Abstract
The protective potential of dandelion on acute hepatitis, lung injury and colorectal cancer has recently been revealed. Importantly, ulcerative colitis (UC), a clinically defined inflammatory bowel disease, accelerates the risk of colorectal cancer. However, studies focusing on the activity of dandelion on UC are extremely limited. In the present study, we found that an aqueous extract of dandelion root increases cell viability and decreases apoptosis in dextran sodium sulfate (DSS)-incubated NCM460 human colonic epithelial cells, probably through removing the production of reaction oxygen species and blocking nuclear factor-kappaB signaling. We then examined the anti-colitis efficacy of this extract in an in vivo study. We detected that dandelion root extract efficiently ameliorates progressive acute injury as demonstrated by a reduction in body weight loss, severity scores of disease index and shortened colon length during DSS treatment, as well as reducing the inflammatory conditions and oxidative stress in the colon of DSS-induced mice. Our study clearly demonstrates that dandelion has a strong cytoprotective effect on NCM460 colonocytes and shows powerful defense on an established experimental mouse model of DSS-induced UC. Therefore, dandelion root extract can be an effective anti-colitis complex mixture and can provide a complementary alternative to currently available therapeutic intervention in UC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory bowel disease (IBD) consists of two major clinical types—ulcerative colitis (UC) and Crohn’s disease (CD). It is a common chronic gastrointestinal disharmony characterized by alternating periods of remission and active intestinal inflammation [1]. Unlike CD, which can impact any portion of the gastrointestinal tract, the pathology of UC is limited to the colonic mucosa [2]. More important, UC is capable of enhancing the risk of colorectal cancer [3]. To date, the precise etiology of UC is still enigmatic, but the widely accepted view is that UC is mainly caused by dysfunction of the intestinal epithelium [3]. Besides the clear function as a physical barrier, the intestinal epithelium has to deal with miscellaneous biological functions, including processing and absorbing food components and maintaining intestinal homeostasis [4]. Exceptional immune responses and inflammatory conditions can occur when the barrier function of the intestinal epithelium is disrupted by damage to the intestinal epithelial cells [3]. Thus, the integrity of the intestinal epithelium is essential for maintaining a healthy gut, and cytoprotective drugs that target the intestinal epithelial cells should be accommodating for the therapeutic intervention of UC. Despite the advances made in the innovation and improvement of numerous therapy drugs, the concomitant toxicities and side-effects [5] suggest that further study is necessary.
Herbs are essential in the development of many drugs [6]. Dandelions (Taraxacum spp., Asteraceae family) have been applied for hundreds of years as therapy for various ailments. As a traditional Chinese medicine, dandelions are frequently utilized for the treatment of tumors as well as hepatitis and digestive diseases [7]. In human leukemia and pancreatic cancer cells, the death receptor-mediated extrinsic pathway of apoptosis can be induced promptly by dandelion root extract and this induction of apoptosis is dependent mostly on activated caspase-8 [8, 9]. Furthermore, multiple death signaling pathways are activated in dandelion root extract-treated colorectal cancer [10]. Recent scientific reports have revealed the anti-inflammatory and anti-oxidative activities of this plant [11, 12]. Water extract of dandelions relieves lipopolysaccharide (LPS)-induced acute injury in the lung through the PI3K/Akt/mTOR signaling pathway [13]. The aqueous extract of dandelion root lowers alcohol-induced toxicity in the liver by improving anti-oxidative potentials and reducing lipid peroxidation [14]. However, studies focusing on the anti-inflammatory and anti-oxidative actions of dandelion on UC are extremely limited. Furthermore, the function of dandelion in the protection of colonic epithelial cells is unknown.
In this study, we evaluated the protective effects of dandelion root extract on human colonic epithelial cells and a mouse model of UC. We studied the effect of dandelion root extract on cell viability, apoptosis, oxidative stress and the Akt/NF-κB/IL-8 signaling pathway induced by dextran sodium sulfate (DSS) in vitro and the effect on inflammatory conditions and oxidative injury induced by DSS in vivo.
Materials and methods
Preparation of dandelion root extracts
The dandelion roots (Asian, Taraxacum officinale) used in the present study were obtained from Premier Herbal Inc. (Lot no. 318121). The root extract was provided according to a procedure described previously [10]. In brief, 100 g of dried dandelion root was immersed and thoroughly frozen in liquid nitrogen for about 5–10 min. The frozen pieces were ground in an impingement grinder to an average particle size of ≤45 μm. After grinding, the dandelion root powder was extracted in boiling water on low heat for 3 h. The total extractive was filtered through a NITEX nylon mesh filter (LAB PAK; Sefar BDH Inc., Chicoutimi, Quebec, Canada) and the filtrate was centrifuged at 800×g for 5 min at room temperature. The supernatant was filtered through a 0.45-μm filter, followed by lyophilization. The resulting powders weighed approximately 15 g. The current yield (15%) from the dandelion roots was consistent with previous results [7]. The dried extractive was then resuspended in water and the concentration of final stock solution was 100 mg/mL. After filtering with a 0.22-μm filter, the aqueous extract of dandelion root was stored at 4 or − 20 °C for long-term storage. This extract was utilized for all the experiments described in this study.
Cell culture and treatment
Human NCM460 colonocyte (INCELL, San Antonio, TX, USA), a non-transfected human colonic epithelial cell line, was cultured in Dulbecco’s Modifed Eagle Medium (HyClone) supplemented with 10% (v/v) fetal bovine serum and 100 units penicillin/streptomycin at 37 °C with 5% CO2 atmosphere in a humidified incubator. The cells were grown in a flask and were allowed to reach approximately 85% confluence. The culture medium was renewed every 2 days and was then rinsed and removed from the flask by incubating with a trypsin–EDTA solution (HyClone), and harvested in a 15-mL centrifuge tube for subsequent study.
To investigate the function of dandelion root extract in an in vitro model, cells were plated and grown to 50–70% confluence prior to treatment with this extract at increasing concentrations (1, 3, and 6 mg/mL) [10]. After 12 h, the cells were exposed to various concentrations of DSS (0.2, 08, and 1.2 μg/mL) for an additional 12 h [3].
Animals and experimental design
Female C57BL/6 mice (pathogen-free) from the same litter, aged 6–8 weeks and weighing 18 ± 2 g, were reared in cages in an environmentally controlled breeding room (temperature 20 ± 2 °C, humidity 60 ± 5%, 12 h dark/light cycle), and fed with sterile water and a standard laboratory rodent diet. They were maintained in accordance with internationally accepted principles for laboratory animal use. All work was conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care (Animal Welfare Assurance Number: GZPPH2017061732). The Minimum Standards of Reporting Checklist contained details of the experimental design, statistics, and resources used in this study.
For the induction of acute UC, the mice were administered 2% DSS (w/v) (36–50 kDa; MP Biomedicals, Illkirch, France) in their drinking water. The mice were randomly separated into 4 groups (n = 10 per group)—a control group (mice received normal drinking water); a DSS group (mice received 2% w/v DSS in drinking water); a DSS + dandelion (20 mg/kg) group (mice received 2% w/v DSS in drinking water together with 20 mg/kg of dandelion root extract by gavage twice a day); and a dandelion group (mice received 20 mg/kg of dandelion root extract by gavage twice a day). The extent of colitis was monitored daily using the modified method [15]. Body weight was recorded every day and the animals were killed on day 10, after which their colons were removed. Diarrhea was scored daily as 0 = normal; 2 = loose stools; 4 = watery diarrhea. Blood in the stool was scored as 0 = normal; 2 = slight bleeding; 4 = gross bleeding. Weight loss was scored as 0 = none; 1 = 1–5%; 2 = 5–10%; 3 = 10–15%; 4 = >15%. The disease activity index was the average of these scores (combined score of stool consistency, bleeding and weight loss)/3.
Cell viability assay
Cell viability was analyzed using a CCK-8 Assay Kit (Dojindo) according to the manufacturer’s instructions. Briefly, NCM460 cells pretreated with indicated levels of dandelion root extract were incubated with indicated concentrations of DSS in 96-well plates. Twenty-four hours later, 5 µl of CCK-8 reagent was added to each well and incubated at 37 °C for 1 h. The cell numbers were assessed by measurement of absorbance at 450 nm. All the experiments were performed in triplicate.
Flow cytometric analysis of apoptosis
Exposure of phosphatidylserine was evaluated to detect early stage apoptosis by analysis of annexin V-FITC binding. Enhanced propidium iodide (PI) was a correlate for increased secondary necrosis. In particular, 2 × 105 cells were manipulated by sequentially harvesting, washing in phosphate-buffered saline (PBS) and resuspending in binding buffer (10 mM HEPES/NaOH, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2). Annexin V-FITC was added to a final concentration of 200 ng/mL prior to incubation in the dark at room temperature (RT) for 10 min, then washed in PBS and reconstituted in 190 µl of binding buffer. PI (10 µl) was loaded to each sample before flow cytometric analysis. Stained cells were analyzed using a FACStar Plus flow cytometer (Becton–Dickinson). The ratio of fluorescence intensities excited at 488 nm was monitored at an emission wavelength of 515 nm for FITC and 560 nm for PI. Data analysis was performed with a BD BioSciences FACSCalibur flow cytometer using CellQuest software [16].
Western blotting
For immunodetection, NCM460 cells were lysed directly in Laemmli’s sample buffer and boiled for 10 min. After centrifugation, 50 µg of total protein extract was resolved on 10% SDS-PAGE, which was then transferred to nitrocellulose membranes for Western blotting. The membranes were first stained with Ponceau S to confirm the transfer efficacy. After blocking with 1% bovine serum albumin dissolved in Tris-buffered saline containing 0.05% Tween-20 for 2 h at RT, the membranes were incubated with anti-caspase-3, anti-Bax, anti-GAPDH, anti-phospho-Akt1-S473, anti-Akt1, anti-phospho-p65-S536 or anti-p65 at appropriate dilutions (ABclonal), followed by goat anti-rabbit secondary antibody conjugated with horseradish peroxidase. Positive band intensities were detected using a gel documentation system (LAS-3000 Fujifilm) [17].
Measurement of reactive oxygen species (ROS), glutathione/glutathione disulfide (GSH/GSSG) ratio, malondialdehyde (MDA) and superoxide dismutase (SOD) activity
MDA [MDA Assay Kit (TBA method)], myeloperoxidase (MPO) activity (MPO Assay Kit), the ratio of GSH/GSSG (GSH and GSSG Assay Kit) and ROS level (Reactive Oxygen Species Assay Kit) were investigated in NCM460 cells and colon homogenate employing commercially available kits according to the manufacturer’s specifications. The MDA and MPO assay kits were purchased from Nanjing Jiancheng Bioengineering Institute, China. The kits for measuring the ratio of GSH/GSSG, and ROS were obtained from Beyotime, China. Total SOD activity included Cu–Zn and Mn SOD activity and was determined by hydroxylamine assay developed from xanthine oxidase, and data were expressed as units per milligram (U/mg) protein.
Measurement of cytokine concentration
The secretion of interleukin (IL)-8 in the medium of control and treated NCM460 cells was measured using the human IL-8 ELISA Kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s specifications. Production of IL-6 (mouse IL-6 ELISA Kit), tumor necrosis factor alpha (TNF-α) (mouse TNFα ELISA Kit), and IL-1β (mouse IL-1β ELISA Kit) was assessed in the colon homogenate employing commercially available kits (Nanjing Senbeijia Biological Technology Co., Ltd) according to the manufacturer’s specifications.
Histology analysis
The colon sections were removed and fixed with 4% paraformaldehyde overnight and then embedded in paraffin and sliced into 5-μm sections. For histological analysis, the paraffin sections were stained with hematoxylin and eosin (H&E) [18]. Histology was scored as epithelium (E), 0 = normal morphology; 1 = loss of goblet cells; 2 = loss of goblet cells in large areas; 3 = loss of crypts; 4 = loss of crypts in large areas; and infiltration (I), 0 = no infiltrate; 1 = infiltrate around the crypt basis; 2 = infiltrate reaching the lamina (L) muscularis mucosae; 3 = extensive infiltration reaching the L muscularis mucosae and thickening of the mucosa with abundant edema; 4 = infiltration of the L submucosa. Total histological score was given as E + I.
Statistical analysis
The histological score was analyzed using the the two-tailed Mann–Whitney test (non-parametric analysis) to measure statistical significance. The unpaired two-tailed Student’s t test was used to analyze the statistical significance of cell viability, ROS, GSH/GSSG ratio, MDA, IL-8 production, and animal model data. All data were analyzed by Prism (GraphPad Software, Inc.), and p values <0.05 and <0.01 were deemed as two significant levels.
Results
Dandelion root extract protects NCM460 colonocytes against DSS-induced injury
We primarily used the CCK-8 assay to assess the protective effects of dandelion root extract against the cell damage induced by DSS. As shown in Fig. 1a, the cell viability of NCM460 was remarkably decreased after DSS treatment. In particular, when the DSS concentration was >0.8 μg/mL, the survival percentage of NCM460 cells was <50%. Upon pretreatment with dandelion root extract, the DSS-incubated cells exhibited a significant increase in cell viability. More important, this protective effect was in a dosage-dependent manner (Fig. 1b). We then questioned whether NCM460 growth saved by dandelion root extract resulted from the decline of cell apoptosis. Using the increase of annexin fluorescence intensity as readout for enhanced apoptosis, we detected that exposure to 0.2 μg/mL DSS resulted in an improvement in the apoptosis level to approximately 24.22% in the NCM460 cells (Fig. 1c, d). Dandelion root extract (3 mg/mL) initiated the decline of DSS-induced apoptosis (15.79%) and 6 mg/mL of dandelion root extract further decreased DSS-induced apoptosis (10.28%). Since activation of caspase-3 and apoptosis regulator BAX (Bax) have been shown to occur in apoptosis [19], and dandelion root extract prevented DSS-induced apoptosis, the abundance of caspase-3 and Bax was measured. As anticipated, DSS triggered the obviously upregulated expression of caspase-3 and Bax, which was downregulated by administration of dandelion root extract (Fig. 1e).
Dandelion root extract increased cell viability and decreased apoptosis in DSS-induced NCM4460 cells. a Protective effect of dandelion root extract against DSS-induced cell injury. The NCM460 cells were pre-loaded with 3 mg/mL of dandelion root extract for 12 h and then exposed to various concentrations of DSS for an additional 12 h. b Dose-dependent protective effect of dandelion root extract against DSS-induced cell injury. The NCM460 cells were pre-loaded with 1–6 mg/mL of dandelion root extract for 12 h and then exposed to 0.8 μg/mL DSS for an additional 12 h. c Flow cytometric analysis of DSS-induced apoptosis. The NCM460 cells were pretreated with 3 and 6 mg/mL of dandelion root extract for 10 h before being exposed to 0.2 μg/mL of DSS for an additional 12 h. d The apoptosis rate of NCM460 cells from three independent biological repeats of flow cytometric analysis. e Western blotting analysis of apoptosis-related proteins, caspase-3 and Bax. Error bars ± SD, *p < 0.05, **p < 0.01
Dandelion root extract inhibits DSS-induced oxidative injury in NCM460 cells
DSS generated excess ROS, thereby inducing oxidative injury [20]; the latter might be a latent driving factor in the progression of UC [3]. To determine the effect of dandelion root extract on DSS-induced oxidative injury, the measurement of ROS generation, GSH/GSSG ratio and MDA production (a biomarker of the lipid oxidation) was employed. The level of ROS increased in the NCM460 cells following exposure to DSS (0.2, 0.8 and 1.2 μg/mL) (Fig. 2a). The ROS increased to six times the baseline in 0.2 μg/mL and to 12 times the baseline in 1.2 μg/mL. Following pretreatment with dandelion root extract, DSS-induced ROS was clearly inhibited (Fig. 2a), and this suppression was concentration-dependent (Fig. 2b). Moreover, administration of dandelion root extract remarkably increased the DSS-induced decrease of the GSH/GSSG ratio and notably reduced the DSS-induced increase of MDA production (Fig. 2c, d), indicating that dandelion root extract could prevent oxidative injury through taking advantage of the GSH-based antioxidant system and suppressing lipid oxidation.
Dandelion root extract was an antagonist for DSS-induced oxidative stress in NCM460 cells. a Dandelion root extract pretreatment lowered the production of DSS-induced ROS. b The production of DSS-induced ROS was reduced by treatment with dandelion root extract in a dosage-dependent manner. c The GSH/GSSG ratio reduced by DSS was rescued by administration of dandelion root extract. d The production of DSS-induced MDA was inhibited by administration of dandelion root extract. Error bars ± SD, *p < 0.05, **p < 0.01
Dandelion root extract inhibits DSS-induced activation of nuclear factor-kappaB (NF-κB) signaling
It has been reported that NF-κB is the one of the central activators of colitis and has been activated in a DSS-administered NCM460 cell model [20, 21]. We therefore tested whether dandelion root extract could affect NF-κB activity. Consistent with previous results [20], the cells exposed to 0.8 μg/mL DSS showed significantly stronger NF-κB action (Fig. 3a); this was indicated by assessing p65 phosphorylation [22]. Activation of the NF-κB signaling pathway was analyzed by detecting the phosphorylation of Akt, an upstream NF-κB regulator, and the production of IL-8, a downstream NF-κB transcriptional product. DSS stimulation induced the phosphorylation of Akt and the level of IL-8 production (Fig. 3a, b). Pretreatment with dandelion root extract markedly decreased the phosphorylation of p65 and Akt, and the secretion of IL-8 (Fig. 3a, b).
The DSS-activated Akt/NF-κB/IL-8 pathway in NCM460 cells was blocked by administration of dandelion root extract. a DSS enhanced the phosphorylated level of p65 and Akt1, which was reduced by pretreatment with dandelion root extract. b Administration of dandelion root extract reduced DSS-induced IL-8 secretion in a concentration-dependent manner. The NCM460 cells were pre-loaded with 1–6 mg/mL of dandelion root extract for 12 h and then exposed to 0.8 μg/mL DSS for an additional 12 h. Afterwards, the cell media were collected and IL-8 production was measured using an ELISA kit. Error bars ± SD, *p < 0.05, **p < 0.01
Dandelion root extract alleviated DSS-induced UC
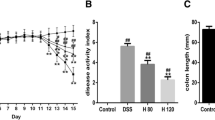
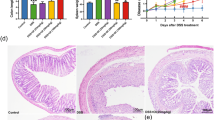
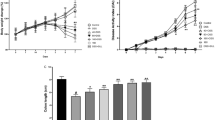
As presented above, the in vitro studies demonstrated that dandelion root extract protected NCM460 cells against DSS-induced injury. The next question was whether dandelion root extract could be effective against DSS-induced UC in mice. Following the establishment of a UC model, the mice were divided into two group—a PBS control group and a dandelion root extract treatment group. The mice were observed for 10 days and their weight and disease index were measured every day. The current results proved that oral administration of dandelion root extract efficiently mitigated progressive injury as described by a reduction in body weight loss (Fig. 4a) and the severity scores of disease index (Fig. 4b) during the DSS treatment, as well as a decline in the shortening of the colon length induced by DSS (Fig. 4c). Additionally, H&E staining of the colon sections indicated more severe epithelial erosion, loss of goblet cells and areas of mucosal ulceration, as well as increased numbers of infiltrating mucosal and submucosal leukocytes in the DSS-induced animals compared with the non-DSS treated group (Fig. 4d), thereby causing higher histological scores for both tissue damage and inflammation (Fig. 4e). Notably, oral administration of dandelion root extract ameliorated this damage and inflammation as indicated by the limited histological disease scores (Fig. 4d, e).
Dandelion root extract protected mice against DSS-induced acute UC. a Body weight was measured daily in control, dandelion-treated, DSS-treated and DSS + dandelion-treated mice (n = 10, per group). b Administration of dandelion root extract reduced the disease severity caused by DSS. c DSS-shortened colon length was restored by administration of dandelion root extract. d Effect of dandelion root extract on DSS-mediated histopathologic changes of the colon. e Histologic inflammatory score calculated from d. Error bars ± SD, *p < 0.05, **p < 0.01
Dandelion root extract mitigated DSS-induced inflammatory conditions in the colon
During the progression of UC, a complex combination of inflammatory signaling processes destroys the intestinal epithelial function and results in the employment of inflammatory cells to the site of injury [3]. Therefore, we also assessed the effects of dandelion root extract on DSS-induced colonic inflammatory conditions by evaluating several inflammatory markers. As shown above, DSS activated NF-κB-dependent IL-8 in colonic epithelial cells, which then triggered the recruitment of inflammatory cells. These inflammatory cells would redundantly generate several proinflammatory cytokines including TNF-α, IL-1β and IL-6. As shown in Fig. 5, all of the inflammatory indicators increased obviously in the DSS-treated animals compared with the control group. Dandelion root extract treatment markedly reduced the expression of the inflammatory cytokines TNF-α, IL-6 and IL-1β compared to the mice given DSS alone (Fig. 5a–c). In the case of MPO, the animals given DSS alone showed a clear increase in MPO activity compared to the control group. Dandelion root extract treatment strongly reduced MPO activity (Fig. 5d).
Quantitative analysis of the inflammatory cytokines TNF-α (a), IL-6 (b), IL-1β (c), and MPO activity (d) in the colon homogenates from C57BL/6 female mice. After DSS administration for 10 days, the levels of TNF-α, IL-6 and IL-1β were measured by ELISA and MPO activity was also measured in the colon tissue. Error bars ± SD, *p < 0.05, **p < 0.01
Dandelion root extract prevents DSS-induced oxidative injury in mice colon
Given that we have demonstrated the protective role of dandelion root extract against DSS-induced oxidative injury in NCM460 colonic cells, we then explored whether dandelion root extract could exert similar protective effects in the colon of the DSS-induced mouse UC model. We discovered that oral administration of dandelion root extract significantly reduced the DSS-induced ROS level (Fig. 6a). SOD activity was reduced in the DSS-induced mice but treatment with dandelion root extract recovered this activity obviously (Fig. 6b). Moreover, we further found that oral administration of dandelion root extract reduced the MDA abundance in the DSS model group (Fig. 6c). Taken together, these data showed that dandelion root extract could alleviate oxidative injury in the DSS-induced mouse model of UC.
Dandelion root extract suppressed DSS-induced oxidative stress in mice colon. a DSS-induced ROS was inhibited by treatment of dandelion root extract in mice colon. b SOD activity reduced by DSS was restored by treatment of dandelion root extract in mice colon. c The production of DSS-induced MDA was inhibited by administration of dandelion root extract in mice colon. Error bars ± SD, *p < 0.05, **p < 0.01
Discussion
This study shows the anti-colitis potential of aqueous dandelion root extract with in vitro and in vivo models. Our results clearly demonstrated that dandelion root extract has a cytoprotective effect against DSS-induced damage in both the NCM460 cells and the mouse colon. DSS, one of the bioreactive sulfated polysaccharides, has frequently been applied for decades to induce inflammation in experimental models. Normally, the initial immune response in the colon against DSS is the vigorous recruitment of neutrophils. Neutrophils accumulated in the colon express proinflammatory cytokines, such as IL-1β, IL-6 and TNF-α, thereby leading to colon injury [3]. In this study, we found that neutrophils and expressed cytokines (IL-6, IL-1β and TNF-α) increased evidently in colon tissue after DSS exposure. As expected, histopathological study indicated that pretreatment with dandelion root extract markedly attenuated neutrophil infiltration in the colon, and ELISA study revealed that pretreatment with dandelion root extract evidently decreased the production of these cytokines. MPO is a main constituent of neutrophil cytoplasmic granules and the total activity of MPO in a tissue is therefore a direct assessment of neutrophil sequestration in that tissue [23]. Consistent with the decreased neutrophils, oral administration of dandelion root extract strongly reduced DSS-induced MPO activity in the colon tissue. Body weight, disease index, and colon length were evaluated as indicators of cytokine-related colon injury [24]. In the present study, it was found that dandelion root extract could decrease DSS-induced colon injury. These results suggested that dandelion root extract has a protective effect on DSS-induced acute colitis through reducing neutrophil accumulation in the colon.
Excess ROS generation contributes substantially to the initiation and progression of inflammation as well as oxidative injury [25]. Oxidative stress is able to produce an accumulation of oxidative DNA damage in the inflamed cell or tissue, finally resulting in cellular dysfunction or death [26]. DSS has two ways to generate ROS—one is the well-known pathway of free radical generation by oxygen species and the other is to impose a sulfate load on cells through a sulfate assimilation pathway [20, 27, 28]. In the experiments presented in this report, ROS was produced excessively in human colonic epithelial cells and mice colon tissue response to DSS-induced stress, thereby accelerating apoptosis of colonocytes. GSH, an endogenous antioxidant that can restore cellular homeostasis by effectively inhibiting the increase in ROS level [29], and SOD, an enzyme that eliminates excess ROS by alternately catalyzing the dismutation of superoxide radical into either ordinary molecular oxygen (O2) or hydrogen peroxide (H2O2) [30], were reduced, respectively, in human colonic epithelial cells and mice colon tissue after DSS treatment, indicating that DSS stimulation breaks the anti-oxidative systems in intestinal epithelium. In addition, lipid oxidation was increased in the proinflammatory stimulus of DSS exposure as evidenced by the MDA level [31]. Interestingly, administration of dandelion root extract could totally reverse all these phenotypes, suggesting that inhibiting apoptosis and colon injury by exogenous dandelion root extract was achieved by restoring the anti-oxidative systems.
Recently, dietary supplementation with generally used antioxidants such as vitamin E, carotenes and other anti-oxidative agents has been demonstrated to virtually increase the risk of cancer and accelerate the progression of tumors [32,33,34]. Thus, enhancing the body’s own endogenous antioxidant defense system may be a better choice for developing a potentially helpful therapy to attenuate or treat UC. Given that dandelion root extract can activate the endogenous anti-oxidative systems and is not toxic according to recent research [10], we believe that dandelion root extract will be beneficial in the treatment of UC.
NF-κB is an inflammation-associated signal that is susceptible to changes in the intracellular reduction–oxidation state [35]. NF-κB modulates transcriptional activity by specifically binding to DNA sequences in IL-8 that are involved in the inflammatory process of UC [36,37,38]. DSS exposure has been reported to induce an increase in NF-κB activation and IL-8 secretion in colonic epithelial cells directly through a distinct pathway activated by ROS, not involving the TLR4-MyD88 pathway of the innate pathway [20]. IL-8 subsequently induces secondary immune responses of inflammatory infiltrate [39]. Our results showed that dandelion root extract could decrease activation of the NF-κB pathway and secretion of IL-8 in human colonic epithelial cells.
The active constituents of dandelion root extracts are various terpenes, phenolic compounds and others [40]. Among the members of terpenes, the reported compound that is involved in anti-inflammatory activity is taraxasterol [12]. Taraxasterol administration ameliorates LPS-induced endotoxic shock in a murine model through meaningfully reducing secretion of inflammatory cytokines including TNF-α, IFN-γ, IL-1β, and IL-6 [41]. Additionally, a recent report revealed that the anti-inflammatory activity of taraxasterol probably results from its ability to block the NF-κB and MAPK signaling pathways [42]. Two ingredients in phenolic compounds that contribute to anti-inflammatory activity are chicoric acid and luteolin, which can relieve LPS-induced oxidative stress and inflammation in RAW 264.7 macrophages [43]. Other potential active ingredients of dandelion root extract are polysaccharides, which represent hepatoprotective activity by modulating inflammatory responses and reducing oxidative stress in Sprague–Dawley rats [44]. All of the above-mentioned active ingredients may play an important role in DSS-induced colitis and need to be determined in the future.
Conclusion
Our results first showed that aqueous dandelion root extract has anti-inflammatory effects on human colonic epithelial cells as evidenced by increasing DSS-reduced cell viability and attenuating DSS-induced apoptosis, ROS, and NF-κB signal activation. In a mouse model of UC, treatment with dandelion root extract markedly ameliorated DSS-induced UC through controlling the inflammatory conditions and oxidative stress. Therefore, we can conclude that dandelion root extract, a complex mixture, can be an effective anti-colitis drug and can provide a complementary alternative to currently available therapeutic intervention in UC.
References
Marchi P, Paiotti APR, Neto RA, Oshima CTF, Ribeiro DA (2014) Concentrated grape juice (G8000™) reduces immunoexpression of iNOS, TNF-alpha, COX-2 and DNA damage on 2, 4, 6-trinitrobenzene sulfonic acid-induced-colitis. Environ Toxicol Pharmacol 37:819–827
Liu TC, Stappenbeck TS (2016) Genetics and pathogenesis of inflammatory bowel disease. Annu Rev Pathol 11:127–148
Lu MC, Ji JA, Jiang YL, Chen ZY, Yuan ZW, You QD, Jiang ZY (2016) An inhibitor of the Keap1–Nrf2 protein–protein interaction protects NCM460 colonic cells and alleviates experimental colitis. Sci Rep 6:26585
Artis D (2008) Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol 8:411–420
Rosenberg LN, Peppercorn MA (2010) Efficacy and safety of drugs for ulcerative colitis. Expert Opin Drug Saf 9:573–592
Abu-Rabia A (2015) Key plants in fighting cancer in the Middle East. Chin Med 6:124
Sigstedt SC, Hooten CJ, Callewaert MC, Jenkins AR, Romero AE, Pullin MJ, Kornienko A, Lowrey TK, Slambrouck SV, Steelant WF (2008) Evaluation of aqueous extracts of Taraxacum officinale on growth and invasion of breast and prostate cancer cells. Int J Oncol 32:1085–1090
Ovadje P, Chatterjee S, Griffin C, Tran C, Hamm C, Pandey S (2011) Selective induction of apoptosis through activation of caspase-8 in human leukemia cells (Jurkat) by dandelion root extract. J Ethnopharmacol 133:86–91
Ovadje P, Chochkeh M, Akbari-Asl P, Hamm C, Pandey S (2012) Selective induction of apoptosis and autophagy through treatment with dandelion root extract in human pancreatic cancer cells. Pancreas 41:1039–1047
Ovadje P, Ammar S, Guerrero JA, Arnason JT, Pandey S (2016) Dandelion root extract affects colorectal cancer proliferation and survival through the activation of multiple death signalling pathways. Oncotarget 7:73080–73100
Yarnell E, Abascal K (2009) Dandelion (Taraxacum officinale and T. mongolicum). Integr Med 8:35–38
Martinez M, Poirrier P, Chamy R, Prüfer D, Schulze-Gronover C, Jorquera L, Ruiz G (2015) Taraxacum officinale and related species−an ethnopharmacological review and its potential as a commercial medicinal plant. J Ethnopharmacol 169:244–262
Ma C, Zhu L, Wang J, He H, Chang X, Gao J, Shumin W, Yan T (2015) Anti-inflammatory effects of water extract of Taraxacum mongolicum hand.-Mazz on lipopolysaccharide-induced inflammation in acute lung injury by suppressing PI3K/Akt/mTOR signaling pathway. J Ethnopharmacol 168:349–355
You Y, Yoo S, Yoon HG, Park J, Lee YH, Kim S, Oh KT, Lee J, Cho HY, Jun W (2010) In vitro and in vivo hepatoprotective effects of the aqueous extract from Taraxacum officinale (dandelion) root against alcohol-induced oxidative stress. Food Chem Toxicol 48:1632–1637
Tang C, Kamiya T, Liu Y, Kadoki M, Kakuta S, Oshima K, Hattori M, Takeshita K, Kanai T, Saijo S, Ohno N, Iwakura Y (2015) Inhibition of dectin-1 signaling ameliorates colitis by inducing Lactobacillus-mediated regulatory T cell expansion in the intestine. Cell Host Microbe 18:183–197
Chen XH, Zhang BW, Li H, Peng XX (2015) Myo-inositol improves the host’s ability to eliminate balofloxacin-resistant Escherichia coli. Sci Rep 5:10720
Chen XH, Liu SR, Peng B, Li D, Cheng ZX, Zhu JX, Zhang S, Peng YM, Li H, Zhang TT, Peng XX (2017) Exogenous l-valine promotes phagocytosis to kill multidrug-resistant bacterial pathogens. Front Immunol 8:207
Dai M, Wang F, Zou Z, Xiao G, Chen H, Yang H (2017) Metabolic regulations of a decoction of Hedyotis diffusa in acute liver injury of mouse models. Chin Med 12:35
Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116:205–219
Bhattacharyya S, Dudeja PK, Tobacman JK (2008) ROS, Hsp27, and IKKβ mediate dextran sodium sulfate (DSS) activation of IκBa, NFκB, and IL-8. Inflamm Bowel Dis 15:673–683
Francescone R, Hou V, Grivennikov SI (2015) Cytokines, IBD and colitis-associated cancer. Inflamm Bowel Dis 21:409–418
Zhong H, May MJ, Jimi E, Ghosh S (2002) The phosphorylation status of nuclear NF-κB determines its association with CBP/p300 or HDAC-1. Mol Cell 9:625–636
Shenkar R, Abraham E (1999) Mechanisms of lung neutrophil activation after hemorrhage or endotoxemia: roles of reactive oxygen intermediates, NF-κB, and cyclic AMP response element binding protein. J Immunol 163:954–962
Kubinak JL, Petersen C, Stephens WZ, Soto R, Bake E, O’Connell RM, Round JL (2015) MyD88 signaling in T cells directs IgA-mediated control of the microbiota to promote health. Cell Host Microbe 17:153–163
Yum H-W, Zhong X, Park J, Na HK, Kim N, Lee HS, Surh YJ (2013) Oligonol inhibits dextran sulfate sodium-induced colitis and colonic adenoma formation in mice. Antioxid Redox Signal 19:102–114
Jena G, Trivedi PP, Sandala B (2012) Oxidative stress in ulcerative colitis: an old concept but a new concern. Free Radic Res 46:1339–1345
Chang TS, Jeong W, Woo HA, Lee SM, Park S, Rhee SG (2004) Characterization of mammalian sulfiredoxin and its reactivation of hyperoxidized peroxiredoxin through reduction of cysteine sulfinic acid in the active site to cysteine. J Biol Chem 279:50994–51001
Kopriva S (2006) Regulation of sulfate assimilation in Arabidopsis and beyond. Ann Bot 97:479–495
Aleksunes LM, Manautou JE (2007) Emerging role of Nrf2 in protecting against hepatic and gastrointestinal disease. Toxicol Pathol 35:459–473
Hayyan M, Hashim MA, AlNashef IM (2016) Superoxide ion: generation and chemical implications. Chem Rev 116:3029–3085
Kenmogne-Domguia HB, Moisan S, Viau M, Genot C, Meynier A (2014) The initial characteristics of marine oil emulsions and the composition of the media inflect lipid oxidation during in vitro gastrointestinal digestion. Food Chem 152:146–154
Risk L (2011) Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 306:1549–1556
Le Gal K, Ibrahim MX, Wiel C, Sayin VI, Akula MK, Karlsson C, Dalin MG, Akyürek LM, Lindahl P, Nilsson J, Bergo MO (2015) Antioxidants can increase melanoma metastasis in mice. Sci Transl Med 7:308re8
Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO (2014) Antioxidants accelerate lung cancer progression in mice. Sci Transl Med 6:221ra15
Ginn-Pease ME, Whisler RL (1998) Redox signals and NF-κB activation in T cells. Free Radic Biol Med 25:346–361
Hegazy SK, El-Bedewy MM (2010) Effect of probiotics on pro-inflammatory cytokines and NF-κB activation in ulcerative colitis. World J Gastroenterol 16:4145–4151
Shin HS, Satsu H, Bae MJ, Zhao Z, Ogiwara H, Totsuka M, Shimizu M (2015) Anti-inflammatory effect of chlorogenic acid on the IL-8 production in Caco-2 cells and the dextran sulphate sodium-induced colitis symptoms in C57BL/6 mice. Food Chem 168:167–175
Dae Park D, Yum H-W, Zhong X, Kim SH, Kim SH, Kim DH, Kim SJ, Na HK, Sato A, Miura T, Surh YJ (2017) Perilla frutescens extracts protects against dextran sulfate sodium-induced murine colitis: NF-κB, STAT3, and Nrf2 as putative targets. Front Pharmacol 8:482
Kryczek I, Wang L, Wu K, Li W, Zhao E, Cui T, Wei S, Liu Y, Wang Y, Vatan L, Szeliga W, Greenson JK, Roliński J, Zgodzinski W, Huang E, Tao K, Wang G, Zou W (2016) Inflammatory regulatory T cells in the microenvironments of ulcerative colitis and colon carcinoma. Oncoimmunology 5:e1105430
González-Castejón M, Visioli F, Rodriguez-Casado A (2012) Diverse biological activities of dandelion. Nutr Rev 70:534–547
Zhang X, Xiong H, Li H, Cheng Y (2014) Protective effect of taraxasterol against LPS-induced endotoxic shock by modulating inflammatory responses in mice. Immunopharmacol Immunotoxicol 36:11–16
San Z, Fu Y, Li W, Zhou E, Li Y, Song X, Wang T, Tian Y, Wei Z, Yao M, Cao Y, Zhang N (2014) Protective effect of taraxasterol on acute lung injury induced by lipopolysaccharide in mice. Int Immunopharmacol 19:342–350
Park CM, Park JY, Song YS (2010) Luteolin and chicoric acid, two major constituents of dandelion leaf, inhibit nitric oxide and lipid peroxide formation in lipopolysaccharide-stimulated RAW 264.7 cells. Int J Food Sci Nutr 15:92–97
Park CM, Youn HJ, Chang HK, Song YS (2010) TOP1 and 2, polysaccharides from Taraxacum officinale, attenuate CCl4-induced hepatic damage through the modulation of NF-κB and its regulatory mediators. Food Chem Toxicol 48:1255–1261
Author information
Authors and Affiliations
Contributions
AGD designed the experiments. AGD performed the experiments. XHW analyzed the data. XHW wrote the paper. All the authors read and approved the final manuscript.
Corresponding author
Rights and permissions
About this article
Cite this article
Ding, A., Wen, X. Dandelion root extract protects NCM460 colonic cells and relieves experimental mouse colitis. J Nat Med 72, 857–866 (2018). https://doi.org/10.1007/s11418-018-1217-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-018-1217-7