Abstract
In pH 5.5 2-(N-morpholino)-ethanosulfonic acid buffer solution containing 0.0125 M NaCl at 80 °C, the single-stranded substrate DNA hybrid with enzyme DNA to form double-stranded DNA (dDNA). The substrate chain of dDNA could be cracked catalytically by UO 2+2 to produce a short single-stranded DNA (ssDNA) that adsorbed on the nanogold (NG) surface to form stable NGssDNA conjugate, and the unadsorbed NG take place aggregation to produce the NG aggregations in blue color. Both NG and NGssDNA exhibited strong catalytic activity on the gold particle reaction between HAuCl4 and ascorbic acid that can be monitored by resonance scattering (RS) spectral technique at 620 nm. However, the catalytic effect of NG aggregation was very weak and it cannot be separated from the cracked reaction solution. When the UO 2+2 concentration increased, the ssDNA increased, the NGssDNA increased, the formed gold particles increased, and the RS intensity at 620 nm increased. The increased RS intensity ΔI 620 nm was linear to UO 2+2 concentration in the range of 3.35–23.45 pM, with a regression equation of ΔI 620 nm = 27.6C + 29.1, and detection limit of 0.1 pM. This new RS assay was applied to analysis of UO 2+2 in water sample with satisfactory results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uranium is a radioactive metal element that exists in the environment [1]. It is one of the main materials in nuclear energy generation, and enriched uranium is a major material in nuclear weapon. If human beings were exposed to uranium-polluted environment, that could threaten the human health [2, 3]. Thus, highly selective and sensitive detection of uranium is very important for environmental protection and human health. At present, some analytical techniques such as inductively coupled plasma, atomic absorption spectrometry and phosphorimetry have been used to detect uranium [4–7], with high sensitivity. However, those methods require expensive and complicated instruments. In recent years, some new methods have been developed for metal ions, including fluorescence [8, 9], surface plasmon resonance (SPR) [10], electrochemistry [11, 12], and colorimetry [13, 14]. However, there are only a few reported methods for trace uranium [15–19], and most of them lack high sensitivity and selectivity, and low cost.
Aptamer is a short single-stranded oligonucleotide containing 20–100 bases. It can bind with high affinity and specificity to a wide range of target molecules including protein, polypeptide or organic compound and metal ion, with very small dissociation constant. Due to the high affinity, easy and quick preparation in vitro, and easy functionalizing, aptamers are used as research tools in specific protein function and interaction study, disease treatment, medical diagnosis, and bioanalysis [20]. At present, spectrophotometry, fluorescence, electrochemistry, SPR, and surface-enhanced Raman scattering have been utilized in aptamer analysis [21–23]. Resonance scattering (RS) spectroscopy is simple, rapid, and sensitive and has been applied to analysis of trace proteins, nucleic acids, and inorganic ions [24–26]. Nanogold (NG), being of easy preparation, high electron density and good biocompatibility, has been applied to protein, nucleic acid, and metal ion analysis [27]. Recently, NG has been also utilized in RS technique to develop immunonanogold RS, immunonanogold catalytic RS, aptamer–nanogold RS, and aptamer–nanogold catalytic RS assays, with high selectivity and sensitivity [28]. Based on our knowledge, there is no report about the catalytic active difference of nanogold and its aggregation, and aptamer RS assay for uranium. In this article, based on UO 2+2 -dDNA catalytic cutting reaction and the NG aggregation reaction, the catalytic active differences both NG and the NG aggregation on the HAuCl4–ascorbic acid (AA) reaction, and RS effect of the gold particles, a new nanogold catalytic RS assay was established for the determination of trace uranium ion in water sample.
Experimental
Apparatus
A model Cary Eclipse fluorescence spectrophotometer (Varian Company, USA) was used to record RS spectra, with the synchronous scanning technique (λ ex − λ em = Δλ = 0), a voltage of 500 V, both excited and emission slit width of 5.0 nm, emission filter of 1%T attenuator. A model TU-1901 double beams UV–visible spectrophotometer (Beijing Purkinje General Instrument Limited Co., China), a model 79-1 magnetic heat agitator (Zhongda Instrumental Plant, Jiangsu, China), a model nanoparticle and zeta potential analyzer (Malvern Company, England), and a model FEI Quanta 200 FEG scanning electron microscopy (SEM, Netherlands) were used.
Reagents
HAuCl4 2.4 × 10−2 mol/L (National Pharmaceutical Group Chemical Reagents Company, China), single-strand DNA (substrate chain 1, SS1) probe (Biological Technology Co., Ltd., Shanghai, China) with sequence of CTCACTATAGGAAGAGATGGACGTG, enzyme chain1 (ES1) of CACGGTCCATCTCTGCAGTCGGGTAGGCTTTCTACTGTT AACCGACC, substrate chain 2 (SS2) of ACTCACTATAGGAAGA ATGGACGG, enzyme chain 2 (ES2) of CACGTCCATCTCTGCAGTCGGGT GTTAAACCGAC TTCAGACAA TGAGT, substrate chain 3 (SS3) of ACTCACTATAGGTCCCGGATA GAGATGGACGTG, and enzyme chain 3 (ES3) of CACGTCCATCTCCGGGTTAAAACCCATAGTGAGT were used. A pH 5.5 2-(N-morpholino)-ethanosulfonic acid buffer solution (MES) with 0.025, 2.0 mol/L NaCl solution, 2.0 mol/L NaOH solution, 0.05 mol/L Tris solution, 1.00 × 10−6 and 1.00 × 10−7 mol/L UO 2+2 standard solution, 0.5 mg/mL NaBH4 solution, 9.6 × 10−3 mol/L HAuCl4 solution, 0.1 mol/L HCl solution, 0.1 mol/L cetyltrimethyl ammonium bromide (CTAMB) solution, and 0.057 mol/L AA solution were prepared. Nanogold particles were prepared by sodium borohydride reduction procedure: A 35 mL doubly distilled water, 0.50 mL 2.4 × 10−2 M HAuCl4 solution, and 3.5 mL 1% sodium citrate solution were added into a 50-mL conical bottle under magnetic stirring. Then, 3.0 mL 0.5 mg/mL NaBH4 solution was dripped slowly, and the color immediately changed from dark red to black red, and to red. After 10 min, the mixture was diluted to 50.0 mL. The nanogold particle concentration was 58.0 μg/mL Au. The NG aggregation was prepared as follows: A 25-μL 2.0 mol/L NaCl solution and 300-μL 58.0 μg/mL NG solution were added into a tube and diluted to 1.0 mL and mixed well. The concentration of NG aggregation was 17.4 μg/mL Au. All reagents were of analytical grade, and the water was doubly distilled.
Hybridization of DNA
A 0.50 mL 0.17 μmol/L of both SS1 and ES1 were added to a 15-mL colorimetric tube. Then 1.0 mL 2.0 mol/L NaCl solution, 2.8 mL of MES buffer solution, and 3.2 mL water were added. After mixed well, the mixture was heated at 80 °C for 15 min and cooled down to room temperature for 1.5 h [29]. The double DNA (dDNA) concentration was 21.5 nm/L.
Procedure
A 75-μL 21.5 nmol/L dDNA solution and a certain amount of UO 2+2 solution were added into a 5-mL marked test tube and mixed well. After 8 min, 25 μL 0.05 mol/L Tris solution was added immediately to stop the cracking reaction. Then 0.20 mL 58.0 μg/mL gold nanoparticle solution was added and diluted to 1.5 mL. The mixture is the cracking reaction solution.
Into a 5-mL tube, 10 μL 9.6 × 10−3 mol/L HAuCl4, 100 μL 0.1 mol/L HCl solution, 30 μL of the cracking reaction solution diluted 20 times, and 150 μL 0.1 mol/L CTMAB solution were added and diluted to 2.0 mL. Then 8 μL 0.057 mol/L AA solutions were added and diluted to 3.0 mL and heated at 60 °C for 15 min. The nanocatalytic reaction was stopped by tap water cooling. The RS spectrum, the I 620 nm, and the blank value (I 620 nm)0 without UO 2+2 were recorded. The value of ΔI = I 620 nm − (I 620 nm)0 was calculated.
Results and Discussions
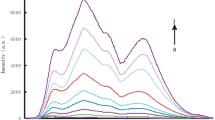
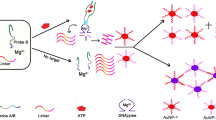
In pH 5.5 MES buffer solution containing 0.0125 mol/L NaCl, the substrate chain of dDNA could cracked by UO 2+2 to release a short single-stranded DNA (ssDNA), also called as cleaved strand. The ssDNA can adsorb on the NG surface to form NGssDNA to prevent its aggregation. When concentration of UO 2+2 increased, the formed ssDNA and NGssDNA increased, the NG aggregation decreased, and the color changed from blue to red gradually. Thus, the RS peak decreased at 610 nm. If no UO 2+2 was added, all NG aggregate, the solution is blue. We observed that the NG and NG aggregation exhibited great catalytic active difference on the gold particle reaction of HAuCl4–CTMAB–AA. As Fig. 1 showed, NG particles had strong catalytic effect with a slope of 36.76, and the NG aggregation was very weak, with a slope of 3.82. If the NGssDNA was used as the nanocatalyst, the catalyzed products had a strong RS peak at 620 nm. Under the chosen conditions, when the concentration of UO 2+2 increased, the content of NGssDNA in the cracking reaction solution increased, the formed gold particles increased, and RS peak at 620 nm increased linearly. On those grounds, a new nanogold catalytic RS assay would be proposed for trace UO 2+2 as in Fig. 2.
Laser Scattering
In the absence of UO 2+2 , the dDNA could not protect the NG particles, which was gathered to form big gold particles by salt, with an average size of 200 nm (Fig. 3a). When UO 2+2 was added to the solution, the dDNA was cleaved to form a short ssDNA that could protect NG particles without aggregation. That is, the NGssDNA and the NG aggregation coexist in the system as in Fig. 3b. The average size of these particles was 135 nm. This was consistent with the analytical principle.
Scanning Electron Microscopy
The SEM of the cracking reaction solution cannot be observed by scanning electron microscope because it contained high concentration of salt. To remove large amount of salt, the cracking reaction solution was centrifuged for 15 min with 15,000 rpm, and the particles were dispersed in water by ultrasonic wave. Then a 20-μL solution was taken into a silicon slice and dried by air. Using a scanning electron microscope, the graph was obtained. The results indicated that the small NG particles in size of 8 nm were aggregated to form large aggregations.
Resonance Scattering Spectra
In pH 5.5 MES buffer solution containing 0.0125 M NaCl, dDNA do not protect NG, and NG formed large NG aggregations that had an RS peak at 610 nm. Upon addition of UO 2+2 , the substrate chain of dDNA could be cracked to a short ssDNA that prevent NG to be aggregated. Thus, the RS peak decreased linearly with the concentration of UO 2+2 increasing and can be used to detect UO 2+2 . But, the sensitivity was not high.
To overcome the above problem and enhance the sensitivity, the nanocatalytic reaction was utilized. When there is no UO 2+2 , the NG aggregations in the cracking reaction solution showed weak catalytic effect on the HAuCl4–CTMAB–AA reaction, and the system exhibited a weak RS peak at 620 nm as in Fig. 4. With the concentration of UO 2+2 increasing, the concentration of NGssDNA increased in the cracking reaction solution, the gold particles increased, and the RS intensity at 620 nm enhanced linearly. Thus, a wavelength of 620 nm was chosen for the catalytic detection of UO 2+2 .
RS spectra of the NGssDNA catalytic system. a 32 μmol/L HAuCl4, 3.3 mmol/L HCl, 5 mmol/L CTMAB, 34 mmol/L AA; b to a 7.74 μg/mL NG, 1.07 nmol/L dDNA, 3.35 pmol/L UO 2+2 ; c to a 7.74 μg/mL NG, 1.07 nmol/L dDNA, 6.7 pmol/L UO 2+2 ; d to a 7.74 μg/mL NG, 1.07 nmol/L dDNA, 10.05 pmol/L UO 2+2 ; e to a 7.74 μg/mL NG, 1.07 nmol/L dDNA, 20.1 pmol/L UO 2+2 ; f to a 7.74 μg/mL NG, 1.07 nmol/L dDNA, 23.45 pmol/L UO 2+2
Selection of Assay Conditions
The NG aggregation reaction need a high concentration of NG, and the NG catalytic reaction takes place at trace NG. Thus, the diluted cracking reaction solution was necessary for the nanocatalytic RS assay. For the cracking reaction, the effect of EMS pH buffer solution on the ΔI 620 nm was tested. When the pH was 5.5, the ΔI 620 nm reached the maximum, so pH 5.5 EMS buffer solution was chosen. The cracking reaction of UO 2+2 is a dynamic process, and the reaction time should be controlled accurately, and a stopping reaction reagent should be used. Tris is a good reagent to stop the UO 2+2 cracking reaction. The effect of Tris concentration on the ΔI 620 nm was considered. A 0.83 mM Tris, giving maximum ΔI 620 nm, was chosen for use. The results showed that when the reaction time was 8 min, the ΔI 620 nm was maximal. Thus, 8 min reaction time was chosen. Figure 5 showed that when NG concentration was 7.74 μg/mL, the ΔI 620 nm had the maximum. Therefore 7.74 μg/mL NG was selected. When the dDNA concentration was 1.07 nmol/L, the ΔI 620 nm of the system was maximum. Thus, 1.07 nmol/L dDNA was selected for use.
Nanocatalytic reaction is an important way to amplify analytical signal. We found that NG and NGssNDA have strong catalytic effect on HAuCl4–CTMAB–AA reaction. We studied the effects of concentration of HCl, HAuCl4, AA, and CTMAB concentrations, reaction time, and temperature on the ΔI 620 nm. The results showed that a 10-μL 9.69 × 10−3 mol/L HAuCl4 solution, 100 μL 0.1 mol/L HCl solution, 150 μL 0.10 mol/L CTMAB solution, 18 μL 0.057 mol/L AA solution, and reaction temperature of 60 °C for 15 min, giving maximum ΔI 620 nm, were chosen for use. We also examined the effect of the volume for cracking reaction solution diluted 20 times on the ΔI 620 nm. The results showed that when the diluted solution was 30 μL, ΔI 620 nm reached maximum. So 30 μL of the diluted solution was chosen for use.
Influence of Coexistent Substances
According to the procedure, the influence of coexistent ions on the determination of UO 2+2 was tested, with a relative error of ±10%. Results indicated that a 1.5-μmol/L Pd2+, 2.1 μmol/L Fe3+, 2.4 μmol/L Ag+, 1.5 μmol/L Co2+, 1.8 μmol/L Ca2+, 2.8 μmol/L Pb2+, 1.6 μmol/L Mn2+, 5.5 μmol/L Mo6+, 2.0 μmol/L Zn2+, 7.5 μmol/L Al3+, 2.03 μmol/L Mg2+, 3.5 μmol/L Cr3+, 251 μmol/L HSA, 25 μmol/L glucose, 150 μmol/L albumin, 512 μmol/L lysine, 145 μmol/L valine, 156 μmol/L phenylalanine, and 148 μmol/L tyrosine do not interfere with the 20 pmol/L UO 2+2 determination. This showed the method had good selectivity.
Linear Relationship
Under the optimal conditions, the analytical features for the three different chain lengths of DNA hybridization systems (SS1-ES1, SS2-ES2, and SS3-ES3) were studied. The SS1-ES1 system was best. For the RS assay, the decreased intensity ΔI 620 nm was linear to UO 2+2 concentration in the range of 0.67–60.3 nmol/L, with a regression equation of ΔI 620 nm = 10.5C + 61.2, coefficient of 0.9972, and detection limit of 0.05 nmol/L. In comparison to the reported assays [15–19, 26], this method was simple, rapid, low cost, sensitive and selective, and the linear range was wider.
Selecting the SS1-ES1 system, an NG catalytic RS assay was proposed, according to the procedure. The working curve was obtained to plot the UO 2+2 concentration C (in picomoles per liter) vs ΔI 620 nm. The increased intensity ΔI 620 nm was linear to the UO 2+2 concentration in the range of 3.35–23.45 pmol/L, with a regression equation of ΔI 620 nm = 27.6C + 29.1, coefficient of 0.9911, and detection limit of 0.1 pmol/L. This NG catalytic RS assay was one of most sensitive, and the signal is linear to UO 2+2 concentration.
Analysis of UO 2+2 in Water Sample
Six water samples, including river, lake, reservoir, fountain, well, and pond, were pretreated according to the reference [30]. The UO 2+2 was determined five times according to the procedure. The results of Table 1 showed that the RS assay was in agreement with that of the laser fluorescence (LF) method. The relative standard deviation was in the range of 3.8%–8.1%. The known 0.10 and 5.00 nmol/L UO 2+2 were added into the sample to measure the recovery. A recovery range of 92.2%–107.5% was obtained.
Conclusions
The catalytic cracking reaction of dDNA-UO 2+2 was studied firstly by nanogold RS spectral method. Results show that the nanogold catalytic activity was stronger than its aggregations on HAuCl4–vitamin C particle reaction that can be also monitored by the RS technique at 620 nm. A novel and highly sensitive and selective nanocatalytic RS assay was proposed for detection of trace UO 2+2 in water, based on the catalytic cracking and nanocatalytic reactions.
References
Gongalsky KB (2003) Impact of pollution caused by uranium production on soil macrofauna. Environ Monit Assess 89:197–219
Craft E, Abu-Qare A, Flaherty M, Garofolo M, Rincavage H, Abou-Donia M (2004) Depleted and natural uranium: chemistry and toxicological effects. J Toxicol Environ Health B Crit Rev 7:297–317
Zhou P, Gu B (2005) Extraction of oxidized and reduced forms of uranium from contaminated soils: effects of carbonate concentration and pH. Environ Sci Technol 39:4435–4440
Abbasi SA (1989) A catalytic beacon sensor for uranium with parts-per-trillion sensitivity and millionfold selectivity. Int Environ Anal Chem 36:163–172
Brina R, Miller AG (1992) Direct detection of trace levels of uranium by laser-induced kinetic phosphorimetry. Anal Chem 64:1413–1418
Boomer DW, Powell MJ (1987) Determination of uranium in environmental samples using inductively coupled plasma mass spectrometry. Anal Chem 59:2810–2813
Mlakar M, Branica M (1989) Stripping voltammetric determination of trace levels of uranium by synergic adsorption. Anal Chim Acta 221:279–287
Chen P, He C (2004) A general strategy to convert the MerR family proteins into highly sensitive and selective fluorescent biosensors for metal ions. J Am Chem Soc 126:728–729
Jiang P, Guo Z (2004) Fluorescent detection of zinc in biological systems: recent development on the design of chemosensors and biosensors. Coord Chem Rev 248:205–229
Homola J, Piliarik M (2006) Surface plasmon resonance (SPR) sensors. Chem Sens Biosens 4:45–67
Xiao Y, Lubin AA, Heeger AJ, Plaxco KW (2005) Label-free electronic detection of thrombin in blood serum by using an aptamer-based sensor. Angew Chem Int Ed 44:5456–5459
Li D, Yan Y, Wieckowska A, Willner I (2007) Amplified electrochemical detection of DNA through the aggregation of Au nanoparticles on electrodes and the incorporation of methylene blue into the DNA-crosslinked structure. Chem Commun 43:3544–3546
Liu J, Lu Y (2003) A colorimetric lead biosensor using DNAzyme-directed assembly of gold nanoparticles. J Am Chem Soc 125:6642–6643
Liu J, Lu Y (2006) A simple and sensitive “Dipstick” test in serum based on lateral flow separation of aptamer-linked nanostructures. Angew Chem Int Ed 45:90–94
Rohwer H, Rheeder N, Hosten E (1997) Interactions of uranium and thorium with arsenazo III in an aqueous medium. Anal Chim Acta 341:263–268
Sessler JL, Melfi PJ, Seidel D, Gorden AEV, Ford DK, Palmer PD, Tait CD (2004) A new colorimetric actinide sensor. Tetrahedron 60:11089–11097
Blake RC, Pavlov AR, Khosraviani M, Ensley HE, Kiefer GE, Yu H, Li X, Blake D (2004) A Novel monoclonal antibodies with specificity for chelated uranium(VI): isolation and binding properties. Bioconjug Chem 15:1125–1136
Safavi A, Bagheri M (2005) A novel optical sensor for uranium determination. Anal Chim Acta 530:55–60
Greene PA, Copper CL, Berv DE, Ramsey JD, Collins GE (2005) Colorimetric detection of uranium(VI) on building surfaces after enrichment by solid phase extraction. Talanta 66:961–966
Hamula CLA, Guthrie JW, Zhang HQ, Li XF, Le XC (2006) Selection and analytical applications of aptamers. Trends Anal Chem 25:681–691
Song SP, Wang LH, Li J, Zhao JL, Fan CH (2008) Aptamer-based biosensors. Trends Anal Chem 27:108–117
Wen JQ, Di W, Jian L, Huang CZ (2010) Visual and light scattering spectrometric detections of melamine with polythymine-stabilized gold nanoparticles through specific triple hydrogen-bonding recognition. Chem Commun 46:4893–4895
Chen JW, Jiang JH, Gao X, Liu GK, Shen GL, Yu RQ (2008) A new aptameric biosensor for cocaine based on surface-enhanced Raman scattering spectroscopy. Chem Eur J 14:8374–8382
Luo HQ, Li NB, Liu SP (2006) Resonance Rayleigh scattering study of interaction of hyaluronic acid with ethyl violet dye and its analytical application. Biosens Bioelectron 21:1186–1194
Jiang ZL, Sun SJ, Liang AH, Huang WX, Qin AM (2006) Gold-labeled nanoparticle-based immunoresonance scattering spectral assay for trace apolipoprotein AI and apolipoprotein B. Clin Chem 52:1389–1394
Xu FG, Dong CQ, Xie C, Ren JC (2010) Ultrahighly sensitive homogeneous detection of DNA and microRNA by using single-silver-nanoparticle counting. Chem Eur J 16:1010–1016
Tao HL, Wei LL, Liang AH, Li JF, Jiang ZL, Jiang HS (2010) Highly sensitive resonance scattering detection of DNA hybridization using aptamer modified gold nanoparticle as catalyst. Plasmonics 5:189–198
Jiang ZL, Fan YY, Chen ML, Liang AH, Liao XJ, Wen GQ, Shen XC, He XC, Pan HC, Jiang HS (2009) Resonance scattering spectral detection of trace Hg(II) using aptamer modified nanogold as probe and nanocatalyst. Anal Chem 81:5439–5445
Lee JH, Wang ZD, Liu JW, Lu Y (2008) Highly sensitive and selective colorimetric sensors for uranyl (UO 2+2 ): development and comparison of labeled and label-free DNAzyme-gold nanoparticle systems. J Am Chem Soc 130:14217–14226
Ma WY, Zhou CL, Han F, Di YM (2001) Laser-fluorescence determination of trace uranium in hot spring water, geothermal water and tap water in Xi'an Lishan region. Radiat Prot 22(2):117–120
Acknowledgments
This work is supported by the National Natural Science Foundation of China (Nos. 21075023, 20865002, 20965002), Natural Science Foundation of Guangxi (No.0991021z), and the Research Funds of Key Laboratory of Ecology of Rare and Endangered Species and Environmental Protection (Guangxi Normal University), Ministry of Education.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, Z., Zhang, Y., Liang, A. et al. Free-Labeled Nanogold Catalytic Detection of Trace UO 2+2 Based on the Aptamer Reaction and Gold Particle Resonance Scattering Effect. Plasmonics 7, 185–190 (2012). https://doi.org/10.1007/s11468-011-9292-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11468-011-9292-6