Abstract
Improvement in blood fluidity leads to the prevention of various lifestyle-related diseases. A raw material for improving blood fluidity has been long desired in the research area of functional and supplemental foods. We successfully showed an improvement in blood fluidity by the Zingiberaceae plant, Kaempferia parviflora. The rhizome of the plant reduced the blood passage time through a micro slit using a disseminated intravascular coagulation model. The mechanism was attributed to the activation of fibrinolysis, as demonstrated by elongation of the euglobulin lysis time and an in-vitro fibrinolysis assay. The active principles were determined to be methoxyflavones. The results show that the rhizome of K. parviflora is a promising candidate preventive agent for treating lifestyle-related diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rhizomes of the Zingiberaceae plant Kaempferia parviflora (RKP) have been used to cure various types of pathological conditions, especially lifestyle-related diseases. RKP is a panax called Krachaidum among specific tribes in Thailand and the tincture is used to treat systemic pain, gastroenteropathy [1] and impotency [2]. In Japan, RKP has been combined in functional foods in an effort to improve lipid metabolic disorders. Thus, RKP has long been considered as a multi-functional raw material since its safety as a food has been guaranteed over a long period of consumption and its various pharmacological activities have been elucidated.
Because of these facts and expectations, extensive studies have been performed to reveal the novel functionalities and pharmacological activities of RKP. We have already reported its inhibitory activities on xanthine oxidase and disclosed the active principle as methoxyflavone [3]. Furthermore, the potency of RKP extract was higher than those of other Zingiberaceae plants, including Zingiber officinale, Curcuma longa and C. zedoaria.
In our continuous search for novel pharmacological activities of RKP, we focused on improvement in blood fluidity. Deficiencies in blood circulation caused by poor diet and lack of exercise can lead to impairment of the basal metabolic system. As a result, obesity, diabetes, lipid metabolic disorders and hypertension can occur. Any combination of these diseases may be fatal.
Blood circulation is closely related to the fluidity of blood. In traditional Chinese medicine, crude drugs originated from Zingiberacea plants have been used to treat blood circulation disorders called “Oketsu”. For example, the rhizome of Z. officinale has been recognized as a perspiratory agent prescribed in some “Kampo” Chinese herbal medicines such as “Keishi-to” and “Kakkon-to”. The active principles causing perspiration have been identified as gingerols and shogaols, which have vasodilatory activities. In addition, these compounds are reported to possess inhibitory activity against aggregated platelets induced by collagen [4, 5].
These facts prompted us to investigate the improvement in blood fluidity due to the activity of RPK, a Zingiberaceae plant. In this report, the effect of an RPK extract on blood in a disseminated intravascular coagulation (DIC) model rat and the elongation of eugobulin lysis time (ELT) were examined. Furthermore, the active principles of fibrinolysis activity were revealed.
Materials and methods
Materials
Chemicals and biochemical reagents were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan), Nacalai Tesque, Inc. (Kyoto, Japan) or Sigma-Aldrich (St. Louis, MO, USA) unless otherwise noted. RKP was purchased from Aoba Trading Co. (Tokyo, Japan). Rhizomes of Z. officinale and C. longa were purchased from Nippon Funmatsu Yakuhin Co. Ltd. (Osaka, Japan). Rhizomes of C. zedoaria were purchased from Maechu (Nara, Japan). Voucher specimens were deposited at the Faculty of Pharmacy, Kinki University.
Preparations of extracts
70 % methanolic extracts from the four plant materials were prepared as reported previously to give KP-ext (K. parviflora), ZO-ext (Z. officinale), CL-ext (C. longa) and CZ-ext (C. zedoaria) [3].
Animals
Male Std-Wistar/ST rats (7 weeks of age) were purchased from Japan SLC (Shizuoka, Japan) and 1 week of acclimation was allowed before administration of samples. They were maintained in an air-conditioned room with lighting from 0700 to 1900 hours. The room temperature (about 23 °C) and humidity (about 60 %) were controlled automatically. Laboratory pellet chow (Labo MR Stock, Nihon Nosan Kogyo Co. Ltd., Tokyo, Japan) and water were freely available. All experimental protocols were approved by the Committee for the Care and Use of Laboratory Animals at Kinki University.
Lipopolysaccharide-induced DIC test
Lipopolysaccharide (LPS)-induced DIC rats were prepared according to the method of Schoendorf et al. [6] with minor modifications. Each KP-ext (200 and 500 mg/kg) suspended in 50 % propylene glycol aqueous solution (PG) was administered orally to rats in the respective groups once a day for 7 successive days (Days 1–7). On Day 7, LPS (1 mg/kg, dissolved in saline) was injected into the tail vein of rats of the vehicle control, KP-ext and heparin (Ajinomoto Pharma Co., Ltd., Tokyo, Japan) groups 1 h after sample administration. PG was administered orally to the control group and the vehicle control group rats once a day for 7 successive days (Days 1–7). Heparin (500 U/kg, dissolved in saline), a reference drug, was administered by injection to the tail vein of rats of the heparin group 1 h before the injection of LPS on Day 7. Blood samples in duplicate were withdrawn from the abdominal vein into plastic syringes 4 h after the injection of LPS, while the animals were anesthetized with pentobarbital [(35 mg/kg, intraperitoneally, (i.p.)]. The following parameters were measured in each blood sample by Osaka Kessei Research Laboratories (Osaka, Japan): fibrinogen, fibrin degradation products (FDP) and platelet counts. Using another whole blood sample, blood passage time, the time taken for the passage of 50 μl of the mixture (1.8 ml of blood and 0.2 ml of 3.8 % sodium citrate solution), of each group (control, vehicle control, KP-ext and heparin group) was measured by a micro channel array flow analyzer (MC-FAN, Hitachi, Tokyo, Japan) with a micro channel array Bloody 6-5 (the array has 8,736 parallel slits, each 4.5 μm wide, 30 μm long and 4.5 μm deep). The whole blood passage time was determined under a negative pressure of 20 cm of water. The standard passage time required for passage of 100 μl of saline through the micro channel array was 14.0 s. In order to avoid deviation in the micro channel array, the saline passage time required for 100 μl of saline was determined just before each blood sample measurement. The whole blood passage time for 50 μl of the mixture was calculated from the following equation:
Measurement of ELT
According to the method of Kaulla and Schultz [7], a test sample suspended in PG was administered orally to the KP-ext groups. To the control group, PG was administered orally. While the animals were anesthetized with pentobarbital (35 mg/kg, i.p.), blood samples were withdrawn from the abdominal vein 1 h after oral administration of the test sample. To the collected blood was added 3.8 % sodium citrate aqueous solution corresponding to one-tenth of the blood volume. The mixture was then centrifuged at 1,520g for 10 min at 4 °C to give plasma for the measurement of ELT. Ice-cold water (9.8 ml) was added to the plasma (0.7 ml) in a tube, and then the tube was incubated at 4 °C for 5 min under carbon dioxide gas blown onto the surface of the plasma. After centrifuging at 1,520g for 10 min at 4 °C, the supernatant was discarded and a precipitate (euglobulin fraction) was retained. The euglobulin fraction was dissolved in 0.7 ml of 1/15 M PBS (pH 7.4), followed by the addition of 40 μl thrombin solution (125 U/ml). The resulting fibrin coagulation was incubated at 37 °C, and the time required for disappearance of fibrin coagulation was recorded as ELT. Dextran sulphate sodium salt solution (5 mg/kg, dissolved in saline), a reference drug, was injected into the tail vein of positive control rats 30 min before withdrawing blood samples.
Fibrin plate assay
Fibrinolysis activity in vitro was evaluated according to the method of Norén et al. [8] with minor modifications. The test sample was dissolved in DMSO and diluted with 0.02 M sodium dihydrogen phosphate buffer saline (0.3 M NaCl, adjusted to pH 7.8 by 1 M NaOH, PBS) to a final DMSO concentration of 0.5 % v/v. Agarose (1 g) was dissolved in 100 ml of boiling distilled water and then allowed to cool in a water bath at 50 °C. Plasminogen-containing fibrinogen (400 mg) was dissolved in 100 ml of agarose at 37 °C. A mixture of agarose solution (5 ml) and fibrinogen solution (5 ml) was placed in a tube with a cap and 0.1 ml of thrombin solution (100 U/ml, dissolved in PBS) was added. The capped tube was quickly inverted, and the contents were immediately poured into a Petri dish (i.d. 90 mm) to give a fibrin plate. After standing for 30 min at room temperature, six separate holes (diameter 6 mm, depth 7–8 mm) were dug in the fibrin plate. A test solution (0.1 ml) of an appropriate concentration and urokinase solution (0.1 ml, 5 U/ml, in PBS) were mixed, and then 20 μl of the mixture was added to each hole. As a control, 0.5 % DMSO in PBS was used. The plates were incubated at 37 °C for 14 h. Dextran sulphate sodium salt (PK Chemicals A/S, Koge, Denmark) was used as a reference drug. The diameter of the transparent rings that appeared was measured by calipers (Mitutoyo Co., Tokyo, Japan), and the fibrinolysis area (mm2) was calculated. The fibrinolysis activity (%) of each sample was expressed as a percentage of the increase in fibrinolysis area compared with that of the control.
Statistical analysis
Results are expressed as the mean ± SD. Significant differences were detected using a multiple comparison test with the Bonferroni/Dunn algorithm (Statcel2, OMS Publishing, Tokyo, Japan) at p < 0.01 and p < 0.05.
Results and discussion
Improvement in blood fluidity in DIC model rats with KP-ext
KP-ext at 500 mg/kg showed a significant improvement in blood fluidity at 952 s compared to that of a vehicle control at 1812 s with successive administration (Table 1). The data suggest that KP-ext is a promising candidate of improving blood fluidity.
Blood fluidity in DIC rats is affected by activation of platelet and erythrocyte aggregation, a decrease in erythrocyte deformability and/or inactivation of fibrinolysis. Of these critical factors, activation of fibrinolysis may be the most critical therapeutic target to improve blood fluidity. Activation of fibrinolysis can improve blood fluidity not only therapeutically, but also preventively. This multiplicity could be an advantage for the development of an effective agent. These considerations prompted us to examine the activation effects of KP-ext on fibrinolysis as one mechanism leading to improvement in blood fluidity.
Activation of a fibrinolytic effect by KP-ext
In order to investigate the mechanism of KP-ext on improvement in blood fluidity, the activation of fibrinolysis was examined on the elongation of ELT. ELT is an in-vivo assay which involves measuring the time to lysis of clotted euglobulin in a simulation of the human blood system. The results are shown in Fig. 1. KP-ext at 200 mg/kg administration significantly shortened ELT. From this data, KP-ext was expected to have activation activity on the fibrinolytic system. We could not investigate the anti-aggregative activity on platelets and erythrocytes due to the por solubility of KP-ext in buffers and water.
Activation of fibrinolysis in in-vitro assays with KP-ext
In order to investigate the active principle, KP-ext was subjected to an in-vitro fibrinolytic test known as the fibrin plate method since the in-vivo assay cannot be adapted to activity-guided fractionation. This method has been widely used to evaluate the degree of fibrinolytic activity with simplified manipulation [8]. As shown in Table 2, KP-ext showed dose-dependent activation of fibrinolysis, evaluated from the increase in transparent area from 132 mm2 (control) to 144, 163 and 177 mm2 at 50, 200 and 500 μg/ml, respectively. These data support the idea that KP-ext enhances urokinase activity to express fibrinolytic activity. Furthermore, KP-ext showed the most potent activity among the extracts obtained from the Zingiberaceae plants tested. These data suggest that KP-ext can be a potent blood improving agent among the popular Zingiberaceae plants in Japan.
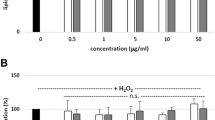
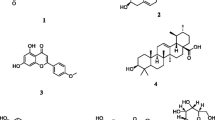
In order to reveal the active principles of KP-ext on fibrinolytic activity, the extract was fractionated using Diaion HP-20 (Mitsubishi Chemicals, Tokyo, Japan) column chromatography with water, and 50 and 100 % methanol as eluents. Of them, the 100 % methanol fraction, which contains mainly methoxyflavones (Fig. 2), showed the most potent activity. From this result, we focused on methoxyflavones as the candidates of active principles. The results are shown in Fig. 3. Of the compounds tested, 5,7,3′,4′-tetramethoxyflavone, 5,7,4′-trimethoxyflavone, 5-hydroxy-3,7,4′-trimethoxyflavone and 3,5,7,4′,5′-pentamethoxyflavone showed significant fibrinolytic activities at 200 μM. These flavones were identified as some of the active principles. Although obvious relationships between the potencies and the structures of the compounds could not observed, the highly methoxylated compounds possessed higher inhibitory activities than the polar compounds. Of the active methoxyflavones, 3,5,7,4′,5′-pentamethoxyflavone was the main constituent in KP-ext. Thus, the activation of fibrinolysis was investigated using the compound. As shown in Fig. 4, the compound showed dose–dependent activity and showed a significant decrease in ELT at 20 μM. This result clearly shows that 3,5,7,4′,5′-pentamethoxyflavone is the most dominant methoxyflavone in terms of fibrinolysis activation by KP-ext. Methoxyflavones have been reported to possess various pharmacological effects and we already reported their moderate inhibitory activity on xanthine oxidase [3]. Here, we successfully discovered a novel function in KP-ext and some other methoxyflavones. KP-ext could be a multi-functional agent to improve blood fluidity, as well as treat obesity, which could be suitable for the prevention of lifestyle-related diseases. Furthermore, in-vivo assays of methoxyflavones are now underway and the results will be discussed elsewhere.
Chemical structures of methoxyflavones used in this study. 1 5,7,3′,4′-tetramethoxyflavone, 2 5,7,4′-trimethoxyflavone, 3 5-hydroxy-3,7,4′-trimethoxyflavone, 4 3,5,7,4′,5′-pentamethoxyflavone, 5 5,7-dimethoxyflavone, 6 5-hydroxy-3,7,3′,4′-tetramethoxyflavone, 7 5-hydroxy-3,7-dimethoxyflavone, 8 3,5,7,4′-tetramethoxyflavone, 9 3,5,7-trimethoxyflavone, 10 5-hydroxy-7-methoxyflavone
Effects of 3,5,7,4′,5′-pentamethoxyflavone on ELT in DIC rats. Each value represents the mean ± SE of triplicates. Significantly different from the C a) group **p < 0.01. Significantly different from the C b) group ## p < 0.01. C a) control for 3,5,7,4′,5′-pentamethoxyflavone (PMF), C b) Control for D, D dextran sulphate sodium salt
Effect of KP-ext on the blood coagulation system using blood from DIC rats
In the same type of DIC rats used in the former experiments, the levels of platelet and fibrinogen were examined in order to investigate the anti-coagulative effect of KP-ext. KP-ext showed suppressive activity against the platelet decrease induced by LPS at 200 mg/kg (Table 3). The decrease in platelet level may be attributable to the coagulation of platelets in the blood. KP-ext may thus have a preventive effect on anti-coagulation. Furthermore, the fibrinogen level also decreased. These data suggest that KP-ext has an anti-coagulative effect in the blood coagulation system, as well as on fibrinolytic activity as discussed above. Further investigation is needed to clarify this point.
References
Rujjanawate C, Kanjanapothi D, Amornlerdpison D, Pojanagaroon S (2005) Anti-gastric ulcer effect of Kaempferia parviflora. J Ethnopharm 102:120–122
Chaturapanich G, Chiyakul S, Verawatnapakul V, Pholpramool C (2008) Effects of Kaempferia parviflora extracts on reproductive parameters and spermatic blood flow in male rats. Reproduction 136:515–522
Nakao K, Murata K, Deguchi T, Itoh K, Fujita T, Higashino M, Yoshioka Y, Matsumura S, Tanaka R, Shinada T, Ohfune Y, Matsuda H (2011) Xanthine oxidase inhibitory activities and crystal structures of methoxyflavones from Kaempferia parviflora rhizome. Biol Pharm Bull 34:1143–1146
Guh JH, Ko FN, Jong TT, Teng CM (1995) Antiplatelet effect of gingerol isolated from Zingiber officinale. J Pharm Pharmacol 47:329–332
Hibino T, Yuzurihara M, Terawaki K, Kanno H, Kase Y, Takeda A (2008) Goshuyuto, a traditional Japanese medicine for migraine, inhibits platelet aggregation in guinea-pig whole blood. J Pharm Sci 108:89–94
Schoendorf TH, Rosenberg M, Beller FK (1971) Endotoxin-induced disseminated intravascular coagulation in nonpregnant rats. Am J Pathol 65:51–58
Kaulla KN, Schultz RL (1958) Methods for the evaluation of human fibrinolysis; studies with two combined technics. Am J Clin Pathol 29:104–112
Norén I, Ramström G, Wallén P (1975) Fibrin plate method with reagents purified by affinity chromatography and its use for determination of fibrinolytic and other proteolytic activity in saliva, bile and plasma. Haemostasis 4:110–124
Acknowledgments
This study was financially supported by the “Antiaging Center Project” for Private Universities from the Ministry of Education, Culture, Sports, Science and Technology, 2008–2012.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murata, K., Deguchi, T., Fujita, T. et al. Improvement in blood fluidity by Kaempferia parviflora rhizome. J Nat Med 67, 719–724 (2013). https://doi.org/10.1007/s11418-012-0729-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-012-0729-9