Abstract
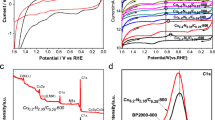
A non-precious metal Co-N/C catalyst for the oxygen reduction reaction (ORR) was synthesized by heating a mechanical mixture of cobalt chloride, urea and acetylene black under a nitrogen atmosphere. The catalyst was characterized by XRD and XPS. The electrocatalytic activity in the ORR was evaluated by linear sweep voltammetry in 0.5 mol L−1 H2SO4 solution. The results show that the Co-N/C catalyst aids the reduction of oxygen. The presence of elemental cobalt in the precursor allows nitrogen atoms to embed themselves in the graphite matrix to form pyridinic and graphitic type C-N structures as the ORR active sites. The effect of heat-treating temperature on the catalytic activity was also investigated. The results also show that the Co-N/C catalyst is most active when pyrolyzed at 600°C. The obtained Co-N/C catalyst loses some activity after initial exposure to the H2SO4 solution because of leaching, but is then stable for up to 20 h immersion. The catalyst is also stable when charged, which is supported by the cyclic voltammetry results.
Similar content being viewed by others
References
Thomas S C, Ren X, Gottesfeld S, et al. Direct methanol fuel cells: Progress in cell performance and cathode research. Electrochim Acta, 2002, 47: 3741–3748
Alexey S, Chan K. Review of non-platinum anode catalysts for DMFC and PEMFC application. Appl Catal B: Environ, 2009, 90: 313–320
Yeager E. Electrocatalysts for O2 reduction. Electrochim Acta, 2002, 29: 1527–1537
Nallathambi V, Lee J W, Kumaraguru S P, et al. Development of high performance carbon composite catalyst for oxygen reduction reaction in PEM Proton Exchange Membrane fuel cells. J Power Sources, 2008, 183: 34–42
Gojkovic S L, Gupta S, Savinell R F. Heat-treated iron(III) tetrame-thoxyphenyl porphyrin chloride supported on high-area carbon as an electrocatalyst for oxygen reduction: Part III. Detection of hydrogen-peroxide during oxygen reduction. Electrochim Acta, 1999, 45: 889–897
Ramya K, Dhathathreyan K S. Direct methanol fuel cells: Determination of fuel crossover in a polymer electrolyte membrane. J Electroanal Chem, 2003, 542: 109–115
Jeon M K, Lee K R, Lee W S, et al. Investigation of Pt/WC/C catalyst for methanol electro-oxidation and oxygen electro-reduction. J Power Sources, 2008, 185: 927–931
Jeyabharathi C, Venkateshkumar P, Mathiyarasu J, et al. Platinum-tin bimetallic nanoparticles for methanol tolerant oxygen-reduction activity. Electrochim Acta, 2008, 54: 448–454
Antolini E, Lopes T, Gonzalez E R. An overview of platinum-based catalysts as methanol-resistant oxygen reduction materials for direct methanol fuel cells. J Alloys Compd, 2008, 461: 253–262
Salvador-Pascual J J, Citalan-Cigarroa S, Solorza-Feria O. Kinetics of oxygen reduction reaction on nanosized Pd electrocatalyst in acid media. J Power Sources, 2007, 172: 229–234
Fu Y, Wei Z D, Chen S G, et al. Synthesis of Pd/TiO2 nanotubes/Ti for oxygen reduction reaction in acidic solution. J Power Sources, 2009, 189: 982–987
Kim J, Momm T, Osaka T. Synthesis of carbon-supported Pd-Sn catalyst by ultrasonic irradiation for oxygen reduction reaction. J Power Sources, 2009, 189: 909–915
Tarasevich M R, Bogdanovskaya V A, Kuznetsova L N, et al. Development of platinum-free catalyst and catalyst with low platinum content for cathodic oxygen reduction in acidic electrolytes. J Appl Electrochem, 2007, 37: 1503–1513
Jasinski R. A new fuel cell catlyst. Nature, 1964, 201: 1212–1213
Zagal J H, Bedioui F, Dodelet J P. N4-Macrocyclic Metal Complexes. New York: Springer, 2006. 45
Faubert G, Cote R, Guay D, et al. Iron catalysts prepared by high-temperature pyrolysis of tetraphenylporphyrins adsorbed on carbon black for oxygen reduction in polymer electrolyte fuel cells. Electrochim Acta, 1998, 43: 341–353
Lefevre M, Dodelet J P. O2 reduction in PEM fuel cells: Activity and active site structural information for catalysts obtained by the pyrolysis at high temperature of Fe precursors. J Phys Chem B, 2000, 104: 11238–11247
Lefevre M, Dodelet J P, Bertrand P. Molecular oxygen reduction in PEM fuel cells: Evidence for the simultaneous presence of two active sites in Fe-based catalysts. J Phys Chem B, 2002, 106: 8705–8713
Schulenburg H, Stankov S, Schulnemann V, et al. Catalysts for the oxygen reduction from heat-treated iron(III) tetramethoxyphenylporphyrin chloride: Structure and stability of active sites. J Phys Chem B, 2003, 107: 9034–9041
Niwa H, Horiba K, Harada Y, et al. X-ray absorption analysis of nitrogen contribution to oxygen reduction reaction in carbon alloy cathode catalysts for polymer electrolyte fuel cells. J Power Sources, 2009, 187: 93–97
Wang P, Ma Z, Zhao Z, et al. Oxygen reduction on the electrocatalysts based on pyrolyzed non-noble metal/poly-o-phenylenediamine/carbon black composites: New insight into the active sites. J Electroanal Chem, 2007, 611: 87–95
Matter P H, Wang E, Arias M, et al. Oxygen reduction reaction activity and surface properties of nanostructured nitrogen-containing carbon. J Mol Catal A: Chem, 2007, 264: 73–81
Zhang H J, Yuan X, Wen W, et al. Electrochemical performance of a novel CoTETA/C catalyst for the oxygen reduction reaction. Electrochem Commun, 2009, 11: 206–208
Matter P H, Zhang L, Ozkan U S. The role of nanostructure in nitrogen-containing carbon catalysts for the oxygen reduction reaction. J Catal, 2006, 239: 83–96
Bezerra C W B, Zhang L, Lee K, et al. Novel carbon-supported Fe-N electrocatalysts synthesized through heat treatment of iron tripyridyl triazine complexes for the PEM fuel cell oxygen reduction reaction. Electrochim Acta, 2008, 53: 7703–7710
Herranz J, Lefevre M, Larouche N, et al. Step-by-step synthesis of non-noble metal electrocatalysts for O2 reduction under proton exchange membrane fuel cell conditions. J Phys Chem C, 2007, 111: 19033–19042
Charreteur F, Jaouen F, Ruggeri S, et al. Fe/N/C non-precious catalysts for PEM fuel cells: Influence of the structural parameters of pristine commercial carbon blacks on their activity for oxygen reduction. Electrochim Acta, 2008, 53: 2925–2938
Bashyam R, Zelenay P. A class of non-precious metal composite catalysts for fuel cells. Nature, 2006, 443: 63–66
Millan W M, Thompson T T, Arriaga L G, et al. Characterization of composite materials of electroconductive polymer and cobalt as electrocatalysts for the oxygen reduction reaction. Int J Hydrogen Energ, 2009, 34: 694–702
Lee K, Zhang L, Lui H, et al. Oxygen reduction reaction (ORR) catalyzed by carbon-supported cobalt polypyrrole (Co-PPy/C) electrocatalysts. Electrochim Acta, 2009, 54: 4704–4711
Garsuch A, Yang R, Bonakdarpour A, et al. The effect of boron doping into Co-C-N and Fe-C-N electrocatalysts on the oxygen reduction reaction. Electrochim Acta, 2008, 53: 2423–2429
Yang R, Stevens K, Dahn J R. Investigation of activity of sputtered transition-metal (TM)-C-N (TM=V, Cr, Mn, Co, Ni) catalysts for oxygen reduction reaction. J Electrochem Soc, 2008, 155: B79–B91
Lalande G, Cote R, Tamizhmani G, et al. Physical, chemnical and electrochemical characterization of heat-treated tetracarboxylic cobalt phthalocyanine adsorbed on carbon black as electrocatalyst for oxygen reduction in polymer electrolyte fuel cells. Electrochim Acta, 1995, 40: 2635–2646
Maldonado S, Stevenson K J. Influence of nitrogen doping on oxygen reduction electrocatalysis at carbon nanofiber electrodes. J Phys Chem B, 2005, 109: 4707–4716
Subramanian N P, Li X, Nallathambi V, et al. Nitrogen-modified carbon-based catalysts for oxygen reduction reaction in polymer electrolyte membrane fuel cells. J Power Sources, 2009, 188: 38–44
Nallathambi V, Lee J W, Kumaraguru S P, et al. Development of high performance carbon composite catalyst for oxygen reduction reaction in PEM Proton Exchange Membrane fuel cells. J Power Sources, 2008, 183: 34–42
Author information
Authors and Affiliations
Corresponding authors
Additional information
This article is published with open access at Springerlink.com
About this article
Cite this article
Si, Y., Chen, C., Yin, W. et al. The synthesis and characterization of a Co-N/C composite catalyst for the oxygen reduction reaction in acidic solution. Chin. Sci. Bull. 56, 1086–1091 (2011). https://doi.org/10.1007/s11434-011-4434-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-011-4434-y