Abstract
Lectins are a class of carbohydrate-binding proteins or glycoproteins and used in the purification and characterization of glycoproteins according to their specificity to carbohydrates. In the present study, the mitogenic activity of Artocarpus lingnanensis lectin (ALL) and its apoptosis induction in Jurkat T cells were explored. MTT assay revealed strong mitogenic potential of ALL. Meanwhile, the anti-cancer activity of ALL was also explored using the human leukemic Jurkat T cell line. ALL exhibited strong binding affinity (97%) to the cell membrane, which could be effectively inhibited by N-acetyl-d-galactosaminide (NAD). ALL induced time- and dose-dependent growth inhibition in Jurkat T cells. ALL could induce morphologic change and increase the hypodiploid cell population with the decreased population of S and G2/M phases. The induction of phosphatidylserine externalization and PARP cleavage further confirmed its apoptosis-inducing activity due to the activation of caspase-8 and -9. The inhibition of caspase-9 but not caspase-8 could rescue ALL-induced apoptotic cells. Further studies showed that ALL enhanced the cleavage of Bid, the release of cytochrome C, the depolarization of mitochondria and the activation of caspase-3. ALL downregulated the expression of Bcl-xl and Bcl-2 without impact on Bax and Bad. In addition, the activation of p38/JNK MAPK signaling pathways was observed to be a requisite for ALL apoptotic activity. In contrast, ALL could not induce apoptosis of normal T cells. These findings present the differential effect of ALL on Jurkat and normal T lymphocytes, suggesting its therapeutic value in leukemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lectins are a group of sugar-binding proteins or glycoproteins, which are classified based on the specificity of carbohydrates such as galactose, glucose and mannose [1]. Lectins have been found in many plants [2, 3] and exhibit different biologic activities including anti-microorganism, anti-tumor and immunostimulation activities, which provide the potential to develop immunomodulatory agents and anti-tumor drugs using lectins. It has been reported that mistletoe lectin (Viscum album coloratum) showed immunostimulatory and cytotoxic effects against tumor cells [4,5,6]. Nevertheless, it is still necessary to screen novel lectins with high bioactivity such as immunomodulatory and anti-tumor activities.
Artocarpus (Moraceae) is a genus of approximately 50 species widely grown in tropical and subtropical areas [7]. It is known as a family containing high-level flavonoids and other phenolic compounds with different biologic activities [8]. It can be used in traditional medicine to treat inflammation, cirrhosis, hypertension, malarial fever, dysentery and tuberculosis and to control the blood sugar levels in diabetic patients [8, 9]. Artocarpus nitidus subsp. lingnanensis, an evergreen tree grown in South China, is a Chinese herbal medicine used to treat gastritis and rheumatism [10].
In the present study, Artocarpus lingnanensis lectin (ALL) purified from Artocarpus nitidus subsp. lingnanensis is explored. The mitogenic activity and apoptosis induction in tumor cells were investigated. The lectin exhibited immunostimulatory functions by inducing a mitogenic response and cytokine secretion in normal splenocytes. In addition, ALL had an anti-proliferative effect on Jurkat T leukemia cells by inducing apoptosis. The immunomodulatory and anti-tumor effects of ALL suggested its potential medicinal applications.
Materials and methods
Purification of Artocarpus lingnanensis lectin
ALL was isolated from the seeds of Artocarpus nitidus subsp. lingnanensis following the method described by Cui et al. [11]. Conjugation of ALL with fluorescein isothiocyanate (FITC) was conducted as previously described by Cui et al. [11].
Assay for mitogenic activity in mouse splenocytes
All animal experiments were approved by the Animal Care and Use Committee of Guangxi Medical University (Nanning, China). BALB/c mice (20–25 g) were killed by cervical dislocation. Splenocytes were separated, and the cell concentration was adjusted to 4 × 106 cells/ml in RPMI 1640 culture medium. In total, 100 µl of splenocytes was seeded to 96-well plates followed by 100 µl of ALL at various concentrations (0.5–40 μg/ml) with or without NAD (20 mM). Concanavalin A (Con A) at 5 μg/ml was used as the positive control. The cells were incubated at 37 °C for 48 h in a humidified atmosphere supplemented with 5% CO2. Cells were pulsed with [3H]-thymidine (0.5 mCi/well, GE Healthcare, Bucks, UK) for 16 h. The cells were subsequently harvested onto glass fiber. [3H] Thymidine incorporation was determined with a β-Scintillation Counter (Wallac, Milton Keynes, Bucks, UK).
Cytokine ELISA assay
Culture supernatants were harvested from splenocytes treated with ALL at 10 μg/ml or Con A at 5 μg/ml or maintained only in culture medium (control) for 48 h. The secretion of IFN-γ, IL-2 and IL-4 was analyzed using an ELISA kit according to the manufacturer’s protocol. All assays were performed in triplicate.
Cultivation of Jurkat T cells and normal T lymphocytes
Human T-lymphoma Jurkat cells were purchased from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). The cell line was cultivated in RPMI-1640 culture medium (Hyclone, Logan, UT, USA) at 37 °C in a humidified atmosphere with 5% CO2.
Peripheral blood mononuclear cells were separated from healthy donors by Ficoll density gradient centrifugation using standard procedures. Individuals were recruited between October 2013 and January 2014. Selected individuals were informed about the study and invited to participate after consenting, and the participants’ identity and records were anonymized prior to analysis. Peripheral T lymphocytes were separated using the RosetteSep human T cell enrichment cocktail (Stemcell Technologies, Vancouver, Canada). The purified T lymphocytes (> 95% CD3+ T cells) were cultivated in RPMI 1640 culture medium (106 cells/ml) at 37 °C in a 5% CO2 incubator before use. The study was conducted after obtaining consent from each of the participants and approved by the Ethics Committee of Guangxi Medical University.
Analysis of lectin binding
Jurkat T cells were blocked with 3% BSA, followed by staining with FITC-labeled ALL at 4 °C for 1 h. Carbohydrate-mediated binding was analyzed by pretreating the FITC-ALL with 20 μM of NAD for 1 h at 37 °C prior to staining. Samples were analyzed by a FACS Calibur cytometer (Becton-Dickinson, Mountain View, CA, USA).
Carbohydrate binding was also analyzed by confocal microscopy. Jurkat cells were incubated in FITC-ALL for 1 h at 4 °C and then fixed with 2% para-formaldehyde for 10 min followed by staining with DAPI. Samples were visualized with confocal laser scanning microscopy (Zeiss LSM 510, Göttingen, Germany).
Cell proliferation assay
Jurkat cells were seeded in a 96-well plate and incubated in RPMI-1640 culture medium (2.5 × 104 cells/ml) overnight. ALL at different concentrations (100 µl, final concentrations of 0–160 μg/ml) was added to the wells. To observe the competitive effect on carbohydrates, ALL (20 μg/ml) was pretreated with 20 μM NAD for 1 h as the described above. After incubation for 48 h, the cell proliferation was determined with cell counting kit-8 (CCK-8; Sigma, USA). The percentage of viable cells was calculated with respect to the control considered as 100%. The IC50 was calculated by the Probit regression analysis in SPSS 13.0 (SPSS Inc., Chicago, IL, USA).
To determine the involvement of caspases or the MAPK signal pathway in ALL-induced apoptosis, Jurkat cells were pre-incubated with the pan-caspase inhibitor ZVAD-FMK, caspase-9 inhibitor Z-LEHD-FMK, caspase-8 inhibitor Z-IETD-FMK (BD Biosciences, Franklin Lakes, NJ, USA), p-p38 inhibitor SB203580 and p-JNK inhibitor SP600125 (Sigma Chemical Co., St. Louis, MO, USA) followed by CCK-8 assay.
Cell cycle analysis
Jurkat T cells were incubated in RPMI-1640 culture medium containing 5, 10 and 20 μg/ml ALL, respectively, for 24 h, harvested by centrifugation, washed with PBS and fixed with ice-cold 70% ethanol at 4 °C overnight, followed by incubating with 0.1% Triton X-100, 0.2 mg/ml RNase A (Sigma Chemical Co., St. Louis, MO, USA) and 50 μg/ml PI (Sigma Chemical Co., St. Louis, MO, USA) at 4 °C for 30 min in a dark environment. The DNA content was then determined using FACSCalibur (Becton-Dickinson, Mountain View, CA, USA).
Hoechst 33258 fluorescent staining
Jurkat cells were collected and fixed with 4% paraformaldehyde for 10 min followed by staining with Hoechst 33258 (Molecular Probes, Eugene, OR, USA) for 10 min. Nuclear alteration-related apoptosis was examined by fluorescence microscope.
Annexin-V and PI staining
After treatment with ALL at various concentrations for 24 h, cells were collected and stained with Annexin V-FITC (BD Phamingen, CA, USA) and PI at room temperature for 15 min. Percentages of apoptotic cells were determined with a FACSCalibur flow cytometer (Becton-Dickinson, Mountain View, CA, USA).
Determination of caspase activity
Caspase activity was determined using a colorimetric assay kit (MBL, MBL International, Woburn, MA, USA) following the manufacturer’s protocol. With or without pretreatment of 20 μM p-JNK inhibitor SP600125 or p-p38 inhibitor SB203580 for 30 min, Jurkat cells were incubated with ALL (20 μg/ml) for a designated time, and then the cells were collected and lysed. After centrifugation at 10,000g for 10 min, the concentration of protein in the supernatant was determined using a BCA protein assay kit (Pierce, Rockford, IL, USA) following the manufacturer’s protocol. Samples (50 μg) were incubated with the colorimetric substrates (LEHD-pNA for caspase-9, DEVD-pNA for caspase-3 and IETD-pNA for caspase-8) for 2 h at 37 °C, and the absorbance was determined at 405 nm.
Mitochondrial membrane potential (MMP) analysis
The alteration in MMP in ALL-treated Jurkat cells was determined using JC-1 dye, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzamidazolocarbocyanin iodide (Molecular Probes, Eugene, OR, USA). Red emission from JC-1 reflects the accumulation of JC-1 monomers in normal polarized mitochondria. Green fluorescence indicated the monomeric form of JC-1 when the mitochondrial membrane depolarized. Cells were stained with JC-1 at 37 °C for 15 min and determined with a FACSCalibur flow cytometer (Becton–Dickinson, Mountain View, CA, USA).
Western blotting
Samples were separated by 10% SDS-PAGE and blotted onto nitrocellulose membrane (Schleicher & Schuell, Keene, NH, USA). The membrane was blocked in TBS-T containing 10% nonfat milk for 1 h and incubated with antibodies for PARP (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), cytochrome C, Bid, Bcl-xl, Bcl-2, Bax, Bad (BD Biosciences, Franklin Lakes, NJ, USA), caspase-3, ERK1/2, JNK1/2 and p38 (Cell Signaling Technology Inc., Beverly, MA, USA) overnight at 4 °C followed by horseradish peroxidase (HRP)-conjugated secondary antibodies (Cell Signaling Technology Inc., Beverly, MA, USA). Proteins were detected with an enhanced chemiluminescence (ECL) kit (Pierce Biotechnology, Rockford, IL, USA).
Statistical analysis
All data were analyzed with GraphPad Prism 3.0, and comparisons were analyzed by one- or two-way ANOVA with Bonferroni’s post hoc tests. p < 0.05 was considered a significant difference.
Results
Mitogenic activity of ALL in mouse splenocytes
To assay the general effect of ALL on immune cells, we examined the proliferation of splenocytes in the presence of ALL at different concentrations with Con A as a positive control. As shown in Fig. 1a, ALL exerted immunomodulatory effects. Treatment with 1–40 μg/ml ALL promoted splenocyte proliferation significantly (p < 0.01); however, 20 mM NAD weakened the mitogenic activity of ALL.
Effect of ALL on murine splenocytes. a Mitogenic response of murine splenocytes induced by ALL. Cells were stimulated by ALL with or without NAD (20 mM) at the designated concentrations or with 5 μg/ml Con A (positive control) for 48 h at 37 °C. Proliferative activity is represented by scintillation counts per minute (CPM). Data represented as mean ± SD (n = 3). *p < 0.05, significant difference compared with control. **p < 0.01, significant difference compared with 20 μg/ml ALL-treated cells. b Cytokine production induced by ALL in mouse splenocyte. Splenocytes were treated with ALL and ConA as described above for 48 h, and then the levels of IL-2 (i), IFN-γ (ii) and IL-4 (iii) in the supernatants were analyzed using ELISA kits. Results were expressed as mean ± SE (n = 3). *p < 0.05, **p < 0.01 compared with untreated cells
Immunologic assays were also performed to analyze the degree of immune response induced by ALL. As shown in Fig. 1b, ALL augmented the secretion of some cytokines. IFN-γ and interleukin-2 were the major cytokines produced by mouse splenocytes treated with ALL, so ALL induced a Th1 preferential response; however, the expression of IL-4 was also upregulated in the presence of 10 μg/ml ALL.
Binding of ALL to Jurkat T cells
FITC-ALL-stained Jurkat T cells were analyzed by flow cytometric analysis. Approximately 97.12% of Jurkat T cells were stained with MFI of 187.96 compared with control cells with MFI of 3.47. Carbohydrate-mediated binding of ALL was analyzed with NAD, which significantly inhibited ALL binding to cells with MFI of 29.61 (Fig. 2a). ALL binding was also observed with confocal microscopy after staining with FITC-ALL. These results indicated that high-level ALL receptors were expressed on the surface of Jurkat T cells (Fig. 2b).
Binding of FITC-ALL to Jurkat T cells. a Cells were treated with FITC-ALL (5 μg/ml) alone or pre-incubated with NAD (20 mM), and ALL binding was analyzed by flow cytometry. X- and Y-axes represent fluorescence intensity and cell counts, respectively. b Jurkat T cells were stained with FITC-ALL and DAPI, followed by visualizing with confocal microscopy. Magnification ×40
The inhibition of ALL on proliferation of Jurkat cells
To evaluate the impact of ALL on cell proliferation, the cells were incubated with ALL (0–160 μg/ml) for 48 h or ALL (20 μg/ml) for an indicated time. The cell proliferation was analyzed using CCK-8 tests. Figure 3 shows that ALL significantly inhibited cell growth from 24 h in a time-dependent manner (Fig. 3a, b) and that ALL (10 μg/ml or above) showed its obvious inhibition in a concentration-dependent manner (Fig. 3c). Lower concentration of ALL (5 μg/ml) did not induce the significant inhibition of cell proliferation. The IC50 of ALL was evaluated as 43.57 μg/ml. However, in the presence of 20 mM NAD, the inhibition of ALL-induced proliferation was attenuated (Fig. 3d). These preliminary results suggest that ALL inhibited Jurkat T cell proliferation dose and time dependently through the interaction with the glycoproteins on cell surfaces.
Effect of ALL on the proliferation in Jurkat T cells. a Cells were incubated with ALL (20 μg/ml) for a designated time. Cell viability was determined using the CCK-8 test. *p < 0.05 and **p < 0.01 compared with control cells. b The total number of cells was counted after ALL (20 μg/ml) treatment for the designated time. *p < 0.05 and **p < 0.01 compared with control cells. c Cells were incubated with ALL at different concentrations (5–160 μg/ml) for 48 h. Cell viability was measured using CCK-8 assays. *p < 0.05 and **p < 0.01 compared with untreated cells. d Cells were incubated with ALL (20 μg/ml) alone or pre-incubated with NAD (20 mM), followed by the analysis of cell viability using CCK-8 test. *p < 0.05 compared with control cells. e Effect of ALL on the proliferation of the EL-4 thymoma cell line. Cells were incubated with ALL at different concentrations (5–160 μg/ml) for 48 h. Cell viability was measured by CCK-8 test. *p < 0.05 and **p < 0.01 compared with untreated cells
In additional, the effect of ALL on other tumor cells was also investigated. Similarly, ALL could significantly inhibit the growth of the EL-4 thymoma cell line in a concentration-dependent manner with the IC50 of 248.2 μg/ml (Fig. 3e).
Effect of ALL on cell cycle
To evaluate whether ALL-mediated inhibition of cell proliferation is correlated with cell cycle progression, the cells treated with different concentrations of ALL were analyzed to evaluate the apoptotic cells and cell cycle distribution by flow cytometry. Treatment with 5 μg/ml ALL for 24 h could not result in an apparent increase in the hypodiploidic cell population (6.28%) compared with untreated cells, but compared with 2.37% of untreated cells, there were 22.37% hypodiploid cells when treated with 20 μg/ml ALL (Fig. 4a). ALL also reduced the cell population at S and G2/M phases to 9.35 and 14.41% compared with 16.85 and 21.56% of untreated controls, respectively.
ALL-induced apoptosis of Jurkat T cells. a ALL induced an increase in the hypodiploid cell population. Cells were incubated with ALL for 24 h followed by staining with PI and then DNA content analysis by flow cytometry. The data were expressed as mean ± SE (n = 3). *p < 0.05 compared with control cells. b Morphologic change of nuclei in ALL-treated Jurkat T cells. Cells were incubated with ALL (20 μg/ml) for 48 h followed by Hoechst 33258 staining. Control cells (left panel) and ALL-treated cells (right panel). Apoptotic nuclei indicated by arrows. c Dose-dependent apoptosis induced by ALL. Cells were incubated with ALL at different concentrations for 48 h followed by Annexin V and PI staining. X- and Y-axes represent Annexin V-positive cells (the percentage was presented in the lower panel) and PI-positive cells, respectively. The data were expressed as mean ± standard deviation (M ± SD) (n = 3). *p < 0.05 and **p < 0.01 vs. control cells. d Cell lysates of Jurkat T cells were incubated with ALL (5–20 μg/ml) for 24 h, and the cleavage of PARP was analyzed by Western blot
ALL-induced apoptosis in Jurkat T cells
To further explore the mechanism of ALL-inhibited proliferation, first we observed the morphologic change in ALL-treated cells stained with Hoechst 33258. As shown in Fig. 4b, ALL induced typical morphologic alteration of apoptosis including karyorrhexis, chromatin condensation and fragmentation. ALL-inhibited proliferation of Jurkat T cells was further studied by flow cytometry analysis using Annexin V/PI staining. With the increase of ALL concentration, the cell population in the lower left quadrant moved to the lower right quadrant, indicating Jurkat T cells suffering apoptotic lesions at the early stage (Fig. 4c). The cell population in the upper right quadrant had a mild increase, suggesting the increased apoptotic cells at the late stage. The graph depicts the apoptosis percentage of Jurkat T cells after ALL treated with various concentrations (Fig. 4c).
PARP cleavage was analyzed by Western blot. A cleaved fragment with a molecular mass of 89 kDa was found in Jurkat T cells with ALL treatment (10 or 20 μg/ml) for 24 h (Fig. 4d), suggesting that ALL-inhibited proliferation may be caused by its induced apoptosis of Jurkat T cells.
ALL-induced apoptosis in a caspase-dependent manner
To explore the mechanisms of ALL-induced apoptosis, caspases-3, -8 and -9 activities were analyzed with a colorimetric assay. As shown in Fig. 5a, there was a difference among different caspase activation: a 1.65-fold increase within 6 h and twofold within 48 h in caspase-9 activity and similarly a 1.98-fold increase within 6 h and 2.3-fold within 48 h in caspase-3 activity were found in ALL-treated cells, respectively. The activity of caspase-8 increased by 1.45-fold in 6 h and maintained a stable level after treatment up to 48 h (Fig. 5a).
Caspase-9 was involved in ALL-induced caspase-dependent apoptosis. a Jurkat T cells were incubated with 20 μg/ml ALL during the designated time, and the activities of caspase-9, -8, -3 were assessed by colorimetric assay, respectively. The activity at 0 h was defined as 1. The data were expressed as mean ± standard deviation (M ± SD) (n = 3). *p < 0.05 vs. 0 h control. b Jurkat T cells were pretreated with pan-caspase inhibitor-Z-VAD-FMK, caspase-8 and -9 inhibitors Z-IETD-FMK and Z-LEHD-FMK for 1 h before ALL (20 μg/ml) treatment for 48 h. Cell viability was assessed by CCK-8 test. The data were depicted as mean ± standard deviation (M ± SD) (n = 3). *p < 0.05 vs. control cells. c Whole cell lysates were exposed to ALL (20 μg/ml) alone or pre-incubated with caspase-9 inhibitor zLEHD-FMK (50 μM) and probed with anti-caspase-3 antibody. The blot showed the total and cleaved caspase-3
To further determine the function of caspases in ALL-induced apoptosis, Jurkat T cells were pretreated with pan-caspase inhibitor-Z-VAD-FMK, caspase-8 and -9 inhibitors Z-IETD-FMK and Z-LEHD-FMK for 1 h before ALL treatment for 48 h. Cell viability after ALL treatment was assessed by the CCK-8 test. Pre-treatment with either Z-LEHD-FMK or Z-VAD-FMK apparently suppressed ALL-inhibited proliferation. Z-IETD-FMK pre-treatment did not reveal an inhibitory effect on ALL-induced cell death (Fig. 5b). Similarly, caspase-9 inhibitior decreased caspase-3 activity significantly (Fig. 5c). These data suggest that caspase-9 was involved in ALL-induced apoptosis.
Loss of mitochondrial membrane potential (MMP)
Caspase-9 is the essential initiator caspase in the mitochondrial apoptotic pathway, and the effect of ALL on the mitochondrial membrane potential was analyzed using fluorescent probe JC-1. As shown in Fig. 6a, ALL induced a dose-dependent depolarization of the mitochondrial membrane as well as the release of mitochondrial cytochrome C into the cytosol (Fig. 6b). Furthermore, ALL treatment caused an apparent reduction of native Bid expression (21 kDa), suggesting the cleavage of Bid and the release of mitochondrial cytochrome C (Fig. 6c). These results indicate that ALL induces mitochondrial perturbation in Jurkat T cells.
ALL induced mitochondrial perturbation and expression of apoptotic-related proteins in Jurkat T cells. a Effect of ALL on MMP. The cells were incubated with ALL at different concentrations for 48 h followed by JC-1 staining (mitochondria-specific dye) and then analyzed with flow cytometry. The percentage of MMP-reduced cells is presented in the lower panel. The data are represented as mean ± standard deviation (M ± SD) (n = 3). *p < 0.05 vs. control cells. b Release of mitochondrial cytochrome C in Jurkat T cells incubated with ALL (20 μg/ml) for different designated times. Organelle and cytosol fractions were isolated, and cytochrome C was analyzed by Western blot. c Jurkat T cells were incubated with ALL (20 μg/ml) for designated times, and the effect of ALL on the cleavage of Bid was analyzed by Western blot. d Jurkat T cells were incubated with ALL (20 μg/ml) for designated times, and Bcl-xl, Bcl-2, Bax and Bad were analyzed by Western blot. e ALL promoted the activation of JNK and p38. Cells were incubated with ALL (20 μg/ml) at the designated time, and the phosphorylation of ERK, JNK and p38 was analyzed by Western blot. f Effects of a JNK inhibitor (SP600125) and a p38 inhibitor (SB203580) on ALL-induced cell death. ALL (20 μg/ml) was incubated in presence of SP600125 (20 μM) or SB203580 (20 μM), and cell viability was analyzed by CCK-8 test after 48 h. Data are representative of three independent experiments performed in triplicate. *p < 0.05 compared with ALL treatment alone. g Effects of SP600125 and SB203580 on caspase-3 activation. Jurkat T cells were incubated with 20 μg/ml ALL for 48 h in the presence of SP600125 (20 μM) or SB203580 (20 μM), and the activity of caspase-3 was assessed by colorimetric assay. The activity of untreated cells was defined as 1. The data were expressed as mean ± standard deviation (M ± SD) (n = 3). *p < 0.05 compared with ALL treatment alone
Downregulation of Bcl-2 and Bcl-xl in ALL-induced apoptosis
The mitochondrial pathway of apoptosis is controlled by Bcl-2 family members [12]. Several proteins of the Bcl-2 family were analyzed in ALL-treated Jurkat T cells. As shown in Fig. 6d, the expression of Bcl-2 and Bcl-xl was downregulated at 12 h after ALL treatment and further decreased at 24 h. However, we did not detect any changes in Bax and Bad. These data indicated that ALL could result in the decrease of anti-apoptotic proteins, but no effect on pro-apoptotic proteins.
ALL induced JNK and p38 phosphorylation
To further explore the apoptotic mechanism of Jurkat T cells induced by ALL, the possible signal transduction pathways were analyzed. As shown in Fig. 6e, JNK1/2 and p38 kinase were activated after 1-h treatment, whereas no change was found in the phosphorylation of ERK. As JNK and p38 are known to induce cell death, we also examined whether JNK and p38 inhibitors can affect ALL-induced cytotoxicity. We confirmed that the phosphorylation of JNK or p38 was inhibited by SP600125 or SB203580, respectively (data not shown). Pretreatment with SP600125 or SB203580 significantly elevated the viability of ALL-treated Jurkat T cells (Fig. 6f) and inhibited caspase-3 activity (Fig. 6g). These data indicate that the p38 and JNK/MAPK signal pathways were involved in ALL-induced apoptosis.
Effect of ALL on normal T lymphocytes
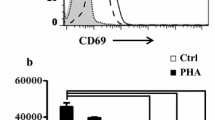
To observe the response of ALL in normal T lymphocytes, human peripheral T lymphocytes were separated by the RosetteSep Ab cocktail, and the purified cells (> 95% CD3+ T cells) were obtained. To evaluate the effect of ALL on normal T lymphocytes, a 3H-thymidine incorporation assay was carried out in ALL-treated T cells. ALL promoted the significant proliferation of normal T cells (Fig. 7a). To confirm that ALL cannot cause apoptosis of normal T cells, the cells were incubated with ALL for 24 h, followed by determining the Annexin-V-positive cells and apoptosis-related proteins. We found that in normal T cells, ALL could not promote Annexin-V binding (Fig. 7b) and change the expression of apoptosis-related proteins (Fig. 7c), indicating that ALL could not cause the apoptosis of normal T lymphocytes.
Effect of ALL on normal human T cells. a T lymphocytes were stimulated with ALL at designated concentrations for 48 h at 37 °C. Proliferation activity was represented by scintillation counts per minute (CPM). The values were expressed as mean ± standard deviation (M ± SD) (n = 3). *p < 0.05 vs. control cells. b T cells were incubated with ALL (20 μg/ml) for 48 h followed by flow cytometry analysis. c T cells were treated with ALL (20 μg/ml) for 24 h, and then the expression of Bcl-2, Bcl-xl and Bax was analyzed by Western blot
Discussion
Plant lectins have huge potential because of their medical and clinical applications [13]. Lectins are a group of proteins with specific sugar-binding specificity. Their multiple functions are tightly correlated with carbohydrate-binding types [14], and their activities are usually suppressed by mono- and oligo-saccharides [15]. This study presents a lectin in seeds of Artocarpus nitidus subsp. lingnanensis, a Moraceae with important applications in traditional folk medicine. Our previous results showed that ALL is an NAD and d-galactose specific lectin [11]. In this study, the mitogenic and anti-proliferative activity of ALL can be inhibited in the presence of NAD. This indicates that ALL's activity depends on carbohydrate recognition. The binding of carbohydrates is reversible so that lectin activity can be modulated by introducing or getting rid of specific carbohydrates, therefore presenting a good strategy to regulate ALL activity.
ALL can induce a mitogenic response in murine splenocytes and stimulate murine splenocytes to produce high-level IL-2, IFN-γ and IL-4 and interferon-γ (IFN-γ). Tumorigenesis usually presents abnormal glycosylation of cell-surface structures that can promote cancer invasion and metastasis [16]. Lectins could bind to the specific carbohydrate and regulate cell proliferation. In the present study, we presented the anti-tumor activity of ALL against a human leukemia cell line, the Jurkat cell line. ALL exhibits the binding to the cell surface. In contrast, its binding can be inhibited in the presence of NAD. ALL can significantly suppress the proliferation and promote the apoptosis of Jurkat cells with typical apoptotic changes, such as nuclear fragmentation and condensation, phosphatidylserine externalization and increased hypodiploid cell population.
Apoptosis is usually involved in death receptor and mitochondrial pathways [17]. Caspases are a family of cysteine proteases that play a key role in the process of apoptosis by cleavage at aspartic acid residues of target proteins [18]. The activation of caspase 3, which acts as the crucial effector caspase, will trigger the occurrence of apoptosis. Many lectins have been reported that can promote apoptosis through a caspase-dependent pathway [19, 20]. Our previous study also demonstrated that ALL can induce human B-lymphoma cell line Raji apoptosis with the involvement of p38 phosphorylation and caspase-3 activation [21]; however, more detailed molecular mechanisms in Raji cells remain unclear.
In the present study, our data demonstrate that ALL could induce the processing of procaspase-9 and procaspase-3, thus leading to the cleavage of PARP. Furthermore, the inhibition of caspase-9 and caspase-3 activity can prevent cell death. However, the inhibition of caspase-8 cannot prevent cell apoptosis. The data indicate that caspase-9 has a key role in ALL-induced apoptosis in Jurkat cells.
ALL-induced apoptosis is accompanied by the downregulation of native Bid. Cleaved BID translocates to mitochondria and interacts with Bcl-2 family proteins, thereby resulting in the release of mitochondrial cytochrome C [22]. In the present study, MMP is significantly lost because of the induction of ALL, indicating the involvement of the mitochondrial apoptotic pathway. The depolarization of the mitochondrial membrane is also similarly observed in Momordica charantia lectin-treated hepatocellular carcinoma cells [23] and P. vulgaris lectin-treated MCF7 cells [20]. The loss of MMP can also promote the release of cytochrome C and pro-apoptotic protein [24]. It has been reported to have downregulated anti-apoptosis proteins, damaged the mitochondrial membrane and released cytochrome C, thereby correspondingly producing the activated caspase-9 and caspase-3 in Polygonatum odoratum lectin-treated cells [25]. Our data indicate that ALL can downregulate anti-apoptosis Bcl-2 family members such as Bcl-2 and Bcl-xl without affecting the proapoptosis proteins, therefore resulting in cell death. These results further suggest that ALL induced a caspase-dependent apoptosis due to the occurrence of mitochondrial perturbation.
Some different mechanisms of the anti-proliferation roles of lectins have been reported. ConA is taken up by tumor cells, gathered in mitochondria and then sequentially downregulates Akt phosphorylation [26]. Polygonatum cyrtonema lectin can cause MTP collapse by regulating Bcl-2 family members, thus inducing apoptosis in a caspase-dependent manner by inactivating ERK, PI3K–Akt ROS-mediated p38–p53 pathways [27, 28]. The activation of p38 and JNK are proapoptotic [29], and the activation of ERK is correlated to both anti- and pro-tumor activity [30, 31]. Our studies show that ALL cannot induce the activation of ERK, but can induce the phosphorylation of p38 and JNK as early as 1 h post-incubation with ALL, indicating that the activation of p38 and JNK should be prior to mitochondrial perturbation. We further confirmed that JNK inhibitor SP600125 or p38 inhibitor SB203580 significantly mitigated ALL-induced cytotoxic effects and caspase-3 activity in Jurkat T cells. Previous reports showed that the activation of JNK and p-38 signaling induced mitochondria-mediated apoptosis through the regulation of the Bcl-2 family [32, 33]. Taken together, the effects of ALL in human T-lymphoma Jurkat cells may occur by activating JNK and p-38 signaling and inducing a caspase-dependent mitochondria-mediated apoptosis.
ALL presents different actions on different cells. It can evoke mitogenic activity toward mouse splenocytes at the dose of 1–40 μg/ml, while it also can accomplish the anti-proliferative activity on the Jurkat T cell line. This shows that ALL can initiate its immunomodulatory effect on splenocytes and its anti-proliferative effect on Jurkat T cells simultaneously. Both activities suggest the therapeutic potential of ALL.
Finally, the effect of ALL on normal T lymphocytes has also been investigated. We have also examined the response of ALL in normal T lymphocytes separated from donors with different blood groups and confirmed that ALL could not induce the apoptosis of T cells; on the contrary, ALL can promote the significant proliferation of the cells without blood group specificities, suggesting that ALL has no cytotoxicity to normal T cells. Although some lectins such as Phaseolus vulgaris lectin have been documented to have the proliferative activity on murine splenocytes and anti-proliferative activity toward tumor cells [19], yet no reports associated with their actions in normal T lymphocytes have been documented.
In summary, a novel lectin from Artocarpus nitidus subsp. lingnanensis is confirmed to have mitogenic activity toward mouse splenocytes and anti-proliferative activity against T-lymphoma (Jurkat) cells. However, further studies are highly required to better understand its action mode on other tumors and to explore its possible applications.
References
Subramanyam S, Smith DF, Clemens JC, Webb MA, Sardesai N, Williams CE (2008) Functional characterization of HFR1, a high-mannose N-glycan-specific wheat lectin induced by Hessian fly larvae. Plant Physiol 147(3):1412–1426
Santiago MQ, Leitão CC, Pereira FN Jr, Pinto VR Jr, Osterne VJ, Lossio CF et al (2014) Purification, characterization and partial sequence of a pro-inflammatory lectin from seeds of Canavalia oxyphylla Standl. & L. O. Williams. J Mol Recognit 27(3):117–123
Hong J, Chen TT, Hu L, Yang J, Hu P, Wang SY (2015) Purification and characterization of a novel lectin from Chinese leek seeds. J Agric Food Chem 63(5):1488–1495
Dong Q, Sugiura T, Toyohira Y, Yoshida Y, Yanagihara N, Karasaki Y (2011) Stimulation of IFN-c production by garlic lectin in mouse spleen cells: involvement of IL-12 via activation of p38 MAPK and ERK in macrophages. Phytomedicine 18(4):309–316
Tatsuta T, Hosono M, Sugawara S, Kariya Y, Ogawa Y, Hakomori S, Nitta K (2013) Sialic acid-binding lectin (leczyme) induces caspase-dependent apoptosis-mediated mitochondrial perturbation in Jurkat cells. Int J Oncol 43(5):1402–1412
Hong CE, Park AK, Lyu SY (2014) Synergistic anticancer effects of lectin and doxorubicin in breast cancer cells. Mol Cell Biochem 94(1–2):225–235
Zhang XS, Wu ZY (1988) Flora reipublicae Popularis Sinicae, vol 23. Beijing Science Press, Beijing, p 53
Hakim EH, Asnizar Yurnawilis, Aimi N, Kitajima M, Takayama H (2002) Artoindonesianin P, a new prenylated flavone with cytotoxic activity from Artocarpus lanceifolius. Fitoterapia 73(7–8):668–673
Fernando MR, Wickramasinghe N, Thabrew MI, Ariyananda PL, Karunanayake EH (1991) Effect of Artocarpus heterophyllus and Asteracanthus longifolia on glucose tolerance in normal human subjects and in maturity-onset diabetic patients. J Ethnopharmacol 31(3):277–282
Editorial Committee of the Chinese Materia Medica (1999) Chinese materia medica, vol 2. Shanghai Science and Technology Publishing Press, Shanghai, p 467
Cui B, Li L, Zeng QY, Lin FQ, Yin L, Liao LJ, Huang M, Wang J (2017) A novel lectin from Artocarpus lingnanensis induces proliferation and Th1/Th2 cytokine secretion through CD45 signaling pathway in human T lymphocytes. J Nat Med 71:409–421
Brunelle JK, Letai A (2009) Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci 122(Pt4):437–441
De Mejia EG, Prisecaru VI (2005) Lectins as bioactive proteins: a potential in cancer treatment. Crit Rev Food Sci Nutr 45(6):425–445
Francis F, Jaber K, Colinet F, Portetelle D, Haubruge E (2011) Purification of a new fungal mannose-specific lectin from Penicillium chrysogenum and its aphicidal properties. Fungal Biol 115(11):1093–1099
Shao B, Wang S, Zhou J, Ke L, Rao P (2011) A novel lectin from fresh rhizome of Alismaorientale (Sam.) Juzep. Process Biochem (Oxford, UK) 46:1554–1559
Hakomori S (2002) Glycosylation defining cancer malignancy: new wine in an old bottle. Proc Natl Acad Sci USA 99(16):10231–10233
Estaquier J, Vallette F, Vayssiere JL, Mignotte B (2012) The mitochondrial pathways of apoptosis. Adv Exp Med Biol 942:157–183
Salvesan GS, Dixit VM (1999) Caspase activation: the induced-proximity model. Proc Natl Acad Sci USA 96(20):10964–10967
Chan YS, Wong JH, Fang EF, Pan W, Ng TB (2012) Isolation of a glucosamine binding leguminous lectin with mitogenic activity towards splenocytes and anti-proliferative activity towards tumor cells. PLoS One 7(6):1–13
Lam SK, Ng TB (2010) First report of a haemagglutinin-induced apoptotic pathway in breast cancer cells. Biosci Rep 30(5):307–317
Luo Y, Liu X, Lin F, Liao L, Deng Y, Zeng L, Zeng Q (2018) Cloning of a novel lectin from Artocarpus lingnanensis that induces apoptosis in human B-lymphoma cells. Biosci Biotechnol Biochem 82(2):258–267
Luo X, Budihardjo I, Zou H, Slaughter C, Wang X (1998) Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94(4):481–490
Zhang CZ, Fang EF, Zhang HT, Liu LL, Yun JP (2015) Momordica charantia lectin exhibits antitumor activity towards hepatocellular carcinoma. Investig New Drugs 33(1):1–11
Munoz-Pinedo C, Guio-Carrion A, Goldstein JC, Fitzgerald P, Newmeyer DD, Green DR (2006) Different mitochondrial intermembrane space proteins are released during apoptosis in a manner that is coordinately initiated but can vary induration. Proc Natl Acad Sci USA 103(31):11573–11578
Ouyang L, Chen Y, Wang XY, Lu RF, Zhang SY, Tian M, Xie T, Liu B, He G (2014) Polygonatum odoratum lectin induces apoptosis and autophagy via targeting EGFR-mediated Ras-Raf-MEK-ERK pathway in human MCF-7 breast cancer cells. Phytomedicine 21(12):1658–1665
Lei HY, Chang CP (2009) Lectin of Concanavalin A as an anti-hepatoma therapeutic agent. J Biomed Sci 16:10
Liu B, Wu JM, Li J, Liu JJ, Li WW, Li CY, Xu HL, Bao JK (2010) Polygonatum cyrtonema lectin induces murine fibrosarcoma L929 cell apoptosis via blocking Ras–Raf and PI3K–Akt signaling pathways. Biochimie 92(12):1934–1938
Liu B, Cheng Y, Zhang B, Bian HJ, Bao JK (2009) Polygonatum cyrtonema lectin induces apoptosis and autophagy in human melanoma A375 cells through a mitochondria-mediated ROS–p38–p53 pathway. Cancer Lett 275(1):54–60
Dasmahapatra G, Lembersky D, Kramer L, Fisher RI, Friedberg J, Dent P, Grant S (2010) The pan-HDAC inhibitor vorinostat potentiates the activity of the proteasome inhibitor carfilzomib in human DLBCL cells in vitro and in vivo. Blood 115(22):4478–4487
Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H et al (2004) BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64(19):7099–7109
Castro J, Ribo M, Navarro S, Nogues MV, Vilanova M, Benito A (2011) A human ribonuclease induces apoptosis associated with p21W AF1/CIP1 induction and JNK inactivation. BMC Cancer 11:9
Park J, Kim I, Oh YJ, Lee K, Han PL, Choi EJ (1997) Activation of c-Jun N-terminal kinase antagonizes an anti-apoptotic action of Bcl-2. J Biol Chem 272(27):16725–16728
Markou T, Dowling AA, Kelly T, Lazou A (2009) Regulation of Bcl-2 phosphorylation in response to oxidative stress in cardiac myocytes. Free Radic Res 43(9):809–816
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 81160366) and Guangxi Natural Science Foundation Grant (no. 0832130). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Rights and permissions
About this article
Cite this article
Zeng, L., Li, L., Zeng, Q. et al. Mitogenic activity of Artocarpus lingnanensis lectin and its apoptosis induction in Jurkat T cells. J Nat Med 72, 745–756 (2018). https://doi.org/10.1007/s11418-018-1212-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-018-1212-z