Abstract
Pigmented villonodular synovitis (PVNS) can recur after complete synovectomy and even after total joint replacement. In the authors’ experience, there is a misconception that MRI may not be useful to diagnose PVNS in the setting of a total joint replacement due to dephasing artifact from metal. While there are case reports of PVNS in patients with total joint replacement diagnosed surgically, to our knowledge, diagnosis of recurrent PVNS by MRI following total joint replacement has not been reported. This report illustrates the utility of MRI in the diagnosis of recurrent PVNS following total joint replacement by reviewing two cases of pathologically correlated PVNS recurrence following arthroplasty, and two cases in which PVNS recurrence is strongly suspected, though pathological correlation is not available.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pigmented villonodular synovitis (PVNS) can be radiographically occult. The most common findings are pressure erosions on both sides of the joint. The joint is preserved early in the disease with uniform joint space narrowing occurring later [1]. Other plain film findings include soft-tissue swelling, joint effusion, and degenerative joint disease [8]. These findings are often non-specific and, as such, MRI is the imaging modality of choice to diagnose PVNS. On MRI, the diffuse form of PVNS appears as a diffuse, nodular, plaque-like thickening of the synovium with heterogeneous low to intermediate signal intensity on T1-weighted imaging and low signal intensity on T2-weighted imaging, owing to the presence of hemosiderin. The focal form appears as a low signal mass on all imaging sequences [1, 11]. On gradient echo imaging, exaggeration of low signal intensity distortion related to magnetic field inhomogeneity from the deposition of the paramagnetic hemosiderin occurs and results in amplified signal dropout [signal void] in the regions of low signal seen on T1- and T2-weighted images. This appearance of marked signal void is known as susceptibility artifact or blooming artifact and is characteristic of PVNS [11]. While a degree of signal dropout occurs on all imaging sequences in the presence of hemosiderin, the effect is amplified on gradient echo sequences as no refocusing pulse is used [2].
Gross pathologic findings of PVNS include a well-defined, lobulated, nodular soft-tissue mass in the focal form or synovial hypertrophy with irregular papillary or villous projections and larger nodular or villonodular protrusions in the diffuse form. The soft-tissue mass or synovium contains mottled yellow to dark brown areas of discoloration depending on the amount of hemosiderin and xanthoma cells. Microscopically, focal and diffuse PVNS contains mononuclear histiocytoid cells with reniform nuclei and eosinophilic cytoplasm, mixed with multinucleated giant cells and xanthoma cells. Hemosiderin deposition is present in various amounts [11].
PVNS can recur after complete synovectomy [10] and even after total joint replacement [3, 9], which is often performed in cases where PVNS has contributed to advanced degenerative arthrosis [11]. While MRI is frequently used to diagnose PVNS in the native joint, in the authors experience, there is a misconception that MRI is not useful to diagnose PVNS in the setting of a total joint replacement due to susceptibility artifact from metal. However, current metal artifact reduction MRI sequences allow for the diagnostic evaluation of the peri-prosthetic soft tissues [12], allowing visualization of the characteristic findings of PVNS, namely low signal proliferative nodular synovial hypertrophy and low signal masses. While gradient echo sequences exacerbate the artifact associated with metal, the effects of magnetic susceptibility artifact (blooming) from hemosiderin deposition in PVNS can be detected on non-gradient sequences.
There are case reports of recurrent PVNS diagnosed with arthroscopy or with arthroplasty revision [3, 9]. To our knowledge, diagnosis of recurrent PVNS by MRI in cases of total joint replacement has not been reported. This report illustrates the utility of MRI in the diagnosis of recurrent PVNS following total joint replacement in two cases of pathologically correlated PVNS recurrence, and two cases in which PVNS recurrence is strongly suspected based on the MRI, though pathological correlation is not available.
Case Reports
Case 1
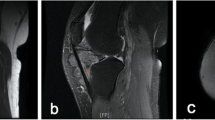
A 19-year-old female underwent total hip replacement with a ceramic on ceramic prosthesis, at an outside institution, for advanced arthritis secondary to PVNS. For 8 years following hip replacement, the patient enjoyed a pain-free athletic lifestyle. She then began to notice a tendon shifting sensation, stiffness, and discomfort in her left hip and tried physical therapy with no success. Plain radiographs were taken at this time and yearly thereafter for 3 years. The radiographs were noncontributory and the initial clinical impression did not include recurrent PVNS as a possibility. The patient's clinical course did not improve and after 3 years following onset of symptoms, the patient was referred to our institution for MRI of the arthroplasty. The MRI demonstrated marked distention of the pseudocapsule by hypointense particulate debris, causing an indolent pattern of erosion of the anterior margin of the proximal femur. There was considerable bone resorption around the stem of the femoral component and periacetabular osteolysis. The pseudocapsule had expanded and decompressed into the iliopsoas bursa as well as through the capsule into the adductor group creating a large heterogeneously hypointense soft-tissue mass. A gradient-recalled sequence was performed through the mass demonstrating blooming (Fig. 1). The imaging findings were consistent with PVNS. While hemorrhage can cause hemosiderin deposition and thus blooming, hemorrhage was excluded in this case due to the uniform, dense, mass-like nature of the findings. An acute hematoma would be of more heterogeneous signal intensity and hemosiderin deposition from previous hemorrhage would not appear mass like or uniform. The lack of a history of a bleeding diathesis also made periarticular hemorrhage highly unlikely. Particle disease and infection would be less likely due to the presence of blooming artifact. Needle biopsy was performed and the pathology revealed predominantly skeletal muscle with a focus of epithelioid cells with hemosiderin pigment. The cytology showed neoplastic cells with hemosiderin, consistent with PVNS (Fig. 2). The patient then underwent surgical resection of the soft-tissue mass with debridement of the pseudocapsule, requiring an 8-inch incision and resulting in 1,200 ml of blood loss related to the extensive and infiltrative nature of her recurrence. Three months following the surgical resection, the patient is recovering well. The patient is scheduled for a follow-up MRI 6 to 8 months following the surgical resection.
MRI images from case 1. a A coronal proton density image demonstrates expansion of the pseudocapsule by low signal particulate debris partially decompressing into the iliopsoas bursa as well as into the adductor group illustrated by a large heterogeneously hypointense soft-tissue mass (asterisks). b An axial proton density demonstrating the large heterogeneously hypointense soft-tissue mass caused by decompression of the pseudocapsule. c An axial gradient-recalled sequence through the soft-tissue mass shows marked signal void (blooming) in the region of low signal seen on the other images (asterisk). The degree of blooming is more pronounced than on the proton density images seen in a and b as no refocusing pulse is used in gradient echo imaging
Image-guided fine needle aspiration biopsy from case 1. Flat sheets and papillary clusters of small, bland, spindled, and epithelioid mononuclear cells composed the majority of the highly cellular specimen. Brown hemosiderin pigment was observed in the cytoplasm of these cells (alcohol-fixed, Papanicolaou-stained smear, ×200 magnification; inset ×400; Slide courtsey of Dr. Carlie Sigel of Memorial Sloan-Kettering Cancer Center)
Case 2
A 49-year-old female with a history of juvenile arthritis since age two underwent left cemented total knee replacement for severe arthrosis. Her arthritis was well controlled at the time. An inflamed synovium most consistent with diffuse PVNS was discovered during the surgery. Grossly, the surgical specimens were reddish brown, focally finely fibrillated, and focally nodular with regions of yellow discoloration. The synovium demonstrated invasion into the periphery of the articular surface and subchondral bone. Microscopically, the synovium showed coarse and delicate papillary hypertrophy and a solid cellular infiltrate of mononuclear epithelioid cells, giant cells, and xanthoma cells with variable degrees of hemosidersosis. These findings were consistent with the diagnosis of PVNS. The patient recovered and was asymptomatic until 2 years later when she started to develop joint stiffness and swelling. She was treated with an intraarticular anesthetic and steroid injection without symptomatic relief. Radiographs revealed a soft-tissue mass, osteopenia, and questionable erosions around both components. MRI examination was performed revealing a nodular mass with blooming and dephasing artifact on gradient-recalled imaging, suspicious for PVNS. Based on the clinical history, presentation and MRI findings PVNS recurrence was suspected. As in case 1, the presence of blooming made particle disease highly unlikely while the patient's history made other causes of hemosiderin deposition unlikely. The patient underwent arthroscopy demonstrating what was described as “burnt out PVNS” surrounding the tibial tray and patella. Arthroscopic synovectomy, scar excision, and a lateral release were performed. The gross pathologic specimen consisted of white, focally tan, and orange-brown soft tissue. Microscopically, the resection contained hypertrophic synovium predominantly composed of polygonal cells with eosinophilic cytoplasm and round nuclei, occasional multinucleated giant cells, hemosiderin deposits, and fibrionous exudate compatible with PVNS. Subsequent to the synovectomy, the swelling decreased and the patient recovered her range of motion. Three years later, the patient again developed swelling and pain in her left knee. Follow-up MRI of the knee was performed, demonstrating exuberant hypointense synovium throughout the joint recesses highly suggestive of PVNS (Fig. 3). Pathological follow-up of this recurrence was not available.
MRI images from Case 2. a Sagittal inversion recovery sequence demonstrating total knee prosthesis with exuberant, nodular synovial proliferation both anterior to the femoral component (asterisk) and posterior to tibial component in a suspected popliteal cyst (arrow). Note the fluid signal (bright signal) intensity surrounding the tibial component reflecting peri-prosthetic bone resorption and component loosening. Also note several foci of signal void (blooming) as indicated by the arrowheads. Figure 1b Sagittal proton density image demonstrating marked heterogeneous signal hypointensity in the areas of synovial proliferation (seen in Fig. 2a) with low to intermediate signal intensity, characteristic of PVNS (arrows). Note the ability to clearly define the peri-prosthetic soft tissues with the metal artifact reduction techniques used on the proton density images
Case 3
A 59-year-old female with a history of seronegative spondyloarthropathy affecting her hand had an MRI of her knee performed due to increasing knee pain and a posterior knee mass. The MRI revealed a heterogeneous mass of predominately intermediate signal, demonstrating blooming artifact on gradient echo imaging. The patient was diagnosed with PVNS of her left knee. She received a synovectomy and total knee arthroplasty. The surgical specimen revealed a soft-tissue mass with multiple fragments of yellow and rusty brown synovium that showed papillary hypertrophy. There were also regions of focal hemorrhage in the resected soft-tissue mass. The hyperplastic synovium microscopically was composed of mononuclear polygonal cells with abundant eosinophilic cytoplasm and round nuclei with interspersed giant cells, collections of lipid laden macrophages and focal hemosiderin deposition. This pathology is consistent with PVNS. She received routine follow-up MRI examinations at Hospital for Special Surgery (HSS) at 2-year intervals following her surgery. Four years following the surgery, PVNS recurrence was suspected based on the MRI findings of a proliferative synovitis distending the synovial recesses, with extension into a large collection at the medial margin of the knee that communicated with the semimembranous bursa and containing large foci of diminished signal intensity. The large foci of low signal combined with the relative lack of peri-prosthetic osteolysis are more in keeping with recurrent PVNS rather than particle disease. Ongoing MRI follow-up has shown only minimal change over the past 2 years. Her most recent MRI is shown (Fig. 4). The patient has thus far received conservative management. Pathologic confirmation of PVNS recurrence is not available.
MR images from Case 3. a An axial proton density and b a coronal proton density image shows a markedly proliferative synovium of low signal with synovial expansion (arrows) and foci of blooming (arrowheads). Osteolysis was not a prominant feature. This finding suggests recurrent PVNS. Once again note the ability to clearly define the peri-prosthetic soft tissues on these images
Case 4
A 25-year-old otherwise healthy female was diagnosed with PVNS following an MRI at an outside institution which demonstrated synovial proliferation with subchondral cysts of the acetabulum and femoral head as well as signal dropout on T1- and T2-weighted images. The MRI was followed by a percutaneous biopsy, which was also consistent with PVNS. She received a synovectomy and total hip arthroplasty at the outside institution. She was referred to HSS 2 years later for recurrent pain. Follow-up MRI demonstrated a marked bulky and dense synovitis of low signal on all sequences (Fig. 5a, b), as well as an extracapsular area of low signal intensity material associated with erosion of the anterior inferior iliac spine (Fig. 5c). While a bulky synovitis and cortical erosion can be seen in particle disease, the combination of the two, along with the low signal intensity proliferative synovitis and patient's history, makes PVNS a far more likely diagnosis. Exploration and synovectomy was planned. Pathological confirmation of PVNS recurrence is not available at this time.
MRI images from case 4. a An axial proton density image and b a coronal proton density image show a low signal intensity bulky and dense synovitis consistent with PVNS (arrows). With foci of signal void (blooming) as indicated by the arrowheads. c An extracapsular focus of tissue of low signal intensity with associated erosion of cortical bone at the anterior inferior iliac spine consistent with PVNS
Discussion
Pigmented villonodular synovitis is a benign, monoarticular proliferation of the synovium involving joints, bursae, and tendon sheaths. PVNS can be focal or diffuse, with the diffuse form more prone to recur following surgical resection and synovectomy, yielding a recurrence rate of up to 30 % [5]. The abnormal synovium is prone to hemorrhage with minor trauma, often resulting in a hemorrhagic effusion and subsequent hemosiderin deposition. Although neoplasia, trauma, and inflammation have all been suggested as possibly etiologies, the true cause of PVNS remains unknown [13].
While synovectomy is the most common treatment for PVNS, total joint replacement along with synovectomy is considered when there is advanced joint degeneration [14]. In cases of joint replacement, particle disease is an important differential diagnosis. Particle disease can present with a soft-tissue mass, [6]; however, this is much less common than in PVNS. The predominant feature of particle disease is progressive bone resorption surrounding the arthroplasty components [4, 6]. In PVNS, loosening of the arthroplasty components can occur but is generally less prominent than with particle disease. Erosion of cortical bone occurs with PVNS while particle disease preferentially extends from the margins of the hardware and medullary space and erodes eccentrically into cortical bone. The synovitis of particle disease is a reactive synovitis and is not as thick, nodular, and exuberant as in PVNS [4]. Hemosiderin deposition is not typically a feature of particle disease and as a result susceptibility artifact and blooming are not usually seen.
Intraarticular hemorrhage can result in hemosiderin deposition and the subsequent appearance of blooming artifact; however, spontaneous intraarticular hemorrhage in the absence of a bleeding diathesis is very rare and can generally be excluded in the absence of an appropriate history. Additionally, on imaging, a bulky nodular synovitis should not be present in these conditions. If the hemorrhage is recent, the signal characteristics will be more heterogeneous than that of PVNS. If intraarticular hemorrhage is a differential possibility following imaging, a distinction can be made pathologically between hemorrhage from hemophilia, hemosidersosis, and other conditions causing intraarticular hemorrhage and PVNS as other conditions will result in hemosiderin largely confined to the synovial cells and macrophages rather than the diffuse hemosiderin deposition seen in PVNS. Additionally, the giant cells and histiocytes seen in PVNS will not be present [1].
In the past, MRI was considered of limited utility following arthroplasty because of severe image degradation caused by metallic components [12]. Such image degradation by susceptibility artifact made it difficult to diagnose recurrent PVNS by MRI. However, given the advances in MRI technology and pulse sequence design in recent years, the amount of artifact has been significantly reduced, allowing for the visualization of the peri-prosthetic soft tissues [12]. Despite the limitations of gradient echo sequences with metallic arthroplasty components, MRI can provide valuable information concerning the recurrence of PVNS with a high degree of confidence, based on the findings of a mass or diffuse synovitis of low signal intensity on all sequences. Furthermore, with the advent of novel MR pulse sequences such as the multi-acquisition with variable resonance image combination and slice-encoding metal artifact correction sequences, which allow for improved visualization of peri-prosthetic tissues, MRI can become the modality of choice for evaluation of recurrent PVNS in the setting of joint replacement [7, 8].
While in the past, MRI was considered of limited utility following arthroplasty because of severe image degradation caused by metallic components, advances in MRI technology and pulse sequence design have resulted in a marked reduction in the amount of artifact from metal. Today, visualization of the peri-prosthetic soft tissues allows diagnosis of recurrent of PVNS with a high degree of confidence, based on the findings of a mass or diffuse synovitis of low signal intensity on all sequences.
References
Al-Nakshabandi, N. A.; Ryan, A. G.; Choudur, H.; Torreggiani, W.; Nicoloau, S.; Munk, P.L. E-mail: plmunk@interchange.ubc.ca; Al-Ismail, K. Pigmented villonodular synovitis. Clin Radiol. 2004 May;59(5):414–20.
Bitar, R, Leung, G, Perng, R, Tadros, S, Moody, A,R, Sarrazin, J, McGregor, C, Christakis, M, Symons, S, Nelson, A, Roberts TP. Radiographics. 2006; 26:513–537
Chung BJ, Park YB. Pigmented villonodular synovitis after TKA associated with tibial component loosening. Orthopedics. 2011 Aug 8;34(8):e418–20.
Cooper J.H., Ranawat A.S., Potter H.G., Foo L.F., Koob T.W., Ranawat C.S. Eary reactive synovitis and osteolysis after total hip arthorplasty. Clin Orthop Relat Res. 2010 Dec;468(12):3278–85.
Granowitz SP, D. J. (1976). The pathogenesis and long-term end results of pigmented villonodular synovitis. Clin Orthop Relat Res, 114: 335–351.
Fabbri N, Rustemi E, Masetti C, Kreshak J, Gambarotti M, Vanel D, Toni A, Mercuri M. Severe osteolysis and soft tissue mass around total hip arthroplasty: description of four cases and review of the literature with respect to clinico-radiographic and pathologic differential diagnosis. Eur J Radiol. 2011 Jan;77(1):43–50.
Hayter CL, Koff MF, Shah P, Koch KM, Miller TT, Potter HG. MRI after arthroplasty: comparison of MAVRIC and conventional fast spin-echo techniques. AJR Am J Roentgenol. 2011 Sep;197(3):W405–11.
Koch KM, Brau AC, Chen W, Gold GE, Hargreaves BA, Koff M, McKinnon GC, Potter HG, King KF. Imaging near metal with a MAVRIC-SEMAC hybrid. Magn Reson Med. 2011 Jan;65(1):71–82.
Ma X, Xia C, Wang L, Zhao L, Liu H, He J. An unusual case of pigmented villonodular synovitis 14 years after total hip arthroplasty. J Arthroplasty. 2011 Feb;26(2):339.e5–6
Mendenhall WM, Mendenhall CM, Reith JD, Scarborough MT, Gibbs CP, Mendenhall NP. Pigmented villonodular synovitis Am J Clin Oncol. 2006 Dec;29(6):548–50.
Murphey MD, R. J.-S. (2008). From the archives of the AFIP: pigmented villonodular synovitis: radiologic-pathologic correlation. Radiographics, 28: 1493–1518.
Potter HG, Nestor BJ, Sofka CM, Ho ST, Peters LE, Salvati EA. Magnetic resonance imaging after total hip arthroplasty: evaluation of periprosthetic soft tissue.J Bone Joint Surg Am. 2004 Sep;86-A(9):1947–54.
Ottaviani S, Ayral X, Dougados M, Gossec L. Pigmented villonodular synovitis: a retrospective single-center study of 122 cases and review of the literature. Semin Arthritis Rheum. 2011 Jun;40(6):539–46
Yoo JJ, Kwon YS, Koo KH, Yoon KS, Min BW, Kim HJ Cementless total hip arthroplasty performed in patients with pigmented villonodular synovitis. J Arthroplasty. 2010 Jun;25(4):552–7
Disclosures
Each author certifies that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the reporting of this case, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Friedman, T., Chen, T. & Chang, A. MRI Diagnosis of Recurrent Pigmented Villonodular Synovitis Following Total Joint Arthroplasty. HSS Jrnl 9, 100–105 (2013). https://doi.org/10.1007/s11420-012-9283-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11420-012-9283-y