system and cervical lymph nodes: a non-invasive treatment strategy for multiple sclerosis. J Neuroimmunol 151:66–77
Ruff MR, Polianova M, Pert CB, Ruscetti FW (2001) Peptide T inhibits HIV-1 infection mediated by the chemokine receptor-5 (CCR5). Antiviral Res 52–63
Ruff MR, Polianova M, Yang QE, Leoung GS, Ruscetti FW, Pert CB (2003) Update on D-ala-peptide T-amide (DAPTA): a viral entry inhibitor that blocks CCR5 chemokine receptors. Curr HIV Res 1:51–67
Schulz C, Paulus K, Lehnert H (2004) Central nervous and metabolic effects of intranasally applied leptin. Endocrinol 145:2696–2701
Shimizu H, Oh-I S, Okada S, Mori M (2005) Inhibition of appetite by nasal leptin administration in rats. Int J Obes 29:858–863
Shingaki T, Sakane T, Yamashita S, Sezaki H, Tokunaga Y, Shibata S (1999) Transnasal delivery of anticancer drugs to the brain tumors: a new strategy for brain tumor chemotherapy. Drug Deliv Syst 14:365–371
Thorne, RG, Frey WH 2nd (2001) Delivery of neurotrophic factors to the central nervous system: pharmacokinetic considerations. Clin Pharmacokinet 40:907–946
Thorne RG, Pronk GJ, Padmanabhan V, Frey WH 2nd (2004) Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 127:481–96
Vazeux R, Brousse N, Jarry A, Henin D, Marche C, Vedrenne C, Mikol J, Wolff M, Michon C, Rozenbaum W, Bureau JF, Montagnier L, Brahic M (1987). AIDS subacute encephalitis. Identification of HIV-infected cells. Am J Pathol 126:403–410
Wang D, Gao Y, Yun L (2005) Study on brain targeting of raltitrexed following intranasal administration in rats. Cancer Chemother Pharmacol 57:97–104
Wang F, Jiang XG, Lu W (2004) Intranasal delivery of methotrexate to the brain in rats bypassing the blood–brain barrier. Drug Deliv Technol 4:48–55
Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB (1986) Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci USA 83:7089–7093
Yu YP, Xu QQ, Zhang Q, Zhang WP, Zhang LH, Wei EQ (2005) Intranasal recombinant human erythropoietin protects rats against focal cerebral ischemia. Neurosci Lett 387:5–10
Acknowledgment
This study was supported by a grant from the NIH to W.H.F. (MH072473).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hanson, L.R., Frey, W.H. Strategies for Intranasal Delivery of Therapeutics for the Prevention and Treatment of NeuroAIDS. Jrnl Neuroimmune Pharm 2, 81–86 (2007). https://doi.org/10.1007/s11481-006-9039-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-006-9039-x