Abstract
This paper studies the effects of sodium-based alkaline activators and class F fly ash on soil stabilisation. Using the unconfined compressive strength test (UCS), the effectiveness of this binder is compared with that of a common cement-based binder. Influence of the activator/ash ratio, sodium oxide/ash ratio and sodium hydroxide concentration was also analysed. Sodium hydroxide concentrations of 10, 12.5 and 15 molal were used for the alkaline-activated specimens (AA), with activator/ash ratios between 1 and 2.5 and ash percentages of 20, 30 and 40 %, relatively to the total solids (soil + ash). UCS was determined at curing periods of 7, 28, 90 and 365 days, and the most effective mixtures were analysed for mineralogy with XRD. The results showed a clear increase in strength with decreasing activator/ash ratio (up to a maximum of 43.4 MPa), which is a positive result since the activator is the most expensive component in the mixture. Finally, UCS results of the cement and AA samples, at 28 days curing, were very similar. However, AA results proved to be just between 20 and 40 % of the maximum UCS obtained at 1 year curing, while cement results at 28 days are expected to be between 80 and 90 % of its maximum.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Soft soils are basically clay soils with reduced shear strength, capable of large deformations under relatively small loads, and are therefore considered one of the biggest concerns in geotechnical engineering. As the construction industry grows, there is a reduction in sites suitable for development. It is therefore of most importance to develop a large spectrum of soil improvement techniques that can respond to increasingly demanding situations. One of the major techniques used to overcome the problems created by soft soils is the mixing with a cementitious binder. Traditionally, these binders are cement and/or lime, which ‘glue’ the soil particles together mainly through chemical and not physical reactions. In the case of cement, the reactions are mainly hydraulic, while with lime they are pozzolanic. This means that cement needs only water to react and increase in strength, since the pozzolanic component is already incorporated into the cement, whereas lime needs water and a pozzolanic material, like clay [19]. Both binders share the fact that their reactions with water depend largely on their specific surface. Moreover, although the type of reaction is different for lime and cement, the final product is very much alike, based on calcium and silica compounds. In terms of mechanical strength, cement-based binders usually deliver significantly better results than lime-based binders. However, environmental and durability issues related to cement production and application in soil constitute a significant motivation to develop new binders. The environmental limitations of ordinary Portland cement (OPC) are related to the high levels of CO2 released during its production, estimated at 7 % of the total anthropogenic CO2 [12], while the chemical vulnerability of OPC is of special concern when dealing with its use in structural foundations or soil improvement, due to the attack by sulphates in the ground or in chemical wastes [27]. Also, concrete made with cement requires very specific aggregates that are increasingly hard to source and lead to the degradation of landscapes.
It seems therefore of most interest to develop new binders, with equal or better performance than cement-based binders, but with lower environmental costs. As such, the use of increasing amounts of waste materials in construction plays an important contribution to the reduction in cement consumption, since it allows its substitution in significant percentages. In particular, the use of fly ash has become more prevalent. It is produced from the combustion of coal and consists of the inorganic matter that did not burn during the process. It can be classified according to the level of calcium present in its composition: if it is a sub-bituminous coal, the resulting ash will be classified as type C (calcium percentage usually above 20 %). The combustion of a bituminous coal will result in class F fly ash (calcium percentage usually not higher than 10 %). The calcium content is known to have a significant influence on the application of fly ash for structural applications [4, 5, 15]. Additionally, the US Department of Energy [17] conducted a thorough study that concluded that fly ash, if used properly, is not a hazard to the environment when used for soil stabilisation.
Materials formed using reactions between silica and alumina and alkali cations like sodium or potassium are very similar, at a molecular level, with natural rocks, sharing their stiffness, durability and strength. Alkaline activation of alumina-silicate materials (geopolymerisation) is already considered an important alternative to OPC, since most of the well-known limitations of OPC are overcome by this new technology [16]. An important advantage of alkaline-activated materials over cement is environmental, since the absence of a high-temperature calcination step in the activation of ashes and/or slags results in a dramatic reduction in CO2 emissions compared to OPC production. Duxson et al. [10] studied the reductions in CO2 emissions of geopolymer compared to OPC based on the dissolved solids (Na2O + SiO2) content of the activating solution and concluded that it can be as high as 80 %. The amorphous chemical structure of fly ash, which is a consequence of the high temperatures it was subjected to during combustion, makes it particularly interesting for alkaline activation. Its high levels of silica and alumina are much more willing to combine in this form with other elements, like sodium or potassium cations, than they were in their original crystalline forms. Several authors have studied the activation of class F fly ash alone with sodium-based or sodium + potassium-based activators [6, 24, 28, 32] and reported values for compressive strength higher than 8 MPa at 14 days curing.
The combination of all these factors highlights the potential for materials based on alkaline activation of waste by-products. According to [6, 9, 22, 29, 31, 32], alkaline-activated materials are, in general, better performing than cement from a mechanical point of view and show increased durability and stability. These research programmes have been carried during the last 10 years to study alkaline activation, but have not considered its application to soil improvement. Not only the binder itself can be better performing than cement binders, but it shows an improved bond between the soil particles and the binder. In fact, studies by Lee and Van Deventer [21] and Temuujin et al. [25] focused on the mixing of geopolymeric binders with mineral aggregates, like sand and/or natural rocks, and particularly the interface between particle and binder. Several compositions were prepared using sodium silicate solution and NaOH and/or KOH solution to activate nine parts of fly ash and one part of kaolin [21]. Sand and other aggregates were added to this grout, and the conclusion was that the interface between the siliceous materials and the binder gel was not well defined. Similar conclusions were obtained when determining the compressive strength of fly ash-based geolopymer mortars with varying levels of sand aggregate [25]. The increase in soluble silicate dosage formed denser binders and stronger binder/aggregate interfaces, leading to stronger products. Therefore, the fact that alkaline activation can actually interact with the soil particles and not just involve them in a strong matrix, like in the case of a cement grout, is particularly interesting not only because usually the interface is the weakest point, but also because the soil particles can add to the final product strength by providing a very stiff natural skeleton, and therefore, it is advantageous that they form an integral part of the final mixture and are not merely surrounded by it.
The aim of this paper is to study the influence of binder composition on mechanical strength of stabilised soil, using a more common soil–cement stabilisation as a reference. A sodium-based activator was used at different concentrations, with different ash percentages and different Na2O/ash ratios, and the samples were tested after different curing periods.
2 Methodology
2.1 Materials characterisation
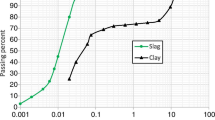
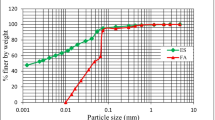
Geotechnical characterisation, according to BS 1377 [3] (Table 1), and mineralogical tests were performed on the soil, which was collected from a natural deposit near Lisbon (Portugal) and was classified as Sandy Clay of low plasticity using ASTM D2487 [2]. Prior to mixing, the soil was sieved down to fractions below 1.18 mm. The X-ray diffraction (XRD) pattern showed the presence of quartz, kaolinite and muscovite (mica). All of these minerals are very common in this type of soil.
The fly ash used had low calcium content (class F) and was obtained from a Portuguese thermo-electric plant. Its characterisation was carried by scanning electron microscopy (SEM), together with chemical analysis by energy dispersive spectroscopy (EDS) and XRD, with results presented in Table 2 and Fig. 1. Its total mass available for dissolution is approximately 74 % (Si and Al), with a specific gravity of approximately 24.2 (± 0.2) kN/m3. It has a small vitreous phase content (halo registered between 2θ = 20° and 2θ = 35°) and therefore significant crystalline phases—quartz and mullite. According to Fernández-Jiménez and Palomo [14], the lower the vitreous phase, the lower the capacity for activation of the fly ash, and therefore, the lower the mechanical strength gain anticipated with alkaline activation. The cement was a Portland cement Type I, class 42.5 R.
The alkaline activator solution used was a combination of sodium silicate and sodium hydroxide, both from Fisher Scientific UK. The sodium silicate was originally in solution form, with a specific gravity of 1.5, a SiO2 : Na2O mass ratio of approximately 2 and a SiO2 : Na2O concentration of 39.5 %. The sodium oxide (Na2O) was originally supplied in flake form with a specific gravity of 2.13 at 20 °C and 95–99 % purity and was dissolved in water to achieve sodium hydroxide with different concentrations of 10, 12.5 and 15 molal, before being mixed with the silicate. It was decided to use a ratio of sodium silicate solution to sodium hydroxide solution, by mass, of 2. This value was chosen not only because the silicate is considerably cheaper than the hydroxide, but also because several studies that have analysed the influence of the activator composition concluded that higher ratios resulted in higher strength levels. For example, Hardjito and Rangan [16] compared the effects on compressive strength of 0.4 and 2.5 silicate/hydroxide ratios and reported a strength increase between 40 and 235 % with the higher ratio. Criado et al. [6] used silicate/hydroxide ratios between 0 and 0.5 and obtained higher strengths with the higher ratios. Villa et al. [29] tested five different ratios and concluded that the best results, in terms of strength gain, were obtained with a 1.5 ratio or higher, with the lowest ratio of 0.4 proving the worst option.
2.2 Sample preparation and testing
To evaluate the effect of the ash/soil ratio (by dry mass) on mechanical strength, three different fly ash percentages, regarding the total solids (soil + ash) weight, were used: 20, 30 and 40 %, corresponding to ash/soil ratios of 0.25, 0.43 and 0.67. A crucial decision then had to be made regarding the use of an activator/solids ratio or an activator/ash ratio as a reference to decide how to change the activator (solution) in the tests. Since it is the most expensive component, it was decided to use just the amount of activator necessary to make the mixture sufficiently fluid to be homogenised and poured in the moulds. However, because the fly ash percentage in the mixtures was changing, and that is in fact the main component that is expected to react with the activator, two possibilities emerged: adapting the quantity of activator to the solids content or to the ash content only. The possibility of keeping constant the activator/ash ratio was then tested, only to find out that it would lead to very different moisture levels when compared to the ash present in the mix. As the ash content increased to its maximum value, the amount of activator necessary to maintain the same ratio would become comparatively very high, to a point where the difference in moisture content of the driest and wettest mixtures was very significant. Therefore, it was decided to use the activator/solids ratio as a reference, which implied that the activator/ash ratio would change every time the ash percentage in the mixture was changed. Initially, an activator/solids ratio of 0.30 was tested, only to reveal that it would be impossible to even start the mixing process due to very poor homogenisation. After several increasing ratios were tested, it was clear that a 0.60 value resulted in higher water content than necessary for homogenisation. That led to the option of using an activator/solid ratio of 0.40. However, this ratio was determined for the 12.5 molal mixtures, and when the 15 molal mixtures were started, it was found that because the reactions were faster than with the lower concentration of 12.5 molal, the increase in stiffness was too fast to allow a proper pouring in the moulds. It was then decided to use an activator/solid ratio of 0.45. Finally, and in order to understand the effect of an increase on this ratio on compressive strength, the 10 molal mixtures were prepared with an activator/solids ratio of 0.50.
These three different activator/solids ratios resulted in liquid/ash ratios, accounting for the variation of the fly ash percentage in the total solids dry mass, of between 1.0 and 2.5. Other authors used smaller solution/ash ratios [6, 30], because the only dry mass available was the fly ash, while in this case, the soil substantially increased the need for a higher liquid phase, for homogenisation purposes. Only one of the mentioned liquid/solid ratios of 0.40, 0.45 and 0.50 was used per hydroxide concentration, ash content and curing period. Full details of the mixtures are contained in Table 3.
The soil and the ash were previously homogenised before the activator was added to the mixture. After mixing for 3 min, the samples were cast into 38-mm moulds by tapping the moulds on the lab counter [26], which were then left in a sealed container. Since the behaviour of the mixtures was that of a viscous fluid, no density control was used during the preparation of the samples. However, when removed from the moulds, every sample was weighted, and an average unit weight of 20 kN/m3 was obtained, regardless of the fly ash percentage in the mixture. The 15 molal mixtures showed a very high viscosity which made the preparation and handling process more difficult than with the remaining concentrations, to a point where this factor should be considered when designing future studies and/or applications. This effect is related to the SiO2 : Na2O mass ratio of the silicate + hydroxide solution which, for the 15 molal activator, is approximately 1, making the metasilicate solution very unstable and favouring crystallisation. This SiO2 : Na2O mass ratio was, in the original silicate solution, approximately 2, but the addition of the hydroxide solution reduced it significantly, especially in the 15 molal mixtures.
After 48 h, the samples were removed from the moulds and wrapped in cling film and left at ambient temperature and humidity conditions (40–50 % RH and 19–23 °C). Immediately before testing, at the ages of 7, 28, 90 and 365 days, the samples were trimmed to 76 mm long and tested for unconfined compressive strength (UCS) on a 250 kN capacity Shimadzu Autograph hydraulic testing machine. Every single result obtained was the average of 3 tested samples.
After being tested, some selected samples at 90 and 365 days curing were micro-structurally studied by X-ray diffraction (XRD), in order to help explain the mechanism of strength gain and determine the structure of the final product. The XRD equipment was a PANalytical X’Pert Pro diffractometer, fitted with an X’Celerator. A secondary monochromator would have eliminated fluorescent scattering from the specimen, resulting in a good peak/background ratio for samples containing transition metals and rare earths. However, for this work, the monochromator was unavailable, so the background scatter could be a little higher. The scans covered the range 5–70 degrees, with a nominal step size of 0.033 deg 2-theta and time per step of 100 s. Radiation is Cu K-alpha with a wavelength of λ = 1.54180 A. Phase identification was carried out using the X’Pert accompanying software program High Score and the ICDD database, Sets 1–49 (1999).
For the cement-based mixtures, initial water/cement ratios of 1, 1.5 and 2 were tested. However, these ratios were changed to 0.5, 0.75, 1.0, 1.25 and 1.5 when the water/cement ratio of 2 produced mixtures with a very high moisture content. In terms of cement percentage in the mixtures, values of 20, 30 and 40 % of the total dry weight were used. These values are higher than the usual cement contents in soil–cement stabilisation, so that a direct comparison with the activated ash could be established. These values also allowed the analysis of a very significant spectrum of soil/cement/water combinations, in order to detect the most performing. The mixtures used for the cement samples are described in Table 4.
The density and viscosity of both grouts were analysed and compared, with the purpose of determining how much time is available before mixing with the soil. Two tests were conducted to compare cement and alkaline grout viscosity. The first test was performed by filling a plastic bucket, with a 1-mm sieve on the bottom end, with sand up to a height of 0.24 m. For the cement test, a grout with a water/cement ratio of 1:1 was released on top of the sand and the time necessary for the grout to start to drop onto the bottom of the bucket was measured, as well as the time taken before the grout stopped to fall. A similar procedure was used for an alkaline grout with an activator/ash ratio of 0.89. This ratio, lower than the minimum value of the range considered in this study, was obtained after a field trial (described in Cristelo et al. [8]) using jet grouting revealed that the viscosity should be taken into consideration when designing the grout. Since the exact same components used in the present grout were grout used in the mentioned field tests, it was decided to present the results on viscosity in this paper. The second test was a Marsh funnel viscometer (ASTM D6910-04 [1]) and consisted of measuring the time taken for a known volume of liquid to flow from the base to the bottom end of the inverted funnel. The liquid was poured through the top, saturating the voids in the sand until it reached the top level, which used approximately 1.5 litres. The bottom exit was then released and the liquid flowed into a measuring container, while the time spent was recorded.
3 Results and discussion
3.1 Compressive strength
Table 5 identifies each of the alkaline-activated and cement/soil mixtures. Designation was determined as a function of ash percentage and sodium hydroxide concentration (for the alkaline activator-based mixtures) and as a function of cement content and water–cement ratio (for the cement-based mixtures).
Based on Fig. 2, the strength of the 10 molal mixtures is still increasing with curing time after 365 days, for every fly ash percentage. There is a similar rate of increase between days 1 and 28 and days 90 and 365, with a small decrease in rate between days 28 and 90. This probably can be related with the necessary period for the nucleation phase to occur, during which the products resulting from the dissolution of the raw silica and alumina accumulate before precipitation. A similar situation was previously reported in soil stabilisation with lime [7]. In addition, there are no significant differences in the strength development for the three fly ash percentages used, although, as expected, the absolute values increased with the amount of fly ash added. A similar pattern, in terms of strength evolution, was produced for the mixtures with hydroxide concentration of 12.5 and 15 molal. The main difference between the three mixtures was the absolute values obtained for each curing period. The higher results for each concentration were obtained with 40 % fly ash. Also, the 15 molal mixtures produced the highest results for every fly ash percentage, with the exception of the 40 % fly ash, for which the highest value was produced with the 12.5 molal concentration. This was indeed the highest value obtained for every test performed, which is a significant fact since a lower sodium hydroxide concentration (12.5 molal) means a reduction in cost and an advantage regarding the preparation and handling of the mixtures compared to 15 molal.
Regarding the cement mixtures, only the 28 days curing values showed in Fig. 3 were obtained through laboratory tests, while the 90 and 365 days results were estimated using the following expression from Eurocode 2 (BS 1992) [11]:
with
where f cm (t) is the mean compressive strength at an age t (in days) and s is a coefficient which depends on the type of cement (0.25 for class CEM 42.5 N).
Figure 3 reveals that the best W/C ratio for the cement mixtures was 0.75 and that higher ratios resulted in strength loss. A similar conclusion was reported by Kasama et al. [20]. This is a significant factor since the W/C ratio is related to the viscosity of the mixture, which in turn has a major influence in such cement-based applications as jet grouting [13]. This means that the 0.75 ratio might not be a viable option in practical terms. Therefore, the advantage of the alkaline activator mixtures becomes more significant since there was a very significant minimum strength loss of 43 % between W/C = 0.75 and W/C = 1.0. The values obtained with the 0.75 W/C ratio were very similar for the 30 and 40 % cement contents. Results compiled by Kasama et al. [20] from several research papers on soil–cement, with a wide range of cement content (between 3 and 45 %), showed that the higher UCS values were obtained for specimens with a cement content of 30 %. Analysis of Fig. 3 also shows that at 28 and even at 90 [predicted using Eqs. (1) and (2)] days curing, the strength values for the cement–soil mixtures were in general higher than those obtained with the alkaline activator–soil mixtures. However, at 365 days, the highest value predicted [using Eqs. (1) and (2)], for the mixtures C075/30 and C075/40, was approximately 29 % lower than the highest value obtained with the alkaline activation (A125/40). Also significant was that at 365 days curing, only three predicted results from the cement mixtures (C050/40, C075/30 and C075/40) were higher than the lowest value from the alkaline-activated mixtures (A100/20).
3.2 Effect of activator concentration
As can be seen from Fig. 4, sodium hydroxide concentration had a significant influence on strength development. Apart from the 40 % fly ash mixtures, there was a slight advantage to use the 15 molal concentration. Hu et al. [18] reached a similar conclusion and even suggested that this is the most important factor regarding strength gain. However, for the 40 % fly ash, the best option was the 12.5 molal, which probably has to do with the increased difficulties that appeared while preparing the 15 molal concentration mixtures, especially in the case of the 40 % ash content. It was clear when preparing the 15 molal mixture that the initial reactions were very fast, resulting in completely hardened samples after only 24 h. When the fly ash is mixed with the activator, the vitreous phase from the ash is quickly dissolved, not allowing enough time for the gel to grow into a well crystallised structure [14]. This effect was more pronounced with the 15 molal mixtures and thus responsible for the higher short-term strength of the 15 molal compared to the 10 and 12.5 molal mixtures. It also prevented the original reactive silica from being more extensively dissolved, and therefore, the resulting geopolymeric gel available in the 15 molal mixture at medium to long term was less than with the 10 and 12.5 molal mixtures, resulting in lower strength values. Hu et al. [18] tested lower NaOH concentrations and only up to 60 days curing and therefore did not report such difficulties. At 7 and 28 days curing, the described effect was not so pronounced since the 10 and 12.5 molal mixtures had not yet developed significant quantities of geopolymeric gel and therefore the 15 molal samples achieved higher strengths.
3.3 Effect of Na2O/ash ratio
Figure 5 shows the variation in UCS with the Na2O/ash ratio for all 4 curing periods. The Na2O present in each mixture was calculated by summing the Na2O from the silicate and the sodium oxide, which are indicated in Sect. 2.1. There is a decrease in UCS with the increase of the Na2O/fly ash ratio up to a ratio of 0.375, when it appears that UCS values start to rise again. The maximum UCS was obtained, for all curing periods, at the lowest ratio of 0.166. An opposite conclusion was obtained by Winnefeld et al. [30], which reported higher UCS results with an increase in activator dosage relatively to the fly ash content. However, the Na2O/ash ratio range in this study was between 0.052 and 0.125, while in the present study, this range was between 0.166 and 0.405, which means that the optimum value might be in the 0.09 and 0.16 range, depending on materials and conditions.
Figure 6 shows the variation in UCS with the activator/ash ratio. At 28 days curing (Fig. 6b) the lower activator/ash ratios are the best performing in terms of strength gain, for every concentration, with the higher activator concentration achieving the best results. At 90 and 365 days (Fig. 6c, d) the lower activator/ash ratio again gave the best results for every concentration. However, for these curing periods, and for the activator/ash low range ratio, the activator/ash seems even more relevant than the hydroxide concentration itself, since for both curing periods a 1.00 activator/ash ratio with a 12.5 molal concentration achieved a higher UCS than a 1.13 ratio with a 15 molal concentration. Further tests are needed to better support this conclusion, specifically on samples with 12.5 and 15 molal but with the same activator/ash low ratio. The positive effect of a lower activator/ash ratio was therefore detected at every curing period tested, except for an early stage, when significant reactions had yet to occur. This is contrary to a previous study by Palomo et al. [23], which analysed the reaction between fly ash and different activators, with different solution/ash ratios, concluding that during the development of the resulting amorphous aluminosilicate gel, the referred ratio had no influence whatsoever in the final mechanical strength.
3.4 XRD analysis
Table 6 summarises the composition of the mixtures analysed with XRD. The focus was on the higher curing periods (90 and 365 days), since those were considered more significant in terms of reaction development between the activator and the fly ash and more likely to yield crystalline products. The 15 molal sodium hydroxide concentration mixtures were chosen for this study for two reasons: the higher concentration results (theoretically) in a greater reaction volume, more visible in the XRD results; and the UCS evolution with the activator/ash ratio showed a similar pattern for all three concentrations, meaning that probably no significant differences should be expected when studying the 10 and 12.5 molal mixtures. Mixture B13 was obtained with a lower quantity of activator than mixture B12, although in both cases 40 % fly ash was used.
The results in Figs. 7 and 8 show an XRD pattern typical of an amorphous to semi-crystalline material, which can be attributed to the lack of time to form a well-crystallised structure after the start of the reactive silica dissolution. Also relevant is that no significant peak representing crystalline hydration products formed after 90 and 365 days curing, which was also reported by Winnefeld et al. [30]. Comparing B12 (activator/ash = 1.13) and B13 (activator/ash = 1.00) samples, both with 40 % fly ash content, a higher dissolution was detected with the lower activator/ash ratio, which also corresponds to the higher UCS. It is therefore clear that there is a correlation between the dissolution level, the activator/ash ratio and mechanical strength, also reported by Criado et al. [6].
The reduction in vitreous phase between the original fly ash and 90 days curing and between 90 and 365 days curing can be observed by comparing Figs. 1, 7 and 8. This was expected since vitreous material is the primary source of the activation process and therefore is also correlated with the UCS increase. Villa et al. [29] reached a similar conclusion when simultaneously evaluating mechanical strength and XRD development on natural zeolite with varying sodium silicate/hydroxide ratios.
3.4.1 Viscosity
The results in Table 7 show that the viscosity of the alkaline grout is higher than that of the cement grout. The higher viscosity can be a factor in the grout/soil mixing levels [8], which can be overcome by increasing the water percentage in the activator. However, in so doing, the activator/ash ratio is increased, while the Na2O/ash ratio is kept constant, therefore justifying the study of the effects on strength which are presented in Sect. 3.3.
4 Conclusions
From this study, it can be concluded that alkaline activation of low calcium fly ash can be used as an alternative binder to Portland cement for soft soil stabilisation. A good comparison with the predicted cement mixtures strength, after longer curing periods, was obtained. However, if short- to medium-term strength gain is required, then alkaline-activated grouts may not be appropriate. The following conclusions can also be drawn:
-
There is a strong dependency between the activator/ash ratio and mechanical strength. Results showed that it is advantageous to reduce this ratio since it has a positive effect on strength results, which has also a positive effect on final cost.
-
The sodium hydroxide concentration has proved to be an important factor regarding strength development; however, there seems to be a limit around 12.5 molal, since a 15 molal concentration resulted in substantial difficulties with the mixing process which probably had a negative influence on strength levels after longer curing periods (90 and 365 days curing).
-
After 90 days curing, the solution/ash ratio is more relevant for the strength gain of the mixtures than the activator concentration, which is another significant cost-related advantage.
-
XRD showed that higher dissolutions of the vitreous phase can be related with strength increase and lower activator/ash ratios.
-
Lowering the viscosity of the grout mixtures to similar values to that of cement grout can have a negative effect on final strength, since it demands an increase in the activator/ash ratio. Therefore, it is recommended that a compromise is made between an optimum viscosity level and the lowest activator/ash ratio possible, whenever the viscosity is a key issue for a particular application.
-
Strength results obtained with cement as the binder strongly depend on the W/C ratio, and a significant minimum strength loss of 43 % was produced when the W/C increased from 0.75 to 1.0 or higher. If it becomes necessary to use higher ratios to decrease viscosity, the strength comparison between cement an alkaline activation grouts will become increasingly favourable to the latter.
References
ASTM (2004) ASTM D6910 standard test method for marsh funnel viscosity of clay construction slurries. doi: 10.1520/D6910_D6910M-09
ASTM (2011) D2487-11 standard practice for classification of soils for engineering purposes (Unified Soil Classification System). doi: 10.1520/D2487-11
BSi (1990) BS 1377-2: 1990—methods of test for soils for civil engineering purposes, Part 2: classification tests. British Standards Institution, London 2
Chindaprasirt P, Jaturapitakkul C, Chalee W, Rattanasak U (2009) Comparative study on the characteristics of fly ash and bottom ash geopolymers. Waste Manag (New York, NY) 29(2):539–543
Chindaprasirt P, Chareerat T, Hatanaka S, Cao T (2011) High-strength geopolymer using fine high-calcium fly ash. J Mater Civ Eng 23(3):264
Criado M, Fernández-Jiménez A, de la Torre AG, Aranda MAG, Palomo A (2007) An XRD study of the effect of the SiO2/Na2O ratio on the alkali activation of fly ash. Cem Concr Res 37(5):671–679
Cristelo N, Glendinning S, Jalali S (2009) Sub-bases layers of residual granite soil stabilised with lime. Soils Rocks 32(2):83–88
Cristelo N, Glendinning S, Teixeira Pinto A (2011) Deep soft soil improvement by alkaline activation. Proc ICE Ground Improv 164(2):73–82
Duxson P, Provis JL, Lukey GC, Mallicoat SW, Kriven WM, van Deventer JSJ (2005) Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf A: Physicochem Eng Aspects 269(1–3):47–58
Duxson P, Provis J, Lukey G, Vandeventer J (2007) The role of inorganic polymer technology in the development of “green concrete”. Cem Concr Res 37(12):1590–1597
EN B (1992) 1-1: 2004 Eurocode 2: design of concrete structures. General rules and rules for buildings (3)
Escalante-Garcia JI, Espinoza-Perez LJ, Gorokhovsky A, Gomez-Zamorano LY (2009) Coarse blast furnace slag as a cementitious material, comparative study as a partial replacement of Portland cement and as an alkali activated cement. Constr Build Mater 23(7):2511–2517
Essler R, Yoshida H (2004) Jet grouting. In: Moseley MP, Kirsch K (eds) Ground improvement, 2nd edn. Taylor & Francis, New York, pp 160–196
Fernández-Jiménez A, Palomo A (2003) Characterisation of fly ashes. Potential reactivity as alkaline cements. Fuel 82(18):2259–2265
Hadi NARA, Khoury HN, Suliman MR (2008) Utilization of bituminous limestone ash from EL-LAJJUN area for engineering applications. Acta Geotechnica 3(2):139–151
Hardjito D, Rangan BV (2005) Development and properties of low-calcium fly ash-based geopolymer concrete. Research Report GC 1. Perth
Hassett DJ, Heebink LV (2001) JV Task 13—environmental evaluation for utilization of ash in soil stabilisation, Final report. US Department of Energy, Pittsburgh
Hu M, Zhu X, Long F (2009) Alkali-activated fly ash-based geopolymers with zeolite or bentonite as additives. Cem Concr Compos 31(10):762–768
Janz M, Johansson S-E (2002) The function of different binding agents in deep stabilization. Report 9. Linkoping
Kasama K, Zen K (2007) High-strengthening of cement-treated clay by mechanical dehydration. Soils Found 47(2):171–184
Lee WK, van Deventer J (2004) The interface between natural siliceous aggregates and geopolymers. Cem Concr Res 34(2):195–206
Pacheco-Torgal F, Abdollahnejad Z, Camões AF, Jamshidi M, Ding Y (2012) Durability of alkali-activated binders: a clear advantage over Portland cement or an unproven issue? Constr Build Mater 30:400–405
Palomo A, Grutzeck M (1999) Alkali-activated fly ashes: a cement for the future. Cem Concr Res 29:1323–1329
Phair JW, Van Deventer JSJ (2002) Characterization of fly-ash-based geopolymeric binders activated with sodium aluminate. Ind Eng Chem Res 41(17):4242–4251
Temuujin J, Van Riessen A, MacKenzie KJD (2010) Preparation and characterisation of fly ash based geopolymer mortars. Constr Build Mater 24(10):1906–1910
Terashi M, Kitazume M (2011) QA/QC for deep-mixed ground: current practice and future research needs. Proc ICE Ground Improv 164(3):161–177
Tomlinson MJ (2001) Foundation Design and Construction. Pearson Education, Harlow
Van Jaarsveld JGS, Van Deventer JSJ (1999) Effect of the alkali metal activator on the properties of fly ash-based geopolymers. Ind Eng Chem Res 38(10):3932–3941
Villa C, Pecina ET, Torres R, Gómez L (2010) Geopolymer synthesis using alkaline activation of natural zeolite. Constr Build Mater 24(11):2084–2090
Winnefeld F, Leemann A, Lucuk M, Svoboda P, Neuroth M (2010) Assessment of phase formation in alkali activated low and high calcium fly ashes in building materials. Constr Build Mater 24(6):1086–1093
Xu H, Van Deventer JSJ (2000) The geopolymerisation of alumino-silicate minerals. Int J Miner Process 59:247–266
Xu H, Van Deventer JSJ (2003) Effect of source materials on geopolymerization. Ind Eng Chem Res 42(8):1698–1706
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cristelo, N., Glendinning, S., Fernandes, L. et al. Effects of alkaline-activated fly ash and Portland cement on soft soil stabilisation. Acta Geotech. 8, 395–405 (2013). https://doi.org/10.1007/s11440-012-0200-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11440-012-0200-9