Abstract
In this study, the structural and optical properties of aluminum oxide thin films were investigated. Aluminum oxide thin films were prepared on silicon and glass substrate by DC magnetron sputtering of aluminum targets with subsequent thermal oxidation of the aluminum-deposited thin films. Important result obtained included the presence of a plasma edge for the individual aluminum atoms. In addition, the temperatures that resulted in the highest concentration of surface plasmons were determined. On other hand, the relationship between the plasma edge and the optical energy gap was investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Silver oxide thin films gained the attention of many research groups in recent years [1,2,3,4,5,6,7,8,9,10,11,12,13], mainly because they exhibit significant applications in gas sensors, passivation, organic field-effect transistors, perovskite solar cells, organic light-emitting diode encapsulation, optical data storage, and surface plasmon optics, etc.
There are numerous deposition techniques for obtaining Al2O3 thin films as thermal evaporation [2],atomic layer deposition (ALD) [13,14,15], magnetron sputtering [16, 17], pulsed-laser deposition (PLD) [18], spray pyrolysis [19, 20], and sol-gel coating [21].
Valence electrons in any conductor act like free electrons, where the collision among the electrons is similar among gas molecules that are described in the kinetic theory of gases [22]. Optical properties of metals are produced due to the interaction between the fallen photons on the surface and the electronic cloud [22]. Optical reflectivity is considered the most important physical property of the metal layers, which generally relates to the interaction between the light and the free electrons which could be expressed by the equation of dispersion as follows [22, 23]:
where N is the concentration of conducting electrons, e the electron charge, ∞ the high frequency (visible wavelength), ɛ the dielectric constant, and m* the electron effective mass.
Both electron concentration and electron effective mass affect this plasma edge [22] and could be directly determined from the reflecting spectra where dramatic change of reflectivity happens at the plasma edge as a result of photon reflection from the conduction band electron plasma oscillations [23]. Furthermore, equation indicates that, when carrier concentration and hence typically the conductivity are increased, the plasma edge moves to high frequencies (shorter wavelengths) [23]. In this paper, we are interested in identifying the factors affecting the plasma edge formed in the reflectance spectra of aluminum oxide thin films prepared by the thermal oxidation of the aluminum thin films deposited by DC magnetron sputtering of aluminum targets.
Experimental
Sample Preparation
Pure Al thin films (99.99%) were deposited at room temperature onto thoroughly cleaned n-type Si (100) and glass substrates from a high-purity Al (99.99%) target using DC magnetron sputtering process. The substrate placed below the source in the direction of ablated material flux. The deposition conditions are given in Table 1.
Each deposited film was annealed in air at specific temperature (100, 200, 300, 400, and 500 °C) in order to oxidize the aluminum atoms. Table 2 contains the thermal treatment conditions for each sample.
Sample Characterization
Crystallite structure of the films was measured by X-ray diffraction (XRD) using (Stoe StadiP) transmission X-ray diffractometer employing a Cu-Kα1 (λ = 1.54060 Å) source. The optical transmittance and reflection spectra were recorded with a UV–Vis spectrophotometer (Cary 5000).
Results and Discussion
X-ray diffraction measurements were carried out on the aluminum oxide thin films. The XRD profiles of these samples are shown in Fig. 1. As seen in this figure, the A sample spectrum contains a large number of peaks and broad bands, some of which are related to aluminum metal and the rest are related to some phases of aluminum oxide (alpha, beta, kappa, gamma). This great diversity of phases makes it difficult to make an accurate description of the structural composition of this sample. On the other hand, when the annealing temperature increases, the number of peaks becomes limited and the spectrum becomes clearer. Therefore, we determined the main peaks in the spectra of samples from B to F. Table 3 contains the main features of all the peaks observed in XRD spectra. We have taken into account the determination of the samples in which each of the main peak clearly shows. It can be seen that the alpha phase peak appears in the spectra of the C, E, and F samples. In addition, the samples that have a high percentage of aluminum metal are A, D, and E. The rhombohedra phase of aluminum oxide has a high percentage in three samples (A, B, and D). It is interesting to note that the peak of the silicon does not show in the spectra of the samples except for the sample (F) spectrum, which indicates the low density of this sample and that it has a porous structure.
The structures we obtained are more diversified than that recorded in previous work [2], which adopted the method of thermal evaporation to obtain aluminum thin films.
The ratio of non-oxidizing aluminum atoms in each sample can be used to investigate the stability of the bonds (Al–O) during thermal treatment. This ratio is proportional to the sum of the relative areas of the aluminum peaks in the XRD spectrum. In order to calculate the relative areas, a fitting process performed for each peak adoption of the Gaussian and Lorentzian shape. Table 4 contains the position and the area of each peak, in addition to the total area (SAl%) of the prepared samples.
Figure 2 illustrates the total area (SAl%) as a function of annealing temperature.
It can be observed that in general, the ratio of aluminum atoms decreases with increasing annealing temperature except for the sample D (400 °C), where the ratio of non-oxidizing aluminum atoms increases. This indicates that oxidation processes are limited at the temperature 400 °C where a low-concentration mixture of the rhombohedra and cubic Al2O3 phases is formed. On the other hand, the annealing at 300 °C (sample C) appears to be suitable for the formation of the phase alpha and kappa p with high oxidation rates. Almost complete oxidation process also occurs in the case of the sample F (500 °C/2 h) where the alpha stable phase predominates.
Figure 3 shows the reflectance spectra of the prepared samples.
By comparing this figure with Fig. 2, we notice that the effect of annealing temperature on reflectance is not the same as its effect on the proportion of aluminum in the film, and this makes us believe that the reflectivity is not related to the ratio of aluminum atoms. On the other hand, we note that in all spectra, there is a wide band to the left of the plasma edge and that this band slopes sharply toward the plasma edge, and we note that the length of the slope decreases with both the increase in annealing temperature and in the reflectance. This can be attributed to the different optical behavior on both sides of the plasma edge. In the case where λ < λp, light interacts with individual aluminum nanoparticles. In the case where λ > λp, light interacts with surface plasmons of larger Al nanoparticles, where, the concentration of surface plasmons depends on the annealing temperature, and accordingly, we can conclude from Fig. 3 that samples C, E, and D have largest Al nanoparticles compared to the rest of the samples.
The presence of two types of aluminum nanostructures leads to an important conclusion, which is the presence of a plasma edge for individual aluminum atoms (λ′p). Depending on Fig. 3, it can be expected that this edge is in the ultraviolet range (λ′p < 300 nm).
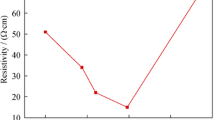
As mentioned in the introduction, samples with plasma edges with short wavelengths have high concentration of charge carriers. By applying this rule to the Fig. 4 that illustrates plasma edge as a function of annealing temperature, we conclude that sample D has the highest concentration of charge carriers followed by sample E. The charge carriers here are intended to be aluminum surface plasmons.
Figure 5 shows plasma edge as a function of the total area (SAl%). We notice that C and F samples are on the left side of the figure and have high plasma edges because of their low concentrations of surface plasmons.
The A and B samples, which are located on the right side of the shape, also have high plasma edges despite having high concentrations of aluminum metal. This is due to the fact that the high concentration of metal particles and their interaction with each other prevents the occurrence of response vibrations of surface plasmons. We conclude from the above that the samples D and E are best suited as Al/Al2O3 composites for surface plasmon applications because they have high concentrations of surface plasmons.
Because the prepared films were opaque, we calculated the optical energy band gap from the reflectance spectra using Kubelka-Munk method [24, 25]. Figure 6 shows the method of calculating the energy band gap in the case of sample E.
Figure 7 shows the optical band gap as a function of annealing temperature. We notice that the band gap increases with increasing annealing temperature, but at the temperature of 400 °C (sample D), the band gap decreases. However, it increases again with doubling the treatment time using the same temperature.
This behavior can be understood by investigating the relationship between the optical energy band gap and the plasma edge (Fig. 8).
We notice that, for λp < 830 nm, the band gap decreases when the plasma edge increases. For higher plasma edges, the band gap becomes independent of the plasma edge, in which case the band gap related only to the structural composition of the thin film. The great effect of the ratio of the non-oxidizing aluminum atoms appears here, as the A and B samples have low values for the band gap because they have a high content of the non-oxidized aluminum atoms. Figure 8 is very important because it helps to precisely determine the state at which the plasma effect begins to appear.
We found that, in the samples A, C, E, and F, the band gap related only to the structural composition of the thin film. This also appears applicable to the plasma edge. Despite the many phases formed in these samples, it appears that the plasma edge is proportional to the concentration of the alpha phase. The formation of this phase is associated with high oxidation rates; this is the reason for obtaining a small concentration of aluminum in the sample F, which leads to a low concentration of surface plasmons, and it will naturally increase the film’s dielectric constant. In addition, the significant effect of the grain size on the surface plasmon resonance [26] must be taken into account.
Finally, the optical measurement of the plasma frequency is seen as a non-contact probe to measure the electrical properties [23]. In our case, this seems to be incorrect for all cases, especially for λp < 300 nm, as the band gap (which can be expressed as a measure of conductivity) decreases with increasing the plasma edge. The diversity of structural phases, along with the diversity of preparation conditions (annealing temperature), is the main factor determining the overall optical and electrical characteristics of the prepared thin films. However, investigating the relationship between the optical band gap and the plasma edge appears necessary for the engineering design of Al/Al2O3 composites, which make them suitable for specific applications.
Conclusions
In this work, aluminum oxide films are deposited by DC magnetron sputtering of aluminum metal on silicon and glass substrates with subsequent oxidation of deposition products at different annealing temperatures. Annealing processes were performed in the air using an oven. The effect of temperature on XRD and reflectance spectra of the prepared films was studied. The results obtained included the following:
-
The structural composition of the prepared films is highly dependent on the annealing temperature.
-
The annealing at 500 °C for 2 h provides almost complete oxidation with a high alpha stable phase ratio.
-
In addition to the plasma threshold (λp) caused by aluminum nanoparticles, we monitored the presence of a plasma edge for the individual aluminum atoms (λ′p).
-
The annealing temperatures in the range 400–500 °C are best suited as Al/Al2O3 composites for surface plasmon applications because they have high concentrations of surface plasmons.
-
The curve Eg vs. λp is very important for the engineering design of Al/Al2O3 composites for surface plasmon applications.
References
Lei D, Yu X, Song L, Gu X, Li G, Yang D (2011) Modulation of atomic-layer-deposited Al2O3 film passivation of silicon surface by rapid thermal processing. Appl Phys Lett 99:5–8
Boratto MH, Scalvi LVA (2014) Deposition ofAl2O3 by resistive evaporation and thermal oxidation of Al to be applied as a transparent FET insulating layer. Ceram Int 40:3785–3791
Mohammadi MR (2014) Semiconductor TiO2-Al2O3 thin film gas sensors derived from aqueous particulate sol-gel process. Mater Sci Semicond Process 27:711–718
Liang Z, Bi Z, Gao K, Fu Y, Guan P, Feng X, Chai Z, Xu G, Xu X (2019) Interface modification via Al 2 O 3 with retarded charge recombinations for mesoscopic perovskite solar cells fabricated with spray deposition process in the air. Appl Surf Sci 463:939–946
Baek Y, Lim S, Kim LH, Park S, Lee SW, Oh TH, Kim SH, Park CE (2016) Al2O3/TiO2 nanolaminate gate dielectric films with enhanced electrical performances for organic field-effect transistors. Organic Electronics 28:139–146
Han YC, Kim E, Kim W, Im HG, Bae BS, Choi KC (2013) A flexible moisture barrier comprised of a SiO2-embedded organic-inorganic hybrid nanocomposite and Al2O3 for thin-film encapsulation of OLEDs. Organic Electronics 14:1435–1440
Ding X, Zhang J, Zhang H, Ding H, Huang C, Li J, Shi W, Jiang X, Zhang Z (2014) ZrO2 insulator modified by a thin Al2O3 film to enhance the performance of InGaZnO thin-film transistor. Microelectron Reliab 54:2401–2405
Takhar K, Upadhyay BB, Yadav YK, Ganguly S, Saha D (2019) Al2O3 formed by post plasma oxidation of Al as a gate dielectric for AlGaN/GaN MIS-HEMTs. Appl Surf Sci 481:219–225
Kayed K (2017) An innovative method for measuring the optical band gap of oxidized surface layer of aluminum tablets based on absorption spectra. IJNeaM 11:71–76
Meza-Arroyo J, Syamala Rao MG, Mejia I, Quevedo-López MA, Ramírez-Bon R (2019) Low temperature processing of Al2O3-GPTMS-PMMA hybrid films with applications to high-performance ZnO thin-film transistors. Appl Surf Sci 467–468:456–461
Song X, Xu J, Liu L, Lai PT, Tang WM (2019) Improved interfacial and electrical properties of few-layered MoS 2 FETs with plasma-treated Al2O3 as gate dielectric. Appl Surf Sci 481:1028–1034
Koda Y, Sugita H, Suwa T, Kuroda R, Goto T, Teramoto A, Sugawa S (2016) Low leakage current Al2O3 metal-insulator-metal capacitors formed by atomic layer deposition at optimized process temperature and O2 post deposition annealing. ECS Trans 72:91–100
Yota J, Shen H, Ramanathan R (2013) Characterization of atomic layer deposition HfO2, Al2O3, and plasma-enhanced chemical vapor deposition Si3N4as metal–insulator–metal capacitor dielectric for GaAs HBT technology. J Vac Sci Technol A 31(1):01A134
Kwak HY, Kwon HM, Jung YJ, Kwon SK, Jang JH, Il Choi W, Ha ML, Il Lee J, Lee SJ, Lee HD (2013) Characterization of Al2O3-HfO2-Al 2O3 sandwiched MIM capacitor under DC and AC stresses. Solid State Electron 79:218–222
Gakis GP, Vahlas C, Vergnes H, Dourdain S, Tison Y, Martinez H, Bour J, Ruch D, Boudouvis AG, Caussat B, Scheid E (2019) Investigation of the initial deposition steps and the interfacial layer of atomic layer deposited (ALD) Al2O3 on Si. Appl Surf Sci 492:245–254
Murray J, Huebner W, O’Keefe MJ, Wilder K, Eatinger R, Kuhn W, Krueger DS, Wolf JA (2011) Sputter deposition of thin film MIM capacitors on LTCC substrates for RF bypass and filtering applications. IMAPS'03 2011:000747–000752
Mao S, Yang H, Huang F, Xie T, Song Z (2011) Corrosion behaviour of sintered NdFeB coated with Al/Al2O3 multilayers by magnetron sputtering. Appl Surf Sci 257:3980–3984
Ben Rabha M, Salem M, El Khakani MA, Bessais B, Gaidi M (2013) Monocrystalline silicon surface passivation by Al2O3/porous silicon combined treatment. MSEB 178:695–697
Adamopoulos G, Thomas S, Bradley DDC, McLachlan MA, Anthopoulos TD (2011) Low-voltage ZnO thin-film transistors based on Y2O3 and Al2O3 high-k dielectrics deposited by spray pyrolysis in air. Appl Phys Lett 98:98–101
Dhonge BP, Mathews T, Sundari ST, Thinaharan C, Kamruddin M, Dash S, Tyagi AK (2011) Spray pyrolytic deposition of transparent aluminum oxide (Al2O3) films. Appl Surf Sci 258:1091–1096.040
Nayak PK, Hedhili MN, Cha D, Alshareef HN (2013) High performance In2O3 thin film transistors using chemically derived aluminum oxide dielectric. Appl Phys Lett 103:1–5
Born M, Wolf E (2005) Principles of optics, 6th edn. Cambridge University Press, Cambridge
Perkins JD, Teplin CW, van Hest MFAM, Alleman JL, Li X, Dabney MS, Keyes BM, Gedvilas LM, Ginley DS, Lin Y, Lu Y (2004) Optical analysis of thin film combinatorial libraries. Appl Surf Sci 223:124–132
Kubelka P, Munk F (1931) Ein beitrag zur optik der farbanstriche. Z Tech Phys 12:593–601
Kubelka P (1948) New contributions to the optics of intensely light-scattering materials. Part I. J Opt Soc Am 38:448–457
Kayed K (2020) The optical properties of individual silver nanoparticles in Ag/Ag2O composites synthesized by oxygen plasma treatment of silver thin films. Plasmonics. https://doi.org/10.1007/s11468-020-01169-9
Acknowledgments
The authors would like to thank the Syrian Atomic Energy Commission and the University of Damascus for providing the facility to carry out this research. They would also like to thank Dr. B. Abdallah for the assistance during working on the DC magnetron sputtering system.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kayed, K., Alberni, L. The Effect of Annealing Temperature on the Plasma Edge in Reflectance Spectra of Al/Al2O3 Composites Synthesized by Thermal Oxidation of Aluminum Thin Films. Plasmonics 15, 1959–1966 (2020). https://doi.org/10.1007/s11468-020-01225-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11468-020-01225-4