Abstract
A sensitive method for simultaneous analysis of nine fourth-generation antidepressants in human plasma by ultra-performance liquid chromatography–tandem mass spectrometry (UPLC–MS–MS) is presented. The method was used with a Waters Acquity UPLC LC system, an Acquity TQD MS–MS system, and a Poroshell 120 EC-C18 separation column. For extraction of the target compounds, solid-phase extraction with Oasis HLB cartridges was used. All compounds were detected with retention times lower than 3 min. The calibration curves for the antidepressants spiked into human plasma showed similar good linearities in the range of subnanogram to nanogram per milliliter. The detection limits (signal-to-noise ratio = 3) were in the range of 0.2–100 pg/ml. The method gave satisfactory recovery rates, accuracy, and precision for quality control samples spiked with these drugs. To further validate the present method, 25 mg of milnacipran was orally administered to a healthy male volunteer, and the drug concentrations in plasma were measured in samples collected 0.5, 1, 2, 4, and 8 h after dosing. The concentrations were in the range of 31.2–56.8 ng/ml. To our knowledge, this is the most sensitive quantitative method for fourth-generation antidepressants so far reported, and should prove very useful in forensic and clinical toxicology. In addition, the quantitative analysis of tianeptine by LC–MS–MS is first described in this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antidepressants are widely used for mental problems such as depression and anxiety, and many effective antidepressants have been developed over the past several decades, almost all of which are known to potently affect central noradrenergic and/or serotonergic neurons [1–4]. Tricyclic and tetracyclic antidepressants [5] emerged from the early 1950s through to the 1970s and together make up the first-generation and second-generation antidepressants. Selective serotonin reuptake inhibitors (SSRIs) are classified as third-generation antidepressants. Recently, fourth-generation antidepressants, such as serotonin and norepinephrine reuptake inhibitors (SNRIs), norepinephrine and dopamine reuptake inhibitor (NDRI), noradrenergic and specific serotonergic antidepressant (NaSSA), serotonin antagonist and reuptake inhibitors (SARIs), selective serotonin reuptake enhancer (SSRE), and reversible inhibitors of monoamine oxidase A (RIMA), have appeared. Milnacipran, sibutramine, duloxetine, and venlafaxine belong to SNRI; bupropion to NDRI; mirtazapine to NaSSA; trazodone and nefazodone to SARIs; tianeptine to SSRE; and moclobemide to RIMA. These antidepressants are almost equipotent to the first-generation antidepressants, have fewer life-threatening side effects such as cardiac and central nervous system toxicities, and are generally safer if overdoses occur [1–4, 6]. However, there are reports of suicide by overdose of milnacipran [6, 7].
Among the fourth-generation antidepressants, analyses of only milnacipran [8], mirtazapine [9, 10], trazodone [11], and moclobemide [12] in human samples by gas chromatography (GC)–mass spectrometry (MS) have been reported.
Recently, analyses by liquid chromatography (LC)–MS or LC–tandem MS (MS–MS) for milnacipran [12], sibutramine [13–16], duloxetine [17–19], bupropion [20, 21], mirtazapine [22–24], trazodone [25–27], nefazodone [28], moclobemide [29], and venlafaxine [30] in human samples have appeared. However, most of them were sporadic analytical studies; there are few reports for simultaneous analysis of antidepressants including fourth-generation antidepressants by LC–MS or LC–MS–MS. In addition, there are no reports for analysis of tianeptine by LC–MS (-MS) to our knowledge. In this report, we present a rapid and extremely sensitive method for simultaneous analysis of nine fourth-generation antidepressants in human plasma by ultra-performance liquid chromatography–tandem mass spectrometry (UPLC–MS–MS).
Materials and methods
Chemicals
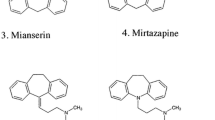
Milnacipran was donated by Asahi Kasei Pharma (Tokyo, Japan). Sibutramine, mirtazapine, and tianeptine were purchased from Tocris Bioscience (Minneapolis, MN, USA); duloxethine, bupropion, and moclobemide from Toronto Research Chemicals (Toronto, DN, Canada); trazodone from Wako Pure Chemical (Osaka, Japan); nefazodone and venlafaxine (internal standard, IS) from LKT Laboratories (St. Paul, MN, USA). The structures of the nine target compounds and IS are shown in Fig. 1. Laboratory distilled water was purified using a Synergy UV apparatus (Millipore, Molsheim, France). Other common chemicals used were of the highest purity commercially available.
Solid-phase extraction procedure
The solid-phase extraction (SPE) of antidepressants was performed according to our previous report [31] with minor modifications. A 1.0-ml volume of plasma containing or not containing antidepressants was mixed with 500 ng of IS and 4.0 ml of 250 mM sodium bicarbonate solution, vortex-mixed for 1 min, and centrifuged at 1,600 g for 5 min. For SPE, the supernatant fraction was applied to an Oasis HLB cartridge (60 mg, 3 cc; Waters, Milford, MA, USA) preconditioned with 3 ml of methanol and 3 ml of distilled water. Each cartridge was washed with 3 ml of distilled water and 3 ml of 5 % methanol aqueous solution. The cartridges were dried under vacuum for 10 s. The analytes were eluted with 3 ml of solvent mixture of chloroform/methanol/acetonitrile (2:1:1, v/v). The eluate was evaporated to dryness under a nitrogen stream at room temperature. The residue was reconstituted in 100 μl of 20 % acetonitrile and 0.1 % formic acid in aqueous solution and vortex-mixed for 1 min.
UPLC–MS–MS conditions
The LC instrument used in combination with an MS–MS detector was a Waters Acquity UPLC system, including an Acquity UPLC binary pump and a sample manager (Waters). The column used for chromatographic separation was a Poroshell 120 EC-C18 (100 × 2.1 mm i.d., particle size 2.7 μm; Agilent, Santa Clara, CA, USA). The column temperature was maintained at 40 °C, and the gradient system was used with mobile phase A (0.1 % formic acid aqueous solution) and mobile phase B (0.1 % formic acid in acetonitrile) delivered at 0.5 ml/min. The linear gradient program was: 80 % A/20 % B (0 min) to 35 % A/65 % B (until 3 min) and to 5 % A/95 % B (until 4 min). The final mobile phase was held for 2 min to avoid carryover from the previous injection. After the elution program, the mobile phase composition was returned to 80 % A/20 % B for the next run in 1 min. The total run time for each sample analysis was 7.0 min. The autosampler was maintained at 4 °C and the injection volume was 5 μl.
The MS–MS detection was made in the positive ionization mode on the tandem quadrupole mass spectrometer (Acquity TQD; Waters) equipped with an electrospray ionization (ESI) interface. Quantitation was performed by selected reaction monitoring (SRM) using peak areas. The optimal MS parameters were: capillary voltage, 3.0 kV; source temperature, 150 °C; desolvation temperature, 450 °C; desolvation and cone gas, nitrogen, with flow rates of 800 and 50 l/h, respectively; collision gas, argon, with a flow rate of 0.15 ml/min. The monitored SRM transitions, together with the cone voltages and optimized collision energies for each antidepressant, are shown in Table 1. All data in the centroid mode were acquired and processed using MassLynx NT 4.1 software with a QuanLynx program (Waters).
Human experiments
The human experiments were approved by the Regional Institutional Board of Aichi Medical University. The main scientist of this research project (healthy 40-year-old man, 65.0 kg) volunteered for the human experiments. He took a single oral dose of 25 mg of milnacipran tablet (Toledomin), and his blood was sampled 0.5, 1, 2, 4, and 8 h after intake. Blank blood was also collected just before drug intake. Plasma samples were immediately prepared by centrifuging the blood samples at 1,600 g for 5 min and were stored at −80 °C until analysis. For quality control samples, blank plasma was separated from the blank blood by centrifugation and stored as described above.
Results and discussion
Product ion mass spectra and selected reaction monitoring chromatograms
After optimization, the protonated precursor molecular ions were chosen to generate product ions. The SRM transitions and their parameters for the antidepressants and IS are shown in Table 1. The product ion mass spectra obtained from the precursor molecular ions of the authentic antidepressants and IS are shown in Fig. 2.
Figure 3 shows SRM chromatograms for the antidepressants and IS spiked into blank human plasma at the concentration of 10 ng/ml. The retention times of milnacipran, sibutramine, duloxethine, bupropion, mirtazapine, trazodone, nefazodone, tianeptine, moclobemide, and IS were 1.34, 2.79, 2.44, 1.40, 0.94, 1.51, 2.75, 2.05, 0.86, and 1.44 min, respectively. The chromatographic peaks were near symmetrical without any impurity peaks, and the retention times were less than 3.0 min, which were shorter than or similar to those in the previous reports [7, 12–33]. The short retention times are useful for high-throughput drug analysis.
Selected reaction monitoring (SRM) chromatograms for milnacipran (a), sibutramine (b), duloxetine (c), bupropion (d), mirtazapine (e), trazodone (f), nefazodone (g), tianeptine (h), moclobemide (i), and IS (j) spiked into blank human plasma obtained by UPLC–MS–MS. The amounts of drugs and IS spiked into 1.0 ml of plasma were 10 and 500 ng, respectively
Reliability of the method
The peak area ratios of each antidepressant to IS obtained from each SRM chromatogram were plotted against the concentration of each compound in human plasma. A linear relationship was observed for all compounds with coefficients of determination (r 2) larger than 0.99 as shown in Table 2. The detection limit, defined as the concentration giving the signal-to-noise ratio of 3, was 0.2–100 pg/ml for quality control samples of human plasma.
The lowest detection limits, which have been described in the literature, are also listed in Table 2 for each compound for comparison. For sibutramine, duloxetine, bupropion, mirtazapine, and moclobemide, the detection limits obtained in this study were in the same order of magnitude as those described in the literature, but not higher than those in the previous ones. For milnacipran, trazodone, and nefazodone, the detection limits obtained in this study were 300–3,000 times lower than those described in the literature. Therefore, this is the most sensitive quantitative method for the fourth-generation antidepressants so far reported.
Table 3 shows accuracy and precision data of the present method. Accuracy values were 77.5–121 %; precision values were not greater than 19.3 %. The recovery rates of antidepressants spiked into human plasma were calculated by comparing each chromatogram area obtained from the spiked sample with the corresponding area obtained from the sample made by adding standard compounds to the final sample extract of blank plasma just before the instrumental analysis. The recovery rates were not less than 60.3 % as shown in Table 4.
Actual analysis of milnacipran in human plasma after a single oral dose
As described above, a 40-year-old volunteer ingested 25 mg of milnacipran, and his blood was sampled at various intervals after ingestion. Intense peaks of milnacipran appeared in all SRM chromatograms obtained at five time points after the intake (Fig. 4). The concentrations of milnacipran in plasma were 43.7, 56.8, 52.8, 51.0, and 31.2 ng/ml after 0.5, 1, 2, 4, and 8 h, respectively.
Conclusions
To our knowledge, this is the first report describing simultaneous quantitation of nine fourth-generation antidepressants in human plasma by LC–MS–MS with full validation. The sensitivity of the present method was highest among those of the existing methods for the drugs; the detection limits obtained in our study were as low as 0.2–100 pg/ml. We expect the present UPLC–MS–MS method with SPE method to prove very useful in forensic and clinical toxicology and pharmacokinetic studies because of its rapidness and high sensitivity.
References
Morilak DA, Frazer A (2004) Antidepressants and brain monoaminergic systems: a dimensional approach to understanding their behavioural effects in depression and anxiety disorders. Int J Neuropsychopharmacol 7:193–218
Wille SM, Cooreman SG, Neels HM, Lambert WE (2008) Relevant issues in the monitoring and the toxicology of antidepressants. Crit Rev Clin Lab Sci 45:25–89
Babinkostova Z, Stefanovski B (2011) Forms of antipsychotic therapy: improved individual outcomes under personalised treatment of schizophrenia focused on depression. EPMA J 2:391–402
Dubicka B, Hadley S, Roberts C (2006) Suicidal behaviour in youths with depression treated with new-generation antidepressants: meta-analysis. Br J Psychiatry 189:393–398
Hayashi D, Kumazawa T, Hasegawa C, Lee X-P, Marumo A, Uchigasaki S, Kawamura M, Sato K (2012) A simple and reliable method for quantifying plasma concentrations of tetracyclic antidepressants using monolithic silica solid-phase extraction tips. Forensic Toxicol 30:98–105
Levine M, Truitt CA, O’Connor AD (2011) Cardiotoxicity and serotonin syndrome complicating a milnacipran overdose. J Med Toxicol 7:312–316
Fanton L, Bévalot F, Grait H, Le Meur C, Gaillard Y, Malicier D (2008) Fatal intoxication with milnacipran. J Forensic Legal Med 15:388–390
Uçaktürk E, Şafak C (2010) Determination of milnacipran in human plasma using GC–MS. Chromatographia 72:111–119
Pietracci E, Bermejo A-M, Álvarez I, Cabarcos P, Balduini W, Tabernero M-J (2013) Simultaneous determination of new-generation antidepressants in plasma by gas chromatography–mass spectrometry. Forensic Toxicol 31:124–132
Wille SM, Maudens KE, Van Peteghem CH, Lambert WE (2005) Development of a solid phase extraction for 13 ‘new’ generation antidepressants and their active metabolites for gas chromatographic-mass spectrometric analysis. J Chromatogr A 1098:19–29
Gaillard Y, Pépin G (1997) Moclobemide fatalities: report of two cases and analytical determinations by GC-MS and HPLC-PDA after solid-phase extraction. Forensic Sci Int 87:239–248
Shinozuka T, Terada M, Tanaka E (2006) Solid-phase extraction and analysis of 20 antidepressant drugs in human plasma by LC/MS with SSI method. Forensic Sci Int 162:108–112
Bhatt J, Shah B, Kambli S, Subbaiah G, Singh S, Ameta S (2007) Rapid and sensitive method for the determination of sibutramine active metabolites in human plasma by reversed-phase liquid chromatography-tandem mass spectroscopy. J Chromatogr Sci 45:91–96
Nirogi RVS, Kandikere V, Shukla M, Mudigonda K, Maurya S (2007) Sensitive and reproducible liquid chromatography-tandem mass spectrometry method for quantification of sibutramine in human plasma. Forensic Toxicol 25:30–36
Kang W, Bae K, Noh K (2010) Enantioselective determination of sibutramine and its active metabolites in human plasma. J Pharm Biomed Anal 51:264–267
Bae J-W, Choi C-I, Jang C-G, Lee S-Y (2011) Simultaneous determination of sibutramine and its active metabolites in human plasma by LC-MS/MS and its application to a pharmacokinetic study. Biomed Chromatogr 25:1181–1188
Satonin DK, McCulloch JD, Kuo F, Knadler MP (2007) Development and validation of a liquid chromatography-tandem mass spectrometric method for the determination of the major metabolites of duloxetine in human plasma. J Chromatogr B 852:582–589
Selvan PS, Gowda KV, Mandal U, Solomon WDS, Pal TK (2007) Determination of duloxetine in human plasma by liquid chromatography with atmospheric pressure ionization-tandem mass spectrometry and its application to pharmacokinetic study. J Chromatogr B 858:269–275
Zhao R-K, Cheng G, Tang J, Song J, Peng W-X (2009) Pharmacokinetics of duloxetine hydrochloride enteric-coated tablets in healthy Chinese volunteers: a randomized, open-label, single- and multiple-dose study. Clin Ther 31:1022–1036
Hu L, Wang Z, Xu R, Ma J, Wang X, Zhang X (2011) Determination of bupropion and its main metabolite in rat plasma by LC-MS and its application to pharmacokinetics. Pharmazie 66:924–928
Parekh JM, Sutariya DK, Vaghela RN, Sanyal M, Yadav M, Shrivastav PS (2012) Sensitive, selective and rapid determination of bupropion and its major active metabolite, hydroxybupropion, in human plasma by LC-MS/MS: application to a bioequivalence study in healthy Indian subjects. Biomed Chromatogr 26:314–326
Paus E, Jonzier-Perey M, Cochard N, Eap CB, Baumann P (2004) Chirality in the new generation of antidepressants: stereoselective analysis of the enantiomers of mirtazapine, N-demethylmirtazapine, and 8-hydroxymirtazapine by LC-MS. Ther Drug Monit 26:366–374
Gerostamoulos D, Beyer J, Staikos V, Tayler P, Woodford N, Drummer OH (2012) The effect of the postmortem interval on the redistribution of drugs: a comparison of mortuary admission and autopsy blood specimens. Forensic Sci Med Pathol 8:373–379
de Santana FJ, Jabor VA, Cesarino EJ, Lanchote VL, Bonato PS (2010) Enantioselective analysis of mirtazapine, demethylmirtazapine and 8-hydroxy mirtazapine in human urine after solid-phase microextraction. J Sep Sci 33:268–276
Sauvage FL, Picard N, Saint-Marcoux F, Gaulier JM, Lachâtre G, Marquet P (2009) General unknown screening procedure for the characterization of human drug metabolites in forensic toxicology: applications and constraints. J Sep Sci 32:3074–3083
Patel BN, Sharma N, Sanyal M, Shrivastav PS (2008) High throughput and sensitive determination of trazodone and its primary metabolite, m-chlorophenylpiperazine, in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B 871:44–54
Rust KY, Baumgartner MR, Dally AM, Kraemer T (2012) Prevalence of new psychoactive substances: a retrospective study in hair. Drug Test Anal 4:402–408
Mao Y, Huang MQ, Xia YQ, Jemal M (2007) High-throughput quantitation of nefazodone and its metabolites in human plasma by high flow direct-injection LC-MS/MS. J Pharm Biomed Anal 43:1808–1819
Choong E, Rudaz S, Kottelat A, Haldemann S, Guillarme D, Veuthey JL, Eap CB (2011) Quantification of 4 antidepressants and a metabolite by LC-MS for therapeutic drug monitoring. J Chromatogr B 879:1544–1550
Jain RT, Panda J, Srivastava A (2011) Two formulations of venlafaxine are bioequivalent when administered as open capsule mixed with applesauce to healthy subjects. Indian J Pharm Sci 73:510–516
Ogawa T, Hattori H, Kaneko R, Ito K, Iwai M, Mizutani Y, Arinobu T, Ishii A, Suzuki O, Seno H (2010) Sensitive analysis of blonanserin, a novel antipsychotic agent, in human plasma by ultra-performance liquid chromatography-tandem mass spectrometry. Anal Sci 26:1099–1102
Ogawa T, Hattori H, Iwai M, Ito K, Iwakura K, Ishii A, Suzuki O, Seno H (2012) A rapid and simultaneous analysis of theophylline and its metabolites in human whole blood by ultra-performance liquid chromatography-tandem mass spectrometry. Forensic Toxicol 30:142–148
Nomura M, Suzuki Y, Kaneko R, Ogawa T, Hattori H, Seno H, Ishii A (2012) Simple and rapid analysis of amatoxins using UPLC–MS–MS. Forensic Toxicol 30:185–192
Conflict of interest
There are no financial or other relations that could lead to a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iwakura, K., Ogawa, T., Hattori, H. et al. Simultaneous and sensitive analysis of fourth-generation antidepressants in human plasma by ultra-performance liquid chromatography–tandem mass spectrometry. Forensic Toxicol 32, 30–38 (2014). https://doi.org/10.1007/s11419-013-0197-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-013-0197-2