Abstract
PTH 1-34, an active form of parathyroid hormone, has been shown to enhance osteoblastic bone formation when administered as a daily subcutaneous injection. The effect of the intermittent administration of PTH (1-34) is an uncoupling of bone turnover with an increase in bone mass and density and decrease in risk of vertebral and nonvertebral fractures. While PTH (1-34) has been used clinically to increase bone mass and reduce fracture risk in postmenopausal women with osteoporosis, there is increasing evidence that PTH (1-34) may promote fracture healing. Animal studies have demonstrated accelerated callus formation with enhanced remodeling and biomechanical properties of the healing fracture. Given these effects, PTH (1-34) will likely be used clinically to enhance fracture union in poor healing situations such as osteoporosis and recalcitrant nonunions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Teriparatide (Forteo®; Eli Lilly, Indianapolis, IN, USA) is a synthetic polypeptide hormone that contains the 1-34 amino acid fragment of recombinant human parathyroid hormone (PTH 1-34). It has been approved by the US Food and Drug Administration for the treatment of postmenopausal women with osteoporosis who are at high risk for sustaining a fragility fracture [1]. Daily treatments with either 20 µg or 40 µg of PTH (1-34) resulted in dose-dependent increases in bone mineral density in the lumbar spine and femoral neck in osteoporotic female and male patients [1–3]. Recent animal studies have suggested that PTH (1-34), when administered intermittently, may be an effective agent to enhance fracture healing [4–12]. There is increasing evidence that treatment with PTH (1-34) enhances callus formation by stimulating the proliferation and differentiation of osteoprogenitor cells and increasing the production of bone matrix proteins [13, 14]. Callus forms earlier in response to PTH treatment and demonstrates superior biomechanical properties compared with controls. These studies suggest a potential clinical role for PTH (1-34) in accelerating fracture healing and treating recalcitrant nonunions.
Basic science
There has been increasing evidence in the basic science literature linking parathyroid hormone (PTH) signaling to osteogenesis. Much of this information has come from studies on the parathyroid hormone-related protein (PTHrP). This protein, originally identified as a factor secreted by several tumors and responsible for the hypercalcemia seen in malignancy, has been shown to be produced in bone and to activate the same receptor as parathyroid hormone (PTH/PTHrP receptor) [13, 14]. Both PTH and PTHrP have anabolic effects on bone in rats and humans [15–17]. Both PTHrP and the PTH/PTHrP receptor are present in chondrocytes and osteoblasts during fracture healing suggesting a role for PTH signaling in this process [18].
PTHrP is a product of the Indian hedgehog (Ihh) pathway, a widely conserved developmental pathway which regulates the differentiation of proliferating chondrocytes into hypertrophic chondrocytes, a necessary step in endochondral ossification [19–22]. There is strong evidence that this Ihh-mediated block in hypertrophic differentiation is executed by PTHrP [22]. Furthermore, loss of function mutations in the PTH/PTHrP receptor, the common receptor for PTH and PTHrP signaling, has also confirmed a role for this signaling pathway in hypertrophic cartilage differentiation and endochondral ossification. Inactivation of the receptor in mice resulted in deregulation of hypertrophic cartilage differentiation with premature differentiation of proliferating chondrocytes into hypertrophic chondrocytes [19–21]suggesting that PTH/PTHrP signaling slows the differentiation of proliferating chondrocytes.
In addition to roles in slowing terminal differentiation of hypertrophic chondrocytes, there is increasing evidence that PTH stimulates the differentiation of mesenchymal cells into osteoblasts. PTH stimulates the expression of osteoblast-specific transcription factors, such as runx2 and osteoblast-specific proteins such as osteocalcin. This signaling may be responsible for an intramembranous type of ossification with PTH directly stimulating osteoblast differentiation, bypassing a cartilage intermediate [13, 14].
Bone density studies in humans
The amino terminal active form of human parathyroid hormone (PTH (1-34); Teriparatide; Forteo®; Eli Lilly, Indianapolis, IN, USA), has been approved by the US Food and Drug Administration for the treatment of postmenopausal women with osteoporosis who are at high risk for sustaining a fragility fracture [1]. Daily treatments with either 20 µg or 40 µg of PTH (1-34) resulted in dose-dependent increases in bone mineral density in the lumbar spine and femoral neck in osteoporotic female patients [1, 3]. The relative risk of sustaining a vertebral fracture was 0.35 (20 µg PTH 1-34) and 0.31 (40 µg PTH 1-34) compared to placebo. The relative risk of sustaining a nonvertebral fracture was 0.47 (20 µg PTH 1-34) and 0.46 (40 µg PTH 1-34) compared to placebo [3]. PTH (1-34) has also been shown to increase the bone mineral density of men with osteoporosis [2].
PTH (1-34) has an anabolic effect on both cortical and cancellous bone with an increase in periosteal, cortical, and trabecular thickness [23–25]. Bone histomorphometry has demonstrated at least fourfold increase in bone formation on the cancellous, endocortical, and periosteal surfaces in postmenopausal women with osteoporosis treated with 50 µg PTH (1-34) compared to controls [24]. Compared to controls, PTH (1-34) treatment increased mineralized perimeter, mineralized apposition rate, and bone formation rate by 659%, 143%, and 300%, respectively. This increased periosteal bone formation may contribute to an increase in bone diameter and cross-sectional moment of inertia [24].
The clinical trials in humans noted above, clearly demonstrate an anabolic effect of PTH signaling (mediated either by PTH or PTHrP) on bone formation [2, 3, 15–17, 23, 25]. The basic science studies suggest at least two mechanisms for this: (1) stimulation of endochondral ossification, perhaps by slowing hypertrophic chondrocyte differentiation and thus maintaining a pool of proliferative chondrocytes and (2) stimulation of mesenchymal cell differentiation into osteoblasts [14]. These mechanisms and the anabolic effect of PTH (1-34) on bone density and periosteal bone formation in osteoporotic patients suggest a role for this agent in accelerating fracture healing.
Fracture healing studies in animals
Effects of PTH (1-34) on the biomechanical properties of healing fractures
There have been several recent studies using rat diaphyseal fracture models to investigate the effectiveness of PTH (1-34) in enhancing fracture healing [4–12]. Holzer and colleagues created closed mid-diaphyseal femur fractures in 20 young (3-month-old) male rats and stabilized these with a retrograde intramedullary pin. Half of the animals received either a daily subcutaneous injection of delivery vehicle (0.9% saline) or 80 µg/kg PTH (1-34). At 3 weeks, the group treated with PTH (1-34) demonstrated significant increases in callus area and strength [8]. Fractures treated with vehicle demonstrated a central fibrous union, while PTH (1-34) treated fractures demonstrated a narrow cartilaginous or fibrous union. In some cases, a central island of new bone was noted to bridge the fracture site. The femoral marrow cavity contained trabecular bone and endosteal new bone suggesting an osteogenic response to PTH (1-34) [8].
Investigations of larger doses of PTH and longer follow-up periods after fracture have demonstrated dose-dependent increases in callus formation and mechanical strength of healed fractures; these properties have been maintained at longer follow-up [4–7, 9]. In a rat tibial shaft fracture model, 200 µg/kg of PTH (1-34) increased the ultimate load and external callus volume of fractures by 175% and 72%, respectively after 40 days of healing. Bone mineral content increased by 108% at 40 days [5]. At 8 weeks after tibial shaft fracture in old rats treated with PTH 200 µg/kg of PTH (1-34), ultimate load, external callus volume, and bone mineral content of fractures increased by 270%, 135%, and 169%, respectively [6].
Similar results were observed in fracture models in which osteoporosis was induced either by ovariectomy or corticosteroid treatment [9, 26]. At 3-months post-ovariectomy, bilateral tibial shaft fractures were created in rats and stabilized with intramedullary Kirschner wires. Saline, 17-estradiol, or recombinant human PTH (1-84) was administered once daily for 1 month during fracture healing. PTH treatment resulted in an increase in the morphologic and biomechanical properties of the fracture callus; this effect was not observed in the group treated with 17-estradiol or saline [9]. RS-66271, a PTHrP analog, has been demonstrated to enhance fracture healing in rabbits on corticosteroid therapy [26]. A 1-mm defect was surgically created in rabbit ulnae; healing was delayed by daily injections of prednisone (0.15 mg/kg) beginning 2 months prior to surgery and continuing throughout fracture healing. RS-66271 (0.01 mg/kg) injected subcutaneously on a daily basis, starting 1 day after surgery demonstrated union in nine of ten ulnae at 6 weeks after osteotomy; only two of ten limbs in the control group (normal saline) achieved union at this time point. Ulnae in the PTHrP-analog-treated group showed increased radiodensity, callus size, and stiffness compared to controls [26]. These findings provide support for the effectiveness of PTH signaling in treating osteoporotic fractures.
Potential mechanisms of PTH (1-34) action on healing fractures
Several studies have made observations suggesting potential mechanisms by which PTH (1-34) may enhance fracture healing. In a rat femoral shaft fracture model, 10 µg/kg of PTH (1-34) increased bone mineral content, bone mineral density, and ultimate load to failure in healing fracture calluses at 28 and 42 days after fracture. Periosteal callus formation occurred earlier in the PTH-treated group than in the control group with visible callus formation by 7 days post-fracture [11]. The callus of the PTH-treated group demonstrated an increased number of proliferating osteoprogenitor cells at day 2. Healing fractures from the PTH-treated group demonstrated increased expression of mRNAs for alkaline phosphatase, type I collagen, and the bone matrix proteins osteocalcin and osteonectin [11]. Taken together, these data suggests that PTH (1-34) enhances fracture healing by stimulating the proliferation of mesenchymal cells and their differentiation into matrix-producing osteoblasts [11].
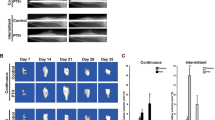
Studies on the rat femur fracture model have also provided evidence that PTH (1-34) may stimulate endochondral ossification by increasing chondrogenesis [4, 12]. Alkhiary and colleagues generated femoral shaft fractures in 270 rats and treated them with daily subcutaneous injections of 5 or 30 µg/kg of PTH (1-34) or vehicle. At 3 weeks post-fracture, plain radiographs of callus from the groups treated with 30 µg/kg of PTH (1-34) demonstrated increased bony bridging over controls (Fig. 1). Cartilage volume, torsional strength, stiffness, bone mineral content, and bone mineral density were maintained at 12 weeks post-fracture [4]. Interestingly, a marked increase in cartilage staining at 3 weeks with the 30 µg/kg dose of PTH (1-34) was followed by a reduction in cartilage staining at 5 weeks suggesting that chondrogenesis occurs earlier than normal with this dose [4]. Similarly, Nakazawa and colleagues have shown in this rat femur fracture model that at 2 weeks after fracture the cartilage area in the callus was significantly increased in the PTH (1-34)-treated group compared to the control (normal saline) group; this increase is not observed at 3 or 4 weeks. In addition, the cartilage transcription factor sox-9 was upregulated in the PTH (1-34)-treated group compared to control; this elevation is not observed at 2 weeks [12]. These data collectively suggest that PTH (1-34) promotes early and transient chondrogenesis and an acceleration of endochondral bone formation.
Radiographs of rat femur fracture calluses from three groups: control (normal saline), 5 µg/kg of PTH and 30 µg/kg of PTH at 21, 35, and 84 days after the generation of closed femur fractures and stabilization with a retrograde intramedullary pin. Bony bridging is seen at 21 days in the group treated with 30 µg/kg of PTH. Osseous bridging is seen in all three groups at day 35 but more abundant callus is seen in the groups treated with PTH. Increased callus remodeling is noted at day 35 in the group treated with 30 µg/kg of PTH. (Reprinted with permission from The Journal of Bone and Joint Surgery, Inc)
Acceleration in bone remodeling from woven to lamellar bone has also been identified as a potential mechanism by which PTH (1-34) accelerates fracture healing. Using the rat femur fracture model, Komatsubara and colleagues have noted that PTH (1-34)-treated rats demonstrated increased lamellar bone formation compared to controls. A dose of 30 µg/kg increased percent cortical shell formation and ultimate load to failure at 3 months compared to controls [10].
Studies in postmenopausal women with osteoporosis have demonstrated that PTH (1-34) increases cancellous, endocortical, and periosteal bone formation. During fracture healing, increases in the mineralized apposition rate and periosteal bone formation rate may contribute to periosteal callus formation and ossification; this in turn will increase in bone diameter and cross-sectional area providing enhanced stability [24].
The basic science studies use doses of PTH several fold greater than the doses recommended for humans (20–40 µg). While the animal data suggest that larger doses correlate with increases in callus volume, bone density, and biomechanical properties, it is difficult to extrapolate these doses to humans. The bioavailability of PTH (1-34) in animal studies is not clear as we lack clear information on the presence, numbers, and activity of PTH receptors in these species. Furthermore, it is unclear if species differences in PTH (1-34) and receptors contribute to signaling inefficiency. One would suspect given lack of 100% conservation of receptors and signaling pathway components that human recombinant PTH (1-34) would less efficiently bind PTH receptors and transduce the PTH (1-34) signal in rats compared to humans [4, 12]. Higher doses of human PTH (1-34), as used in the animal studies, may overcome the species differences to exert a measurable effect on bone density and strength. In humans, the limitation to increasing doses is safety; it is unclear if higher doses (greater than 40 µg) will proportionally increase bone density and reduce fracture risk without increasing the incidence of adverse effects.
Side effects and contraindications
Several side effects have been noted in patients treated with PTH (1-34). Nausea, vomiting, and headaches have been reported in 18% of women taking 40 µg PTH (1-34) [3]. Mild elevations in serum calcium may occur within 6 h of administration [27]. If this occurs repeatedly, treatment should be discontinued [1]. Patients treated with either 20 µg or 40 µg PTH (1-34) demonstrated an increased incidence of elevated uric acid; the incidence was highest in patients with moderately impaired renal function. Urinary calcium excretion is also increased with PTH (1-34) treatment for up to 12 months, compared to placebo [28]. However, there was no increase in the incidence of gout, arthralgia, or nephrolithiasis in PTH-treated patients with normal, mild, or moderate renal impairment [27].
Other side effects reported by the manufacturer (Eli Lilly) include dizziness, leg cramps, lightheadedness, constipation, low energy, and muscle weakness. These may be signs of hypercalcemia. At the injection site patients may note transient redness, swelling, pain, itching, a few drops of blood, and bruising which should resolve in a short time. The medication is self-administered through a once-daily injection of 20 µg PTH with a pre-filled pen. This may be difficult for patients who have trouble injecting themselves [1, 29].
Given its anabolic effect on bone formation, there is concern that prolonged PTH (1-34) administration may result in bone cancers [30]. Osteosarcoma is a potential complication of long-term PTH (1-34) treatment in rats. Vahle and colleagues identified osteosarcomas in rats treated with daily subcutaneous injections of PTH (1-34) for 2 years at doses of 5, 30, or 75 µg/kg. While treatment resulted in increases in bone mass, bone proliferative lesions, including osteosarcomas, were noted in all PTH-treated groups [30]. This is unlikely to be a problem in humans when treating fracture healing as the course of PTH (1-34) should not extend beyond 3 to 6 months. Furthermore, the human dosage is far smaller than the experimental dosage in rats. The recommended human dosage for the treatment of osteoporosis or fractures is 20 µg/day administered subcutanously. Treatment is continued for 6 months for fractures and for up to 24 months for osteoporosis [1]. Osteosarcomas were seen in rats treated with over 200 times the recommended human dosage of PTH (1-34) [1, 3] Because of the concerns raised by the animal studies, human studies have not been extended beyond 2 years. During this period, no osteosarcomas have been identified. However, it is unknown whether PTH treatment beyond 2 years can induce osteosarcomas: for this reason, treatment beyond 2 years is currently not recommended [1]. Interestingly, in monkeys PTH (1-34) treatment at 5 µg/kg for 18 months did not result in bone tumors over a 4.5-year observation, including the 18 months administration period or the following 3 months [31], suggesting that at least in the primate skeleton, the risk of osteosarcoma after long-term treatment with PTH may be minimal.
Because of the theoretical risk of bone cancer with prolonged PTH (1-34) administration [27], the manufacturer recommends avoiding Forteo® in patients who have a history of bone tumors (either primary or metastatic), Paget's disease of the bone, unexplained high levels of alkaline phosphatase in the blood, a history of radiation therapy involving the bones, or metabolic bone diseases other than osteoporosis [1, 29]. Forteo® should also be avoided in women who are pregnant or nursing [1, 29]. There are no reports investigating the safety or efficacy of PTH treatment beyond 2 years: therefore, treatment beyond this length of time is not recommended [1].
Future studies
The above investigations suggest that PTH (1-34) may be effective clinically in accelerating the fracture healing. Similar studies investigating the effects of PTH (1-34) on fracture healing rates in nonunion models will help confirm a clinical role for this agent in enhancing union. Because clinical trials have already been conducted examining the safety of PTH (1-34) in treating osteoporosis, many of the human safety concerns associated with introducing this drug have already been addressed [1]. The promising results of PTH (1-34) in animal fracture healing models have set the stage for prospective randomized controlled clinical trials to scientifically test the effectiveness of this agent in healing human fractures.
Conclusion
The existing basic science data suggest a role for PTH signaling in the regulation of chondrogenesis and osteogenesis. Investigations in humans have confirmed an anabolic role for PTH (1-34) in enhancing bone density and reducing fracture risk. Animal studies on fracture healing suggest that PTH signaling improves the biomechanical properties of fracture callus and accelerates callus formation, endochondral ossification, and bone remodeling. Based on these data, PTH (1-34) is likely to be a potent agent for enhancing fracture healing in patients with poor fracture healing potential such as those with osteoporosis, prolonged steroid use, and recalcitrant nonunions. At this point, prospective randomized clinical trials are needed to determine the safety, dosage, and efficacy of PTH (1-34) in augmenting fracture healing in humans.
References
Madore GR, Sherman PJ, Lane JM (2004) Parathyroid hormone. J Am Acad Orthop Surg 12:67–71
Finkelstein JS, Hayes A, Hunzelman JL et al (2003) The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med 349:1216–1226
Neer RM, Arnaud CD, Zanchetta JR et al (2001) Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441
Alkhiary YM, Gerstenfeld LC, Krall E et al (2005) Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1-34). J Bone Joint Surg Am 87:731–741
Andreassen TT, Ejersted C, Oxlund H (1999) Intermittent parathyroid hormone (1-34) treatment increases callus formation and mechanical strength of healing rat fractures. J Bone Miner Res 14:960–968
Andreassen TT, Fledelius C, Ejersted C et al (2001) Increases in callus formation and mechanical strength of healing fractures in old rats treated with parathyroid hormone. Acta Orthop Scand 72:304–307
Andreassen TT, Willick GE, Morley P et al (2004) Treatment with parathyroid hormone hPTH(1-34), hPTH(1-31), and monocyclic hPTH(1-31) enhances fracture strength and callus amount after withdrawal fracture strength and callus mechanical quality continue to increase. Calcif Tissue Int 74:351–356
Holzer G, Majeska RJ, Lundy MW et al (1999) Parathyroid hormone enhances fracture healing. A preliminary report. Clin Orthop Relat Res 366:258–263
Jahng JS, Kim HW (2000) Effect of intermittent administration of parathyroid hormone on fracture healing in ovariectomized rats. Orthopedics 23:1089–1094
Komatsubara S, Mori S, Mashiba T et al (2005) Human parathyroid hormone (1-34) accelerates the fracture healing process of woven to lamellar bone replacement and new cortical shell formation in rat femora. Bone 36:678–687
Nakajima A, Shimoji N, Shiomi K et al (2002) Mechanisms for the enhancement of fracture healing in rats treated with intermittent low-dose human parathyroid hormone (1-34). J Bone Miner Res 17:2038–2047
Nakazawa T, Nakajima A, Shiomi K et al (2005) Effects of low-dose, intermittent treatment with recombinant human parathyroid hormone (1-34) on chondrogenesis in a model of experimental fracture healing. Bone 37:711–719
Kronenberg HM (2006) PTHrP and skeletal development. Ann N Y Acad Sci 1068:1–13
Martin TJ, Quinn JM, Gillespie MT et al (2006) Mechanisms involved in skeletal anabolic therapies. Ann N Y Acad Sci 1068:458–470
Everhart-Caye M, Inzucchi SE, Guinness-Henry J et al (1996) Parathyroid hormone (PTH)-related protein(1-36) is equipotent to PTH(1-34) in humans. J Clin Endocrinol Metab 81:199–208
Hock JM, Fonseca J, Gunness-Hey M et al (1989) Comparison of the anabolic effects of synthetic parathyroid hormone-related protein (PTHrP) 1-34 and PTH 1-34 on bone in rats. Endocrinology 125:2022–2027
Weir EC, Terwilliger G, Sartori L et al (1992) Synthetic parathyroid hormone-like protein (1-74) is anabolic for bone in vivo. Calcif Tissue Int 51:30–34
Okazaki K, Jingushi S, Ikenoue T et al (2003) Expression of parathyroid hormone-related peptide and insulin-like growth factor I during rat fracture healing. J Orthop Res 21:511–520
Chung UI, Lanske B, Lee K et al (1998) The parathyroid hormone/parathyroid hormone-related peptide receptor coordinates endochondral bone development by directly controlling chondrocyte differentiation. Proc Natl Acad Sci U S A 95:13030–13035
Kobayashi T, Chung UI, Schipani E et al (2002) PTHrP and Indian hedgehog control differentiation of growth plate chondrocytes at multiple steps. Development 129:2977–2986
Lanske B, Karaplis AC, Lee K et al (1996) PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science 273:663–666
Vortkamp A, Lee K, Lanske B et al (1996) Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science 273:613–622
Dempster DW, Cosman F, Kurland ES et al (2001) Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res 16:1846–1853
Lindsay R, Zhou H, Cosman F et al (2007) Effects of a one-month treatment with PTH(1-34) on bone formation on cancellous, endocortical, and periosteal surfaces of the human ilium. J Bone Miner Res 22:495–502
Misof BM, Roschger P, Cosman F et al (2003) Effects of intermittent parathyroid hormone administration on bone mineralization density in iliac crest biopsies from patients with osteoporosis: a paired study before and after treatment. J Clin Endocrinol Metab 88:1150–1156
Bostrom MP, Gamradt SC, Asnis P et al (2000) Parathyroid hormone-related protein analog RS-66271 is an effective therapy for impaired bone healing in rabbits on corticosteroid therapy. Bone 26:437–442
Miller PD, Schwartz EN, Chen P et al (2007) Teriparatide in postmenopausal women with osteoporosis and mild or moderate renal impairment. Osteoporos Int 18:59–68
Miller PD, Bilezikian JP, Diaz-Curiel M et al (2007) Occurrence of hypercalciuria in patients with osteoporosis treated with teriparatide. J Clin Endocrinol Metab 92(9):3535–3541
Medication Guide. Indianapolis I Eli Lilly and Company 2002
Vahle JL, Sato M, Long GG et al (2002) Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol Pathol 30:312–321
Vahle JL, Zuehlke U, Schmidt A et al (2008) Lack of bone neoplasms and persistence of bone efficacy in cynomolgus macaques after long-term treatment with teriparatide [rhPTH(1-34)]. J Bone Miner Res 18:9–17
Author information
Authors and Affiliations
Corresponding author
Additional information
One or more of the authors (JML) has received funding from (Eli Lilly)
Rights and permissions
About this article
Cite this article
Cipriano, C.A., Issack, P.S., Shindle, L. et al. Recent Advances Toward the Clinical Application of PTH (1-34) in Fracture Healing. HSS Jrnl 5, 149–153 (2009). https://doi.org/10.1007/s11420-009-9109-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11420-009-9109-8