Abstract
Artemisia argyi leaf is a well-known species in traditional Chinese medicine. However, the anti-inflammatory and activating blood stasis activities of its essential oil (AAEO) have not been explored in vivo. The present study measured the contents of three chemical components by gas chromatography (GC). The anti-acute inflammatory effects of AAEO were investigated in dimethyl benzene, glacial acetic acid and carrageenan-induced animals through skin administration or by oral gavage, respectively. The effects of AAEO on haemorheology were studied in a rat acute blood stasis model. The contents of eucalyptol, camphor and borneol in AAEO were 254.4, 51.6 and 58.7 mg/g, respectively. All dosages of AAEO by skin administration significantly decreased the swelling in dimethyl benzene-induced ear oedema and carrageenan-induced paw oedema, and reduced the permeability in glacial acetic acid-induced abdominal blood capillary (p < 0.01). Meanwhile, haemorheology indexes such as whole blood viscosity and the erythrocyte aggregation index significantly decreased only in the high dosage group. In addition, the effects of AAEO by oral gavage were weaker than skin administration at the medium dose in the experiments. It suggests that AAEO has better absorption bioavailability and pharmacological effects through skin administration due to the better skin permeability of essential oil than gastrointestinal absorption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Artemisia argyi Folium (Argy Wormwood Folium) is the leaf of Artemisia argyi, which is a herbaceous perennial plant from the Compositae family. It has a long history of medical application as a traditional Chinese medicine in ‘Yellow Emperor’s Internal Canon of Medicine,’ ‘Treatise on Febrile Diseases’ and ‘Synopsis of Golden Chamber.’ Artemisia argyi leaf is also widely used in other countries, such as Japan and Korea. In China, A. argyi leaf from Qichun, Hubei province, China (Qi Zhou, in ancient times), is a famous species and called ‘QiAi leaf’ for short. As a geo-authentic medicine, QiAi was first recorded in ‘Compendium of Materia Medica’ written by Li Shi-Zhen in the Chinese Ming Dynasty. Now, QiAi is cultivated in Qichun, China and well known around the world.
In the Chinese Pharmacopoeia, A. argyi leaf is recorded to cure lower abdomen cold and pain, irregular menstruation, infertility because of the uterus being cold, haematemesis and external use in relieving itching of the skin (Chinese Pharmacopoeia Commission, 2015 edition). It is often used as traditional medical material or original material of pharmaceutical preparations. For example, A. argyi leaf is well known as the original material of Moxa floss (made by grinding A. argyi leaf) in moxibustion, which is one of the international famous Acupuncture and Moxibustion therapy for diminishing inflammation, relieving pain, promoting blood circulation and removing obstruction in channels [1]. It is also extracted for the volatile oil to prepare Moxa essential oil capsule and aerosol for medical products. These preparations are used to eliminate phlegm and relieve asthma and cough. Additionally, the essential oil from A. argyi leaf is widely used as incense for its aromatic odour to relieve uneasiness of the body and mind. Moreover, A. argyi leaf is used as the original material of healthcare products, healthcare food, cosmetics etc., for antimicrobials, relieving itching and improving blood circulation of the skin.
Artemisia argyi has many reports on chemical components including flavones and terpenes, biological activities including anti-tumour and product preparations [2–13]. Previous researches have shown that the essential oil extracted from A. argyi leaves is the main effective composition and possesses anti-histamine, eliminating phlegm, relieving cough, anti-fungal and anti-viral effects [14–16]. Recently, the chemical composition, extracting yield and quality evaluation of essential oils extracted from A. argyi leaves (AAEO) have also been studied [17–19]. Our laboratory has previously focused on the components analysis and the quality evaluation of essential oil extracted from QiAi (Quality Standards of traditional Chinese medicines in Hubei province, China).
However, as a geo-authentic medicine, the research on AAEO is relatively scant. Particularly, it has not been explored regarding the anti-inflammatory and activating blood stasis activities in animals considering its treatment for asthma and application of Moxa floss. The former activity is obviously related to airway inflammation. Regarding the latter, when the Moxa floss made of A. argyi leaves is heated in the moxibustion therapy, the volatile components are released and might have the effects through the skin on diminishing inflammation, relieving pain, promoting blood circulation and removing obstruction in channels. Thus, in this study, the biological activities of AAEO were evaluated to support the application of its products. We examined the anti-inflammatory and improving haemorheology effects of AAEO in animals by skin administration and measured the contents of its main chemical compositions by gas chromatography (GC).
Materials and methods
Plant materials and extraction of essential oil
Artemisia argyi was harvested from Qichun, Hubei province, China. The fresh leaves (QiAi leaf) were subjected to water distillation for 6 h using a Clevenger-type apparatus. The obtained essential oil was dried over anhydrous sodium sulphate, filtered and stored in a sealed vial. The yield of AAEO was 1.13 % (w/w).
Chemicals
Eucalyptol, camphor, borneol and cyclohexanone were purchased from National Institutes for Food and Drug Control (Beijing, China). Carrageenan was bought from Shanghai Aladdin Industrial Co., and Evans blue was obtained from Shanghai Kayon Biological Technology Co. (Shanghai, China). Dimethyl benzene, glacial acetic acid, ethanol and carboxymethylcellulose sodium were purchased from Sinopharm Chemical Reagent Co. (Beijing, China). Epinephrine hydrochloride, Vitalin Emulgel and asprin were obtained from Grand Pharma (China) Co. Ltd., Beijing Novartis Pharma Ltd. and Bayer Healthcare Ltd., respectively.
GC analysis
The three principal components, including eucalyptol, camphor and borneol, in AAEO were determined using an Agilent 6890N gas chromatograph (Agilent Technologies Inc., Santa Clara, CA, USA). The gas chromatograph was equipped with a HP-INNOWax capillary column with a 30 m × 0.25 mm × 0.5 μm film thickness. The oven temperature was held at 110 °C for 8 min and was programmed to increase to 158 °C at a rate of 25 °C per min, and held for 8 min, then from 158 to 230 °C at a rate of 25 °C per min; the temperature was then held at 230 °C for 9 min (71-min analysis time). The injector and detector temperatures were 250 and 280 °C, respectively. The flow rate of the carrier gas (N2) was 0.7 mL/min and the split ratio was 1:10. For the injection, 3 mL of essential oil (2.6 mg/mL) and 1 mL of cyclohexanone (1.4 mg/mL, as internal standard) was diluted in 5 mL of ethanol solution. One microlitre of the diluted solution was injected and the contents of the three components were calculated by comparing to the standard substances of eucalyptol, camphor and borneol.
Animals
Kunming mice (20 ± 2 g) and Sprague-Dawley rats (180 ± 20 g) were purchased from Hubei Province Center for Disease Control and Prevention (Wuhan, China). All animals were housed at room temperature (22–24 °C) and constant humidity (50–60 %) under a 12-h light–dark cycle in an specific-pathogen-free (SPF) grade laboratory. The animal study was performed according to the international rules considering animal experiments and the internationally accepted ethical principles for laboratory animal use and care. Animal experimentation and the corresponding protocol were approved by the Animal Ethics Committee of South-Central University for Nationalities (Wuhan, China). All the procedures were in strict accordance with the People’s Republic of China legislation on the use and care of laboratory animals.
Dimethyl benzene-induced ear swelling in mice
The anti-inflammatory effect of AAEO on dimethyl benzene-induced ear swelling was studied in mice. They were randomly assigned to six groups (n = 10 each). Fifty animals were barbered in the abdomen by a shaver and administered through the skin, including the control group (75 % ethanol solution as vehicle), positive group (Vitalin Emulgel containing diclofenac diethylammonium 100 mg/kg) and the tested drug groups (low, medium and high doses of AAEO of 0.25, 0.50 and 1.00 mL/kg, respectively). The concentrations of AAEO in 75 % ethanol solution were 0.05, 0.10 and 0.20 mL/mL, respectively. In the last group, mice received AAEO (0.50 mL/kg, 0.05 mL/mL) by oral gavage. After treatment for 3 days, the anti-inflammatory activity was tested and evaluated. Half an hour after administration on day 3, the two sides of the right ear was embrocated with 40 μL of dimethyl benzene and the left ear without any treatment was used as a control. After 1 h, the mice were sacrificed and both ears were cut. Round ear pieces (diameter 8 mm) produced by a hole puncher were weighed and the swelling percentage was defined as (W r − W l)/W l × 100 %, where W l is the weight of the left ear without any treatment and W r is the weight of the right ear with the dimethyl benzene embrocation.
Carrageenan-induced paw oedema in rats
The anti-inflammatory effect of AAEO on carrageenan-induced paw oedema was studied in rats. The animals were randomly assigned to six groups (n = 10 each), and five groups of rats were barbered in the abdomen by a shaver and administered by the external route, including a control group (75 % ethanol solution as vehicle), positive group (Vitalin Emulgel containing diclofenac diethylammonium 50 mg/kg) and the tested drug groups (low, medium and high doses of AAEO of 0.125, 0.25 and 0.50 mL/kg, respectively). The concentrations of AAEO in 75 % ethanol solution were 0.075, 0.15 and 0.30 mL/mL, respectively. In the last group, rats received AAEO (0.25 mL/kg, 0.0225 mL/mL) by oral gavage. One hour after administration on day 3, 0.20 mL of 1 % carrageenan was injected into the hind paw of each rat. Before the carrageenan injection, the normal paw volumes of the rats were measured with a plethysmometer. The carrageenan-induced increase in the paw volume was measured at 1, 2, 4 and 6 h after injection. The oedema percentage was defined as (V b − V a)/V a × 100 %, where V a is the paw volume before the carrageenan injection and V b is the paw volume after the carrageenan injection.
Glacial acetic acid-induced abdominal blood capillary permeability in mice
The anti-inflammatory effect of AAEO on glacial acetic acid-induced abdominal blood capillary permeability was studied in mice. The animals were randomly assigned to six groups (n = 10 each) and five groups of rats were barbered in the abdomen by a shaver and administered through the skin, including a control group (75 % ethanol solution as vehicle), positive group (Vitalin Emulgel containing diclofenac diethylammonium 100 mg/kg), the tested drug groups (low, medium and high doses of AAEO of 0.25, 0.50 and 1.00 mL/kg, respectively). The concentrations of AAEO in 75 % ethanol solution were 0.05, 0.10 and 0.20 mL/mL, respectively. In the last group, rats received AAEO (0.50 mL/kg, 0.05 mL/mL) by gavage. One hour after administration on day 3, 0.20 mL of 0.5 % Evans blue was intravenously injected through the tail and then 0.20 mL of 0.6 % glacial acetic acid was injected immediately into the abdominal cavity. After half an hour, the mice were sacrificed and normal saline was injected into the abdominal cavity. The fluid in the abdomen was collected and the absorption value at 590 nm measured.
Ice water bath-induced acute blood stasis in rats
The effects of AAEO on haemorheology were studied in a rat acute blood stasis model being induced by subcutaneous injection of epinephrine combined with an ice water bath. The animals were randomly assigned to seven groups (n = 10 each) and administered by oral gavage, which included a control group and a model group (0.5 % CMC-Na solution as vehicle), positive group (asprin 200 mg/kg) and the tested drug groups (low, medium and high doses of AAEO of 0.125, 0.25 and 0.50 mL/kg, respectively). The concentrations of AAEO in 0.5 % CMC-Na solution were 0.075, 0.15 and 0.30 mL/mL, respectively. In the last group, rats received AAEO (0.25 mL/kg, 0.0225 mL/mL) by oral gavage. Before 12 h on the last and fifth day of administration, all the rats except for those in the control group were subcutaneously injected with epinephrine (0.80 mL/kg). After 2 h, they were placed in the ice water bath for 4 min and then injected with epinephrine again. One hour after administration on day 5, the whole blood in rats was sampled under anaesthetisation. Haemorheology indexes such as whole blood viscosity, plasma viscosity and erythrocyte aggregation index were measured with an automatic haemorheology Tester (SA-6000, SuCCeeder Sid Technology Development Company Limited, Beijing, China).
Data analysis
All experimental results are expressed as mean ± standard deviation (SD) and statistical analysis was done by one-way analysis of variance (ANOVA) with the Turkey test using the Origin 7.0 software. A p-value less than 0.05 was considered to be statistically significant.
Results
Determination of the main components in AAEO by GC
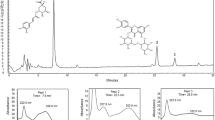
The three principal components, including eucalyptol, camphor and borneol, in AAEO were determined using GC (Fig. 1). Identification and quantification of the compounds was achieved by comparison of their retention time (t R) and peak area with those of the standard substances eucalyptol, camphor and borneol (National Institutes for Food and Drug Control, China). The standard curve equations of eucalyptol (peak 1), camphor (peak 3) and borneol (peak 4) were Y = 3.9689X + 0.0113, Y = 4.1289X + 0.0021 and Y = 4.2625X + 0.0028, respectively, where Y is the ratio of the peak area of the standard substances to that of cyclohexanone (peak 2) as an internal standard and X is the concentration of the standard substances. The correlative coefficients were 1.0000. By calculation, the contents of eucalyptol, camphor and borneol in AAEO were 254.4, 51.6 and 58.7 mg/g, respectively.
Anti-inflammatory effect in dimethyl benzene-induced ear swelling mice
The anti-inflammatory effect of AAEO was evaluated in the dimethyl benzene-induced ear swelling mice. All dosages of AAEO (0.25, 0.50 and 1.00 mL/kg) by skin administration decreased the swelling in dimethyl benzene-induced ear oedema (Fig. 2). The percentages of ear swelling at the lower (0.25 mL/kg), medium (0.50 mL/kg) and high (1.00 mL/kg) dosages were 50.3 ± 17.8, 46.2 ± 13.2 and 40.2 ± 16.2 %, respectively. They showed significant differences (p < 0.01) compared to that of the control (111.6 ± 20.9 %). Moreover, the medium concentration-induced effect was similar to that of the positive control (diclofenac diethylammonium 100 mg/kg, 47.0 ± 16.2 %). In addition, the ear swelling percentage in the oral gavage group (0.50 mL/kg) was reduced by 53.2 ± 19.4 %, being higher but not by much compared to that of the low-dosage AAEO by skin administration (0.25 mL/kg).
Effect of control (1), positive diclofenac diethylammonium 100 mg/kg (2), 0.25, 0.50 and 1.00 mL/kg AAEO by skin administration (3, 4 and 5, respectively) and 0.50 mL/kg AAEO by oral gavage group (6) on the swelling percentage in dimethyl benzene-induced ear oedema mice. Each point value represents the mean ± standard deviation (SD) of ten experiments. **p < 0.01, compared with vehicle control
Anti-inflammatory effect in carrageenan-induced paw oedema rats
The anti-inflammatory effect of AAEO was also carried out in the carrageenan-induced paw oedema rats. In order to observe further the anti-inflammatory action, the effect of AAEO on carrageenan-induced paw oedema was measured in rats (Fig. 3). After the injection of carrageenan, the paw of rats became swollen and the swelling rate reached the highest at 4 h and then reduced gradually. By the skin administration of AAEO, all dosages of AAEO (0.125, 0.25 and 0.50 mL/kg) significantly decreased (p < 0.01) the swelling in the paw at 1, 2, 4 and 6 h. At 4 h after inducing inflammation, the percentages of paw swelling at the lower (0.125 mL/kg), medium (0.25 mL/kg) and high (0.50 mL/kg) concentration were 27.6 ± 4.9, 20.3 ± 3.7 and 16.3 ± 4.8 %, respectively. They showed significant differences (p < 0.01) compared to that of the control group (41.6 ± 9.3 %). The high concentration-induced effect was similar to that of the positive control (diclofenac diethylammonium 50.0 mg/kg, 18.7 ± 4.9 %).
Effect of control (1), positive diclofenac diethylammonium 50 mg/kg (2), 0.125, 0.25 and 0.50 mL/kg AAEO by skin administration (3, 4 and 5, respectively) and 0.25 mL/kg AAEO by oral gavage group (6) on the carrageenan-induced paw oedema in rats. Each point value represents the mean ± SD of ten experiments. **p < 0.01, compared with vehicle control
However, the after administration of AAEO (0.25 mL/kg) by oral gavage, the swelling rates were reduced by 15.2 ± 2.6, 21.8 ± 3.2, 28.5 ± 1.6 and 15.6 ± 2.7 % at 1, 2, 4 and 6 h, respectively. Except for at 1 h, they showed different effects (p < 0.01 by ANOVA). Furthermore, all the values were higher than those of the same dosage by skin administration. This showed that there are lesser effects through skin administration than the gastrointestinal route.
Anti-inflammatory effect in glacial acetic acid-induced abdominal blood capillary mice
Glacial acetic acid by intraperitoneal injection increased the blood capillary osmotic energy of the mouse abdominal cavity. It resulted in the rapid permeability of Evans blue and high absorbance of the fluid in the abdomen cavity under visible light. After the skin administration of AAEO at the lower (0.25 mL/kg), medium (0.50 mL/kg) or high (1.00 mL/kg) dosages, the optical density (OD) 590 nm values were 0.101 ± 0.009, 0.075 ± 0.007 and 0.044 ± 0.009, respectively (Fig. 4). All dosages of AAEO showed a significant decrease (p < 0.01) compared to that of the control group (0.181 ± 0.014). In addition, the absorbance in the oral gavage group (0.50 mL/kg) was reduced by 0.107 ± 0.013, being similar to that of the high dosage AAEO by skin administration and positive control (diclofenac diethylammonium 100 mg/kg, 0.114 ± 0.008).
Effect of control (1), positive diclofenac diethylammonium 100 mg/kg (2), 0.25, 0.50 and 1.00 mL/kg AAEO by skin administration (3, 4 and 5, respectively) and 0.50 mL/kg AAEO by oral gavage group (6) on the glacial acetic acid-induced abdominal blood capillary permeability in mice. Each point value represents the mean ± SD of ten experiments. **p < 0.01, compared with vehicle control
Haemorheology activity in the ice water bath-induced acute blood stasis rats
The activating blood stasis actions of AAEO were investigated in the ice water bath-induced acute blood stasis rats. All the AAEO groups induced an inhibition of the whole blood viscosity in rats with acute blood stasis (Fig. 5). The high dosage of AAEO showed a bigger reduction on the blood viscosity of low shear rate (36.9 ± 5.7, p < 0.01) and medium shear rate (6.3 ± 0.6, p < 0.01) than other dosages compared to that of the model (51.6 ± 5.6 and 7.6 ± 0.6, respectively). And the medium dosage of AAEO showed an effect only on the blood viscosity of low shear rate (42.5 ± 5.2, p < 0.05). However, the blood viscosity was not inhibited significantly in the lower dosage group administrated by skin and the oral gavage group. The results showed that there was a little decrease but no significant difference on the whole blood reductive viscosity and plasma viscosity (data not shown). Moreover, in Fig. 6, AAEO reduced the value of the erythrocyte aggregation index and had a significant difference (p < 0.05) only at the high dosage of 1.00 mL/kg by skin administration (7.5 ± 0.7) compared to that of the model (9.1 ± 0.6). The high concentration-induced effect was similar to that of positive control (asprin 200 mg/kg, 7.4 ± 1.1).
Effect of control (1), model group (2), positive diclofenac diethylammonium 50 mg/kg (3), 0.125, 0.25 and 0.50 mL/kg AAEO by skin administration (4, 5 and 6, respectively) and 0.25 mL/kg AAEO by oral gavage group (7) on the whole blood viscosity at low, medium and high shear rates (1, 50 and 200 s−1, respectively) in the ice water bath-induced acute blood stasis rats. Each point value represents the mean ± SD of ten experiments. **p < 0.01, compared with vehicle control. # p < 0.05 and ## p < 0.01, compared with model group
Effect of control (1), model group (2), positive diclofenac diethylammonium 50 mg/kg (3), 0.125, 0.25 and 0.50 mL/kg AAEO by skin administration (4, 5 and 6, respectively) and 0.25 mL/kg AAEO by oral gavage group (7) on the erythrocyte aggregation index in the ice water bath-induced acute blood stasis rats. Each point value represents the mean ± SD of ten experiments. **p < 0.01, compared with vehicle control. # p < 0.05 and ## p < 0.01, compared with model group
Discussion
Artemisia argyi leaf is characteristic of strong fragrance, indicating that there are rich aromatic compounds. As the main chemical composition, AAEO was extracted from A. argyi leaves and the yield was 0.45–1.00 %. Additionally, chemical components such as sesquiterpenes, esters, monoterpenes, ketones and aromatic compounds have been reported in AAEO [15, 20–25]. The three main components, including eucalyptol, camphor and borneol, have shown antimicrobial, anti-inflammatory and antioxidant activities [19, 20]. In our experiments, eucalyptol, camphor and borneol of AAEO were isolated by GC and quantified with the contents of 254.4, 51.6 and 58.7 mg/g, respectively. The total amount of each them was high by up to 36.7 %, which suggests that the three chemical constituents in A. argyi leaves may play major roles in the biological activities and pharmacological properties.
Artemisia argyi leaf is widely used in traditional Chinese medicine (TCM) and has the effects of antimicrobial, relieving itching, improving blood circulation of the skin in folk medicine and dietary therapy. The plant, belonging to the Compositae family, has a strong aromatic smell, which attracts research on its volatile composition. In the study, we extracted AAEO and investigated its protective effects on acute inflammatory models and an acute blood stasis model. In the acute inflammatory models, dimethyl benzene, as a chemical stimulator, can prompt the release of inflammatory mediates; for example, histamine, kinins and fibrinolytic enzyme. These substances immediately improve the increase of blood capillary permeability and infiltration of inflammatory cells, resulting in ear swelling in mice. And the injection of carrageenan in rats can produce an inflammatory response that peaks at 3–4 h, resulting in hyperalgesia [26]. In this experimental inflammation model, the level of prostaglandin E2 (PGE2) increases, associating with the inflammatory response. These models are often used to evaluate the activities of steroidal and non-steroidal anti-inflammatory drugs. In Figs. 2 and 3, it can be seen that the low, medium and high dosages of AAEO significantly inhibited the inflammatory symptoms in animals through either skin administration or oral gavage. Considering the results in Fig. 4, AAEO prevented the increase of blood capillary permeability, which resulted in reducing the inflammatory swelling.
Furthermore, the effects of AAEO on haemorheology were studied in a rat acute blood stasis model. The model was established by subcutaneous injection of epinephrine combined with an ice water bath, inducing cold accumulation and stagnation of the circulation of vital energy in accordance with the characteristics of TCM syndrome. Figures 5 and 6 showed that AAEO decreased the blood viscosity of low, medium and high shear rates, and the action was related to the ability of reducing the erythrocyte aggregation. In addition, all the effects of AAEO through skin administration were concentration-dependent, and greater than oral gavage of the medium dose in the acute inflammatory and acute blood stasis experiments. It implied that AAEO had better absorption and pharmacological effects through skin administration that might have contributed to the better skin permeability of essential oil than via gastrointestinal absorption [27–29]. Meanwhile, as the main composition, the pharmacological activities of AAEO through skin administration were verified and consistent with the traditional external usage of A. argyi leaf.
In conclusion, AAEO possesses anti-inflammatory activity and improves haemorheology effects in the acute inflammatory and acute blood stasis animals, respectively. It also suggests that AAEO administrated via the skin has better bioavailability than oral gavage.
References
Deng HY, Shen XY (2013) The mechanism of moxibustion: ancient theory and modern research. Evid Based Complement Alternat Med 2013, 379291
Lao AN, Fujimoto Y, Tatsuno T (1984) Studies on the constituents of Artemisia argyi Levl et Vant. Chem Pharm Bull 32:723–727
Tan RX, Jia ZJ (1992) Eudesmanolides and other constituents from Artemisia argyi. Planta Med 58:370–372
Nakasugi T, Nakashima M, Komai K (2000) Antimutagens in gaiyou (Artemisia argyi levl. et vant.). J Agr Food Chem 48:3256–3266
Seo JM, Kang HM, Son KH, Kim JH, Lee CW, Kim HM, Chang SI, Kwon BM (2003) Antitumor activity of flavones isolated from Artemisia argyi. Planta Med 69:218–222
Shoemaker M, Hamilton B, Dairkee SH, Cohen I, Campbell MJ (2005) In vitro anticancer activity of twelve Chinese medicinal herbs. Phytother Res 19:649–651
Lee HG, Yu KA, Oh WK, Baeg TW, Oh HC, Ahn JS, Jang WC, Kim JW, Lim JS, Choe YK, Yoon DY (2005) Inhibitory effect of jaceosidin isolated from Artemisia argyi on the function of E6 and E7 oncoproteins of HPV 16. J Ethnopharmacol 98:339–343
Adams M, Efferth T, Bauer R (2006) Activity-guided isolation of scopoletin and isoscopoletin, the inhibitory active principles towards CCRF-CEM leukaemia cells and multi-drug resistant CEM/ADR5000 cells, from Artemisia argyi. Planta Med 72:862–864
Khan M, Yu B, Rasul A, Al Shawi A, Yi F, Yang H, Ma T (2012) Jaceosidin induces apoptosis in U87 glioblastoma cells through G2/M phase arrest. Evid Based Complement Alternat Med 2012, 703034
Kim MJ, Kim DH, Lee KW, Yoon DY, Surh YJ (2007) Jaceosidin induces apoptosis in ras-transformed human breast epithelial cells through generation of reactive oxygen species. Ann N Y Acad Sci 1095:483–495
Lim JC, Park SY, Nam Y, Nguyen TT, Sohn UD (2012) The protective effect of eupatilin against hydrogen peroxide-induced injury involving 5-lipoxygenase in feline esophageal epithelial cells. Korean J Physiol Pharmacol 16:313–320
Zhang YH, Xue MQ, Bai YC, Yuan HH, Zhao HL, Lan MB (2012) 3,5-Dicaffeoylquinic acid isolated from Artemisia argyi and its ester derivatives exert anti-leucyl-tRNA synthetase of Giardia lamblia (GlLeuRS) and potential anti-giardial effects. Fitoterapia 83:1281–1285
Bao X, Yuan H, Wang C, Liu J, Lan M (2013) Antitumor and immunomodulatory activities of a polysaccharide from Artemisia argyi. Carbohydr Polym 98:1236–1243
Edris AE (2007) Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother Res 21:308–323
Huang HC, Wang HF, Yih KH, Chang LZ, Chang TM (2012) Dual bioactivities of essential oil extracted from the leaves of Artemisia argyi as an antimelanogenic versus antioxidant agent and chemical composition analysis by GC/MS. Int J Mol Sci 13:14679–14697
Hu Y, Yang Y, Ning Y, Wang C, Tong Z (2013) Facile preparation of Artemisia argyi oil-loaded antibacterial microcapsules by hydroxyapatite-stabilized Pickering emulsion templating. Colloids Surf B Biointerfaces 112:96–102
Guan WQ, Li SF, Yan RX, Huang YF (2006) Comparison of composition and antifungal activity of Artemisia argyi Lévl. et Vant inflorescence essential oil extracted by hydrodistillation and supercritical carbon dioxide. Nat Prod Res 20:992–998
He ZY, Zhang YH, Wei D, Yu RH, Yuan HH, Lan MB (2009) Chemical composition of essential oil from fresh and dried Folium Artemisia argyi from Hubei Province. Chinese Tradit Patent Med 31:1079–1082
Li N, Mao Y, Deng C, Zhang X (2008) Separation and identification of volatile constituents in Artemisia argyi flowers by GC-MS with SPME and steam distillation. J Chromatogr Sci 46:401–405
Abad MJ, Bedoya LM, Apaza L, Bermejo P (2012) The Artemisia L. genus: a review of bioactive essential oils. Molecules 17:2542–2566
Yoshikawa M, Shimada H, Matsuda H, Yamahara J, Murakami N (1996) Bioactive constituents of Chinese natural medicines. I. New sesquiterpene ketones with vasorelaxant effect from Chinese moxa, the processed leaves of Artemisia argyi Levl. et Vant.: moxartenone and moxartenolide. Chem Pharm Bull (Tokyo) 44:1656–1662
Jeong MA, Lee KW, Yoon DY, Lee HJ (2007) Jaceosidin, a pharmacologically active flavone derived from Artemisia argyi, inhibits phorbol-ester-induced upregulation of COX-2 and MMP-9 by blocking phosphorylation of ERK-1 and -2 in cultured human mammary epithelial cells. Ann N Y Acad Sci 1095:458–466
Wang S, Li J, Sun J, Zeng KW, Cui JR, Jiang Y, Tu PF (2013) NO inhibitory guaianolide-derived terpenoids from Artemisia argyi. Fitoterapia 85:169–175
Zeng KW, Wang S, Dong X, Jiang Y, Tu PF (2014) Sesquiterpene dimer (DSF-52) from Artemisia argyi inhibits microglia-mediated neuroinflammation via suppression of NF-κB, JNK/p38 MAPKs and Jak2/Stat3 signaling pathways. Phytomedicine 21:298–306
Lee SH, Kim HK, Seo JM, Kang HM, Kim JH, Son KH, Lee H, Kwon BM, Shin J, Seo Y (2002) Arteminolides B, C, and D, new inhibitors of farnesyl protein transferase from Artemisia argyi. J Org Chem 67:7670–7675
Romero A, Planas E, Poveda R, Sánchez S, Pol O, Puig MM (2005) Anti-exudative effects of opioid receptor agonists in a rat model of carrageenan-induced acute inflammation of the paw. Eur J Pharmacol 511:207–217
Williams AC, Barry BW (1989) Essential oils as novel human skin penetration enhancers. Int J Pharmaceut 57:R7–R9
Williams AC, Barry BW (1991) Terpenes and the lipid-protein-partitioning theory of skin penetration enhancement. Pharm Res 8:17–24
Cal K (2006) Skin penetration of terpenes from essential oils and topical vehicles. Planta Med 72:311–316
Acknowledgements
This work was supported by the Natural Science Foundation of the People’s Republic of China (grant 81470181) and the Innovation Group Project of the Natural Science Foundation of Hubei Province, People’s Republic of China (grant 2013CFA013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Ge, Yb., Wang, Zg., Xiong, Y. et al. Anti-inflammatory and blood stasis activities of essential oil extracted from Artemisia argyi leaf in animals. J Nat Med 70, 531–538 (2016). https://doi.org/10.1007/s11418-016-0972-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-016-0972-6