Abstract
In this article, we experimentally investigated the plasmonics interaction in the system composed of Ag nano-cubes on Ag film with controlled distance. The distance is controlled by Rhodamine B (RhB)-doped polymethylmethacrylate (PMMA) film as the spacer, whose fluorescence intensity was then enhanced by the plasmonics interaction. Experimental results show that the fluorescence enhancement is sensitive to the thickness of the spacer. The largest enhancement factor obtained is 521 with the RhB-doped PMMA film of 10 nm thickness. For comparison, we also presented the fluorescence enhancement caused by only the localized surface plasmons from Ag nano-cubes on glass substrate coated with RhB-doped PMMA film, which gives out lower enhancement factors at the same thick spacer. Our experimental results are consistent with previous theoretical investigation and shows promising applications in fluorescence based bio-sensing or bio-imaging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fluorescence spectroscopy is considered to be useful research tool in biochemistry and biophysics [1], which has been used to study fluorophores, fluorophore-labeled bio-molecules, and diagnostic protein antigens and so on. The detection of fluorescence molecules is usually limited by its quantum yield, background signals from the samples, photostability, and brightness. Recently, the interactions of fluorophores with metallic particles and surfaces (metals) have been used to increase fluorescence intensity, which was defined as metal-enhanced fluorescence (MEF) [2–7]. MEF occurs primarily due to the near-field interaction of the excited-state fluorophore with the local electric field on the metallic particles or thin film [8, 9]. For the metallic nano-particles, localized surface plasmons (LSPs) can be excited whereas surface plasmons (SPs) can be excited on the metallic films, which both can induce the field enhancement in the near-field region and be used to increase the fluorescence intensity or signal-to noise ration. Known to all, the interaction of the SPs and LSPs (plasmonics interaction) will lead to hybrid surface plasmons, which is more intense and sensitive to surrounding mediums. Recently theoretical and experimental works verify that the electric intensity between the metallic nano-particles and metallic film can be greatly increased when their distance is optimized. In this article, the plasmonics interaction between the LSPs and SPs was investigated through the fluorescence method. Rhodamine B (RhB)-doped polymethylmethacrylate (PMMA) films of different thickness were used as the spacer layer between the Ag nano-cubes and Ag film, the field enhancement in the spacer region will greatly increase the fluorescence from the RhB molecules. On the other hand, the fluorescence detection can be used to display the interaction of LSPs and SPs.
Experiments
Synthesis of Ag Nano-Cubes
The Ag nano-cubes were synthesized by reducing AgNO3 with ethylene glycol (EG) [10]. In the reaction, a trace amount of Na2S was added to the traditional polyol where EG serves as the solvent and reducing agent. EG (18 ml) was placed in a capped flask, and heated with stirring (260 rpm) in oil bath at 150 °C for 1 h. 270 μl Na2S (3 × 10−3 M solution in EG) was quickly put in, and the bottle was recapped. Then 4.5 ml PVP (20 mg/ml) in EG solution and 1.5 ml AgNO3 (48 mg/ml) in EG solution were simultaneously added to the stirring solution in 3 min. After 2 h, the reaction was quenched. When the reaction bottle has been cooled, 1.5 ml of reactants was transferred into 7-ml centrifuge tube with approximately 5-ml acetone solution. The product was centrifuge at 3,000 rpm for 30 min and the supernatant was removed. Then 5 ml of DI water was added to the centrifuge tube, which was agitated via ultrasonic machine to re-disperse the products, followed by another centrifugation at 3,000 rpm for 20 min. This process was repeated for three times. At last, silver nano-cubes in DI water was obtained.
Figure 1a shows the scanning electron microscopy (SEM) image of the Ag nano-particles, which shows that most of the particles are of cube shape with side length about 100 nm. Figure 1b presents the absorption spectrum of the Ag nano-cubes in water solution. The full width at half maximum of the spectrum is about 280 nm, which means that LSPs of the Ag nano-cubes can be excited in broad-band wavelength.
Coupling System Composed of Ag Nano-Cubes and Ag Film
The Ag thin film was sputtered onto the glass substrate (thickness 0.17 mm) with vacuum thermal deposition technique. Figure 2a is the SEM image of the Ag film. Figure 2b presents atomic force microscopy (AFM, diInnova Scanning Microscope, Veeco Inc.) image at the edge of the Ag film and the line measurement shows that the thickness is about 57 nm.
The RhB-doped PMMA films were prepared in the following manner. A sample of RhB powder (0.1 mg/ml) was dissolved in PMMA solutions (950 K PMMA, Anisole solvent, from Micro Chem.) with different concentrations (1%, 0.2%, and 0.05%). The mixed solutions were agitated by ultrasonic disrupter for 30 min. Subsequently it was allowed to stand for 48 h, to ensure that the RhB molecules dissolved totally in solutions. The RhB doped complete PMMA films were obtained by spin coating the solutions onto Ag thin films at speed 5,000 rpm for 60 s. The films were then baked for 10 min at 105 °C to remove the solvent. Different concentrations of the PMMA solutions resulted in the different thickness of the films. The thickness of the RhB-doped PMMA films were about 65, 10, and 5 nm respectively based on AFM measurement. Our previous experiment results displayed that the RhB molecules were distributed uniformly in the PMMA films by this preparation method [11–13].
Then a drop of the solution containing Ag nano-cubes was put onto each RhB-doped PMMA film of different thickness. The samples were then placed in the dry box (25 °C) for 12 h to insure that the solvent (water) evaporated totally. In our experiment, the Ag nano-cubes solution was diluted so that only one layer of Ag nano-cubes distributed on part of the films. For comparison, we also prepared three samples without Ag films where the Ag nano-cubes were put on the glass substrates coated with RhB-doped PMMA films of thickness 65, 10, and 5 nm, respectively.
Results and Discussions
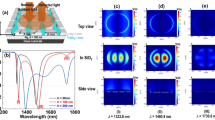
Figure 3a and e give out the sketch of the samples with or without Ag film under the Ag nano-cubes and RhB-doped PMMA films respectively. Figure 3b–d and f–h show the fluorescence spectra of RhB-doped PMMA (thickness 65, 10, 5 nm) on Ag films and glass substrates respectively, which were measured with confocal Raman microscope (LabRAM HR from HORIBA Jobin Yvon). The wavelength of the excitation laser is 514.5 nm. The excitation laser was focused by an objective (×50, numerical aperture N.A., 0.50) from the top of the samples. Fluorescence emission was collected by the same objective and directed into the spectrometer. For each sample, two fluorescence spectra were collected at different position on the PMMA film, one is with Ag nano-cubes on the RhB-doped PMMA film, another is bare RhB-doped PMMA film without the Ag nano-cubes as shown in Fig. 3a and e. Because the two spectra were taken from the same PMMA film, the RhB molecule concentration under excitation was the same and can be used for comparison.
a, e Sketch of the samples. t represents the thickness of the RhB doped PMMA film. b, c, and d present the fluorescence spectra from RhB-doped PMMA films on the Ag film. f, g, and h represent the fluorescence spectra from RhB-doped PMMA films glass substrate. The thicknesses of the PMMA films are about 65 nm (b, f), 10 nm (c, g), and 5 nm (d, h), respectively
From Fig. 3b and f, c and g, and d and h, we can find that the fluorescence intensity of RhB-doped PMMA film on Ag film is weaker than that on glass substrate, which means that the Ag film caused fluorescence emission quenching in the upper directions of the film. Although the Ag film caused the fluorescence quenching, the fluorescence intensity was greatly enhanced after putting Ag nano-cubes on PMMA films as shown in Fig. 3 b–d. When comparing Fig. 3b and f, c and g, d and h, we can find that fluorescence intensity with the Ag nano-cubes on the Ag film are higher than that from Ag nano-cubes on glass substrates in the case of equally thick PMMA film. Based on the radiating plasmon model [3], fluorescence quenching on Ag film substrate is due to induced electron oscillations which cannot radiate to the far-field because of wavevector mismatching, and fluorescence enhancement on glass substrate due to electric field enhancement by LSPs around Ag nano-cubes [14, 15]. As for the system of Ag nano-cubes coupled with Ag film, the fluorescence can be further enhanced with the plasmonics coupling between Ag nano-cubes and Ag film.
Here we defined the fluorescence enhancement factor (EF) as the ratio between the peak of fluorescence intensity curve with Ag nano-cubes and that without Ag nano-cubes. The EF are 52, 521, and 350 for PMMA thickness 65, 10, and 5 nm on Ag film substrate, respectively, and the EF for glass substrate are 7, 19, and 13, respectively, with corresponding PMMA film. The experimental results show that EF is larger on Ag film substrate than that on glass substrate with the same thick RhB-doped PMMA film. The EF is also related with the thickness of the RhB-doped PMMA film.
According to the theoretical investigation [16], for the coupling system between the Ag nano-cubes and Ag film, the electric field enhancement and resonant wavelength are sensitive to the spacer thickness. The largest field enhancement can be obtained in case of optimized spacer thickness under excitation of resonant wavelength. In our experiments, the thickness of the spacer is controlled by the RhB-doped PMMA film. When the spacer thickness is larger, such as 65 nm, the coupling between Ag nano-cubes and Ag film is very weak due to the localized properties of the SPs and LSPs. Whereas, if the spacer is too thin, the fluorescence molecules will be in very near region of the metallic structures, the fluorescence quenching effect would be dominant although the electric field was highly enhanced, such as the 5-nm-thick PMMA in our experiment. So there is an optimized thickness of the spacer thickness to obtained largest fluorescence enhancement. In our experiments, the largest EF happened at the 10 nm thickness spacer.
For the Ag nano-cubes on the glass substrate coated with RhB-doped PMMA film, the fluorescence enhancement is caused only due to the LSPs of the Ag nano-cubes. The EF is also related with the thickness of the PMMA. This can be explained as following: When the PMMA film becomes thinner, the ratio of the number of RhB molecules closed to the Ag nano-cubes vs. the total number of the RhB molecules doped in the PMMA film increases, which results in the fluorescence quenching. If the fluorescence molecules are all too closed to the metallic particles in the case of very thin PMMA film, the fluorescence quenching effect becomes obviously.
It is observed in Fig. 3c and d that the Raman signals of RhB molecules become obvious in the Ag nano-cubes and Ag film composite structure. The narrow peaks of the spectrum with Ag nano-cubes in Fig. 3c and d present the typical Raman signal (1,650 cm−1) from RhB molecules. Figure 4 shows than full Raman spectra of the RhB molecules with the PMMA thickness 10 nm. Two Raman spectra were taken on different position of the film, one is with the Ag nano-cubes (Ag nano-cubes and Ag film composite system as the substrate) and another is without Ag nano-cubes (Ag film as substrate). Experimental results clearly verify that the Raman signals become more obviously and stronger with the Ag nano-cubes on Ag film as the substrate than that on bare Ag film. The details on the Raman enhancement will be further investigated and discussed in the future work.
Conclusions
In summary, we have used the plasmonics coupling between Ag nano-cubes and Ag film to enhance the fluorescence emission from RhB molecules. The influence of the thickness of the spacer on the plasmonics interaction and resulted in fluorescence enhancement are mainly investigated. The highest fluorescence EF obtained is 521. Our experimental works demonstrate a new method to enhance the fluorescence or Raman signal, which is useful in bio-sensing or imaging.
References
Alfano RR, Pradhan A, Tang GC, Wahl SJ (1989) Optical spectroscopic diagnosis of cancer and normal breast tissues. J Opt Soc Am B 6(5):1015–1023
Zhuo S-J, Shao M-W, Cheng L, Que R-H, Ma DDD, Lee S-T (2010) Silver/silicon nanostructure for surface-enhanced fluorescence of Ln3+ (Ln = Nd, Ho, and Er). J Appl Phys 108:034305
Lakowicz JR (2005) Radiative decay engineering 5: metal-enhanced fluorescence and plasmon emission. Anal Biochem 337:171–194
Zhang J, Fu Y, Chowdhury MH, Lakowicz JR (2007) Metal-enhanced single-molecule fluorescence on silver particle monomer and dimer: coupling effect between metal particles. Nano Lett 7(7):2101–2107
Zhang Y, Dragan A, Geddes CD (2010) Metal-enhanced fluorescence from tin nanostructured surfaces. J Appl Phys 107:024302
Aslan K, Leonenko Z, Lakowicz JR, Geddes CD (2005) Annealed silver-island films for applications in metal-enhanced fluorescence: interpretation in terms of radiating plasmons. J Fluoresc 15(5):643–654
Aslan K, Malyn SN, Zhang YX, Geddes CD (2008) Conversion of just-continuous metallic films to large particulate substrates for metal-enhanced fluorescence. J Appl Phys 103:084307
Som T, Karmakar B (2010) Surface plasmon resonance and enhanced fluorescence application of single-step synthesized elliptical nano gold-embedded antimony glass dichroic nanocomposites. Plasmonics 5(3):149–15
Sokolov K, Chumanov G, Cotton TM (1998) Enhancement of molecular fluorescence near the surface of colloidal metal films. Anal Chem 70:3898–3905
Skrabalak SE, Au L, Li X, Xia YN (2007) Facile synthesis of Ag nanocubes and Au nanocages. Nat Protoc 2(9):2182–2190
Zhang DG, Yuan X-C, Bouhelier A, Wang P, Ming H (2010) Excitation of surface plasmon polaritons guided mode by Rhodamine B molecules doped in PMMA stripe. Opt Lett 35:408–410
Zhang DG, Yuan X-C, Bouhelier A (2010) Direct image of surface plasmon-coupled emission by leaky radiation microscopy. Appl Opt 49(5):875–879
Zhang DG, Yuan X-C, Yuan GH, Wang P, Ming H (2010) Directional fluorescence emission characterized with leakage radiation microscopy. J Opt 12:035002
Lin C-Y, Chiu K-C, Chang C-Y, Chang S-H, Guo T-F, Chen S-J (2010) Surface plasmon-enhanced and quenched two photon excited fluorescence. Opt Express 18(12):12807–12817
Anger P, Bharadwaj P, Novotny L (2006) Enhancement and quenching of single-molecule fluorescence. Phys Rev Lett 96:113002
Leveque G, Martin OJF (2006) Optical interactions in a plasmonic particle coupled to a metallic film. Opt Express 14(21):9971–9981
Acknowledgments
This work is supported by the Key Program of National Natural Science Foundation of China under Grant (No. 610360050) and National Natural Science Foundation of China under Grant (No. 10704070, 11074241, 11004182.).
D. G. Zhang acknowledges helpful discussions with Dr. J. R. Lakowicz and Dr. M. H. Chowdhury of the Center for Fluorescence Spectroscopy, University Maryland School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yi, M., Zhang, D., Wen, X. et al. Fluorescence Enhancement Caused by Plasmonics Coupling Between Silver Nano-Cubes and Silver Film. Plasmonics 6, 213–217 (2011). https://doi.org/10.1007/s11468-010-9190-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11468-010-9190-3