Abstract
Diabetes mellitus is a common health issue in human beings. It is very important to develop a highly selective and sensitive biosensor for the detection of glucose concentration in the human body. The proposed sensor is based on localized surface plasmon resonance (LSPR) technique. An optical tapered fiber structure is used to develop the proposed sensor. Gold nanoparticles (AuNPs) are used to increase the sensitivity of the sensor probe. AuNPs of size ~ 10 nm is synthesized for this purpose. The characterization of AuNPs was performed with the UV-vis spectroscopy, transmission electron microscope (TEM), and scanning electron microscope (SEM). Further, glucose oxidase enzyme is used to increase the selectivity of the sensor. The selectivity of sensor is tested with different analytes such as cholesterol, urea, L-cysteine, ascorbic acid, and galactose that are generally found in the serum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is one of the severe diseases in human beings. The symptom of diabetes mellitus is high blood glucose level that results in complications such as kidney failure and diabetic retinopathy [20]. Glucose sensors have an important role in diabetes diagnosis, food industry, and biotechnology. There are wide advantages of optical fiber-based glucose sensors such as miniature, cost-effectiveness, remote access, and multiplexing ability [25]. There are two types of glucose sensors, i.e., nonenzymatic [5] and enzymatic [3, 10]. Sun et al. [19] developed the nonenzymatic optical fiber glucose sensor that is based on the evanescent wave absorption. Taper optical fiber region was functionalized with poly (phenylboronic acid) (polyPBA) film. The reflected light intensity through sensor probe is increased with the increase in glucose concentration. Zhang et al. [25] proposed a tilted fiber Bragg-grating-based optical fiber sensor for the detection of hydrogen peroxide (H2O2) and glucose concentration. The experiments were performed for 0 mM to 12 mM glucose concentration in which 500 μl of 10 μM glucose oxidase (GOx) was added in the glucose solution separately during the measurement means; the sensor probe is nonenzymatic. The nonenzymatic sensors have good stability, reproducibility and are free from oxygen consumption, but a major limitation is a low selectivity [2]. The selectivity can be enhanced with the help of functionalization of sensor probe with GOx enzyme because enzymatic sensors have high selectivity and sensitivity [23]. Huang et al. [3, 4] proposed the temperature-dependent enzymatic sensor whose results varied with the influence of external temperature. Till now, various optical fiber glucose sensors based on long-period grating (LPG) [12, 22], tilted fiber grating [7, 11], coreless silica fiber [14], silver nanoparticles (AgNPs) [13], graphene [15], reduced graphene oxide (rGO) [21], and graphene quantum dots (GQDs) [17] have been developed. But these sensors are complex in nature, and the fabrication cost is high. In this work, a tapered fiber is fabricated to develop the enzymatic glucose sensor. Tapered fibers are mostly used to fabricate the optical biosensors because evanescent waves can be easily coupled between core modes to cladding modes in the tapered fibers [6, 15]. The mode structure of the sensor can vary with the interaction of the evanescent field and surrounding molecules, which can be observed through transmission spectra [12]. The taper fiber structure is coated with AuNPs to enhance the sensitivity of sensor. There are many advantages of AuNPs such as easy synthesis, large specific area, biocompatibility, and high chemical stability [24]. The AuNPs electrons coherently oscillate in the electromagnetic field during localized surface plasmon resonance (LSPR) phenomena. LSPR enhances the sensitivity of sensor by creating a local dielectric environment on the AuNPs surface.
In this paper, a glucose sensor is developed using AuNPs-immobilized tapered fiber. The fiber probe is also functionalized with GOx to increase the selectivity of sensor probe. The manuscript is divided into four sections. Section 1 consists the introduction of work. Section 2 shows the material and methods, in which fabrication of taper fiber, synthesis of AuNPs, coating of AuNPs, characterization of AuNPs and AuNPs-coated taper fiber, and functionalization of GOx are described. The experimental setup for characterization of sensor probe is also discussed in this section. Section 3 consists of the results and analysis of sensor. In last, Section 4 comprises the conclusion of work.
Materials and Methods
Materials
Hydrogen tetrachloroaurate (HAuCl4), trisodium citrate (Na3C6H5O7), hydrogen peroxide solution (H2O2), sulfuric acid (H2SO4), (3-mercaptopropyl)trimethoxysilane (MPTMS), glucose, glucose oxidase (GOx), phosphate-buffered saline (PBS), N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), N-Hydroxysuccinimide (NHS), 11-Mercaptoundecanoic acid (MUA), and ultrapure water were used to develop the enzymatic sensor probe. Most of the reagents were purchased from Merck, Shanghai. These reagents are of analytical grade and were used in the experiment without further purification.
Fabrication of Tapered Fiber
The combiner manufacturing system (CMS) was used to fabricate the tapered fiber structure by tapering a single-mode fiber (as shown in Fig. 1). The CMS operates in a partial vacuum and is capable of easy control of taper profile, low surface roughness, fast fabrication, and economical [9]. The CMS was based on the exclusive technology of a highly controllable plasma field that completely surrounds the fiber for even heat distribution and tapering process. The CMS was operated in three-electrode mode, and pulling stages were used to stretch the fiber.
The stretching length and velocity were controlled with a computer program. Tapered fiber structures with a waist diameter of 40 μm, waist-length (tapered sensing region) of 4 mm, and transition length of 5 mm were fabricated to develop the proposed LSPR sensor probe.
Synthesis of Gold Nanoparticles
AuNPs was synthesized with the help of Turkevich method as discussed in [8]. A total of 15 ml of AuNPs solution was synthesized with the help of hydrogen tetrachloroaurate (150 μL, 100 mM) and trisodium citrate (1.8 mL, 38.8 mM). The solution was heated until boil, and then the trisodium citrate was added. The red color of the solution was obtained after 5 min of vigorous stirring. This solution was stable up to 3 months at room temperature and used in an experiment without further purification.
Characterization
The absorption spectrum of aqueous AuNPs solution was obtained by a UV-visible spectrophotometer (HITACHI-U-3310) at room temperature. The particle size analysis was performed using the transmission electron microscope (TEM) (Talos L120C, Thermo Fisher Scientific). Further, coating of AuNPs was verified by the scanning electron microscope (SEM) (Gemini, Carl Zeiss Microscopy). The SEM of fiber was performed at an optimum 3 kV. The complete tapered section was observed at lower magnification, and AuNPs particles were visible over the tapered fiber structure using SEM at higher magnification.
Coating of AuNPs over Taper Fiber Structure
The tapered fibers were cleaned before coating of AuNPs over taper fiber structure. The cleaning process was carried out in three steps. In first step, the tapered fibers were kept in acetone for 20 min. Thereafter, it was kept in a piranha solution made of three part of H2O2 and seven part of H2SO4 for 30 min. The piranha solution is highly oxidizing, and the surface of the tapered fiber treated with it will have hydroxyl groups, which can be used for subsequent modification. The fibers were rinsed with ultrapure water in the third step of cleaning process. Then, fibers were dried in an oven at 70 °C for 30 min. After cleaning process, the fibers were kept in MPTMS for 12 h for soaking process. MPTMS is an adhesive layer used for coating of AuNPs-over fiber structure. The unbound monomers of MPTMS were removed by rinsed the fiber with ethanol solution and dried by nitrogen gas. Finally, the MPTMS-coated fiber was kept in AuNPs solution for 48 h for nanoparticles (NPs) coating process. The sulfhydryl group on MPTMS was used to connect the AuNPs by Au-S covalent bond. Figure 2 shows the schematic of AuNPs coating. The unbound AuNPs particles were removed by rinsed the fiber with ethanol solution and dried by nitrogen gas.
Functionalization of Sensor Probe in Glucose Oxidase
The AuNPs-coated fibers were rinsed by ultrapure water and then kept in MUA solution (5 ml, 0.5 mM) for 5 h. MUA solution was used to modify a layer of molecules with carboxyl groups on the surface of AuNPs-coated tapered fiber structure. Then, fibers were kept in EDC (2 ml, 200 mM) and NHS (2 ml, 50 mM) solution for 10 min to activate the carboxyl group.
Thereafter, fibers were dipped in GOx (5 ml, 2 mM) for enzyme coating. The amino group of GOx is covalently connected to the activated carboxyl group. Finally, enzyme GOx is attached over the surface of tapered fiber. Figure 3 shows the schematic of enzyme coating over tapered fiber structure [7]. The enzyme coated sensor probe was kept at 4 °C for further use. It was dipped in the PBS solution for 15 min before measurement of glucose solution.
Preparation of Glucose Solution
For the characterization of sensor probe, different glucose solutions were prepared in the range of 0 mM to 10 mM concentrations. The 10 mM of stock solution of glucose was prepared in 1× PBS (pH 7.4) solution as a stock solution. Thereafter, the glucose solutions of 0 mM, 1 mM, 2 mM, 4 mM, 6 mM, 8 mM, and 10 mM concentration were prepared by diluting the stock solution in PBS for detection and measurement. 1× PBS solution (pH 7.4) was taken as a buffer and reference solution. Different solutions of cholesterol, urea, galactose, L-cysteine, and ascorbic acid of 10 mM concentration were also prepared for the selectivity test of the sensor probe.
Experimental Setup for Detection of Glucose Solution
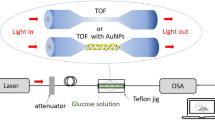
The sensor probe was characterized by the tungsten-halogen white light source (HL-2000, Ocean Optics Inc., USA), whose wavelength lies in the visible and near-infrared region. Figure 4 shows the setup to measure the LSPR spectra through a tapered fiber sensor probe. The other end of the fiber was connected with the USB2000+ spectrometer (Ocean Optics Inc., USA) that works in the range of 200 to 1100 nm.
Results and Discussion
Characterization of Gold Nanoparticles
The range of the optical source to excite the LSPR spectra was decided with the help of SPR wavelength of AuNPs. The absorbance peak of synthesized AuNPs is 519 nm as shown in Fig. 5a.
Characterization of gold nanoparticles. (a) Absorbance spectrum, (b) TEM image. The TEM image of AuNPs is shown in Fig. 5b. The diameter of the synthesized AuNPs is ~ 10 nm
Characterization of Sensor Probe
SEM was used for the characterization of a sensor probe that verifies the coating of AuNPs over taper fiber structure. A small section of taper fiber is placed on a 3 × 3 mm wafer for SEM. Here, Fig. 6a shows the AuNPs-coated taper fiber structure and Fig. 6b shows that AuNPs are uniformly coated over fiber structure.
LSPR Sensor
For the characterization of sensor probe, 0–10 mM of glucose concentration solutions were prepared. This range is crucial for health monitoring. Metal nanoparticles exhibit a strong absorption band due to LSPR phenomena. The peak absorption wavelength of synthesized AuNPs is 519 nm in the UV-visible range. The electric field of light excites localized surface plasmons (LSPs) that produce strong absorption and scattering and increase the electric field near the AuNPs. The LSP absorption band arises due to resonance of the incident light with the oscillation of conduction electrons. The resonance frequency depends on the characteristics of the NPs such as the material, size, shape, and local surrounding medium. Particularly, LSPR is highly sensitive to the refractive index (RI) of the surrounding medium. The LSPR wavelength shift ∆λ due to the RI change Δn is given by Cao et al. [1] and Semwal and Gupta [16]:
where m is the bulk RI response of AuNPs, d is the effective thickness of the adsorbed layer, and ld is the decay length of electromagnetic field.
The AuNPs are coated over the surface of a tapered sensing fiber, as shown in Fig. 7a. The light is guided into the tapered fiber structure via total internal reflection (TIR). The evanescent field near the tapered fiber surface interacts with the AuNPs and thus exhibits LSPR. The GOx decomposes the glucose on the surface of the AuNPs (as shown in Fig. 7a) and produces H2O2 that changes the RI of the medium surrounding the nanoparticles (as shown in Fig. 7b). The change in RI shifts the LSPR peak wavelength.
The RIs of the glucose solutions were measured using the Abbe refractometer with an accuracy of 0.001, and the RIs of all the samples before enzymatic reaction appeared to be identical within the resolution limit of the refractometer. The RI of the 1 mM glucose solution was also measured after the enzymatic reaction with GOx, and we found the RI changed from 1.3395 (without GOx) to 1.3400 (with GOx), thus verifying that there is a change in the RI over the surface of AuNPs due to production of H2O2 during the enzymatic reaction of glucose with GOx. The change in the RI of the medium surrounding the AuNPs shifts the peak wavelength of the spectrum. It can be observed from Fig. 8 that wavelength shifts toward the higher side with the glucose concentration.
Reproducibility and Reusability of Sensor Probe
Reproducibility analysis was carried out with three different sensing probes for 1 mM glucose concentration. The fabrication and characterization parameters were kept same for all sensor probes.
The sensor shows better reproducibility as shown in Fig. 9a. The sensor probe was reused after rinsed with a PBS solution. The 1 mM and 10 mM glucose concentrations were measured twice with the same sensor probe as shown in Fig. 9b. The developed sensor probe shows good reusability as well.
Selectivity of Sensor
Different solutions, such as glucose, cholesterol, urea, L-cysteine, ascorbic acid, and galactose that are generally found in the serum, were measured to verify the selectivity of sensor probe. The specific enzyme GOx determined the selectivity of sensor that was functionalized with a sensor probe. The GOx converts the glucose into gluconic acid and produces H2O2 during sensing (as shown in Fig. 7). The amount of H2O2 varies with the glucose concentration and changes the RI at the surface of LSPR sensor probe. The change in effective RI shifts the resonance wavelength of the sensor [18]. The GOx-immobilized sensor probe is nonreactive for other analytes present in the serum. Figure 10 shows the selectivity test of sensor with respect to different biomolecules present in serum. The 1 mM and 10 mM concentration of biomolecules were measured using a developed sensor probe. It has been found that the difference between the peak absorbance wavelength of 10 mM to 1 mM is higher in case of glucose solution (9.20 nm) than cholesterol (0.95 nm), galactose (0.76 nm), urea (0.99 nm), ascorbic acid (1.77 nm), and L-Cysteine (1.1 nm).
Sensitivity, Linearity and Detection Limit of Sensor
The peak absorbance wavelength of LSPR spectra is measured with different glucose concentrations (mM). The peak wavelength shifts toward higher wavelength with the glucose concentration as shown in Fig. 11. The linear variation of the peak wavelength with glucose solution can be shown as
where λ is the peak absorbance wavelength in nm and C is glucose concentration in mM. The sensitivity of the sensor probe is 0.9261 nm/mM. The detection limit of sensor is calculated with the mean value of PBS (blank sample), and three times maximum standard deviation among all the experimental data is multiplied by the inverse of the fitting function of the calibration curve. The detection limit of the developed sensor is 322 μM, and its linearity range is 0–10 mM.
Comparison of Proposed Sensor with Developed Sensors
Till now, many optical fiber-based glucose sensors have been developed. But, the fabrication procedure was complex and expensive. The sensing performance of the sensor has been compared with the existing glucose sensors based on material used for sensing, linear range, detection limit, and sensitivity of the sensors. The sensitivity of the developed sensor is high, and fabrication cost of the sensor is very low (Table 1).
Conclusion
The LSPR based fiber optic biosensor is developed for the detection and measurement of glucose concentration in blood. The advanced plasma field technique is used to fabricate the taper fiber structure. The sensitivity of sensor probe has been enhanced by the coating of AuNPs of size ~ 10 nm over the tapered fiber. Thereafter, selectivity of the sensor probe is enhanced by functionalization of GOx enzyme. The various steps of fabrication, synthesis, and characterization have been discussed in detail. The sensor shows a linear response in the range of 0 mM–10 mM glucose concentration. The limit of detection of sensor is 322 μM, and sensitivity of sensor probe is 0.9261 nm/mM. The developed sensor probe is robust, immune to electromagnetic interference, miniature, low cost, and designed for real-time measurement.
References
Cao J, Sun T, Grattan KTV (2014) Gold nanorod-based localized surface plasmon resonance biosensors: a review. Sensors Actuators B Chem 195:332–351. https://doi.org/10.1016/j.snb.2014.01.056
Hassan HU, Nielsen K, Aasmul S, Bang O (2015) Polymer optical fiber compound parabolic concentrator tip for enhanced coupling efficiency for fluorescence based glucose sensors. Biomed Opt Express 6:5008–5020. https://doi.org/10.1364/BOE.6.005008
Huang J, Li M, Zhang P, Zhang P, Ding L (2016a) Temperature controlling fiber optic glucose sensor based on hydrogel-immobilized GOD complex. Sensors Actuators B Chem 237:24–29. https://doi.org/10.1016/j.snb.2016.06.062
Huang J, Zhang P, Li M, Zhang P, Ding L (2016b) Complex of hydrogel with magnetic immobilized GOD for temperature controlling fiber optic glucose sensor. Biochem Eng J 114:262–267. https://doi.org/10.1016/j.bej.2016.07.012
Hwang D-W, Lee S, Seo M, Chung TD (2018) Recent advances in electrochemical non-enzymatic glucose sensors – a review. Anal Chim Acta 1033:1–34. https://doi.org/10.1016/j.aca.2018.05.051
Idris S, Azeman NH, Noor Azmy NA, Ratnam CT, Mahdi MA, Bakar AA (2018) Gamma irradiated Py/PVA for GOx immobilization on tapered optical fiber for glucose biosensing. Sensors Actuators B Chem 273:1404–1412. https://doi.org/10.1016/j.snb.2018.07.053
Jiang B et al (2018) Label-free glucose biosensor based on enzymatic graphene oxide-functionalized tilted fiber grating. Sensors Actuators B Chem 254:1033–1039. https://doi.org/10.1016/j.snb.2017.07.109
Kumar S et al (2019) LSPR-based cholesterol biosensor using a tapered optical fiber structure. Biomed Opt Express 10:2150–2160. https://doi.org/10.1364/BOE.10.002150
Li Y, Ma H, Gan L, Liu Q, Yan Z, Liu D, Sun Q (2018) Immobilized optical fiber microprobe for selective and high sensitive glucose detection. Sensors Actuators B Chem 255:3004–3010. https://doi.org/10.1016/j.snb.2017.09.123
Lin T-Q, Lu Y-L, Hsu C-C (2010) Fabrication of glucose fiber sensor based on immobilized GOD technique for rapid measurement. Opt Express 18:27560–27566. https://doi.org/10.1364/OE.18.027560
Luo B, Yan Z, Sun Z, Li J, Zhang L (2014) Novel glucose sensor based on enzyme-immobilized 81° tilted fiber grating. Opt Express 22:30571–30578. https://doi.org/10.1364/OE.22.030571
Luo B, Yan Z, Sun Z, Liu Y, Zhao M, Zhang L (2015) Biosensor based on excessively tilted fiber grating in thin-cladding optical fiber for sensitive and selective detection of low glucose concentration. Opt Express 23:32429–32440. https://doi.org/10.1364/OE.23.032429
Maruthupandy M, Rajivgandhi G, Muneeswaran T, Vennila T, Quero F, Song J-M (2019) Chitosan/silver nanocomposites for colorimetric detection of glucose molecules. Int J Biol Macromol 121:822–828. https://doi.org/10.1016/j.ijbiomac.2018.10.063
Novais S, Ferreira CIA, Ferreira MS, Pinto JL (2018) Optical fiber tip sensor for the measurement of glucose aqueous solutions. IEEE Photon J 10:1–9. https://doi.org/10.1109/JPHOT.2018.2869944
Qiu HW et al (2015) A novel graphene-based tapered optical fiber sensor for glucose detection. Appl Surf Sci 329:390–395. https://doi.org/10.1016/j.apsusc.2014.12.093
Semwal V, Gupta BD (2018) LSPR- and SPR-based fiber-optic cholesterol sensor using immobilization of cholesterol oxidase over silver nanoparticles coated graphene oxide nanosheets. IEEE Sensors J 18:1039–1046. https://doi.org/10.1109/JSEN.2017.2779519
Shehab M, Ebrahim S, Soliman M (2017) Graphene quantum dots prepared from glucose as optical sensor for glucose. J Lumin 184:110–116. https://doi.org/10.1016/j.jlumin.2016.12.006
Srivastava SK, Verma R, Gupta BD (2012) Surface plasmon resonance based fiber optic glucose biosensor. In: Asia Pacific Optical Sensors Conference, vol 8351. SPIE, Bellingham
Sun X et al (2018) Non-enzymatic glucose detection based on phenylboronic acid modified optical fibers. Opt Commun 416:32–35. https://doi.org/10.1016/j.optcom.2018.01.064
Tokuda T et al (2014) CMOS image sensor-based implantable glucose sensor using glucose-responsive fluorescent hydrogel. Biomed Opt Express 5:3859–3870. https://doi.org/10.1364/BOE.5.003859
Wang Y et al (2017) The woven fiber organic electrochemical transistors based on polypyrrole nanowires/reduced graphene oxide composites for glucose sensing. Biosens Bioelectron 95:138–145. https://doi.org/10.1016/j.bios.2017.04.018
Yin M-J, Huang B, Gao S, Zhang AP, Ye X (2016) Optical fiber LPG biosensor integrated microfluidic chip for ultrasensitive glucose detection. Biomed Opt Express 7:2067–2077. https://doi.org/10.1364/BOE.7.002067
Yuan Y et al (2017) Investigation for terminal reflection optical fiber SPR glucose sensor and glucose sensitive membrane with immobilized GODs. Opt Express 25:3884–3898. https://doi.org/10.1364/OE.25.003884
Yuan H et al (2018) Fiber-optic surface plasmon resonance glucose sensor enhanced with phenylboronic acid modified Au nanoparticles. Biosens Bioelectron 117:637–643. https://doi.org/10.1016/j.bios.2018.06.042
Zhang X et al (2018) Hydrogen peroxide and glucose concentration measurement using optical fiber grating sensors with corrodible plasmonic nanocoatings. Biomed Opt Express 9:1735–1744. https://doi.org/10.1364/BOE.9.001735
Funding
This work was supported by the National Natural Science Foundation of China (No.61501214), National Key R&D Program of China (2016YFB0402105), the Belt and Road Special Project approved by Shandong Province for Introduction of Foreign Experts in 2018, and Liaocheng University, China (31805180301, 31805180326).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, Q., Zhang, X., Kumar, S. et al. Development of Glucose Sensor Using Gold Nanoparticles and Glucose-Oxidase Functionalized Tapered Fiber Structure. Plasmonics 15, 841–848 (2020). https://doi.org/10.1007/s11468-019-01104-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11468-019-01104-7