Abstract
We examined the influence of Ginkgo biloba leaf extract (EGb) on the infectivity of influenza viruses in Madin–Darby canine kidney (MDCK) cells. Plaque assays demonstrated that multiplication of influenza viruses after adsorption to host cells was not affected in the agarose overlay containing EGb. However, when the viruses were treated with EGb before exposure to cells, their infectivity was markedly reduced. In contrast, the inhibitory effect was not observed when MDCK cells were treated with EGb before infection with influenza viruses. Hemagglutination inhibition assays revealed that EGb interferes with the interaction between influenza viruses and erythrocytes. The inhibitory effect of EGb was observed against influenza A (H1N1 and H3N2) and influenza B viruses. These results suggest that EGb contains an anti-influenza virus substance(s) that directly affects influenza virus particles and disrupts the function of hemagglutinin in adsorption to host cells. In addition to the finding of the anti-influenza virus activity of EGb, our results demonstrated interesting and important insights into the screening system for anti-influenza virus activity. In general, the plaque assay using drug-containing agarose overlays is one of the most reliable methods for detection of antiviral activity. However, our results showed that EGb had no effects either on the number of plaques or on their sizes in the plaque assay. These findings suggest the existence of inhibitory activities against the influenza virus that were overlooked in past studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Influenza viruses, members of the Orthomyxoviridae family, cause epidemics in the human population every year despite the availability of effective vaccines. In a severe pandemic year, millions of people die from the infection. Influenza viruses are classified on the basis of the antigenic properties of two surface glycoproteins: hemagglutinin (HA) and neuraminidase (NA). Sixteen HA subtypes (H1–H16) and nine NA subtypes (N1–N9) have so far been defined. Influenza virus infection is initiated by the interaction between HA and sialic acid moieties of glycoconjugates on host cells [16].
Several synthetic drugs such as amantadine and rimantadine (M2 ion channel inhibitors) and oseltamivir and zanamivir (NA inhibitors) have been available for decades, but all have side effects and thus somewhat limited usefulness [6, 11]. Therefore, novel substances and approaches are needed to control and prevent this viral disease. Various natural products have distinct anti-influenza virus activities [14]. We have demonstrated that a high-molecular-weight lignin-related fraction extracted from cones of Pinus parviflora Siebold et Zucc. suppresses the multiplication of influenza viruses by preventing viral RNA synthesis [9, 15]. We also reported that Sanicula europaea L. leaf extract contains an anti-influenza virus substance(s) that selectively inhibits influenza A viruses, but not influenza B viruses [13]. Studies on the anti-influenza virus activity of natural products have dramatically increased over the past several years [14].
Ginkgo biloba leaf extract (EGb) is a potential phytomedicine with various pharmacologic effects: in particular, anticoagulant, vasodilator, and anti-inflammatory effects [17]. In many countries, EGb and similar products are prescribed as therapeutic agents for cerebral or peripheral vascular inefficiency and for cognitive impairments associated with aging [2, 3]. Unlike other herbal drugs, however, EGb has hardly been tested for its anti-influenza virus activity. In the present study, we examined the inhibitory effect of EGb on influenza viruses.
Materials and methods
Reagents
The powder of Ginkgo biloba leaf extract was prepared by Mitsubishi Paper Mills Co., Ltd., Japan. In brief, the dried Ginkgo biloba leaves were finely ground and then extracted with water containing alcohol. After removing the residue, the extracts were concentrated under reduced pressure. The concentrate was then filtered and treated with adsorption resin to eliminate the impurities. Finally, the extracts were concentrated under reduced pressure again and then dried to use as powder. The powder of Ginkgo biloba leaf extract was dissolved in DMSO at a concentration of 100 mg/ml and stored at −30 °C until use.
As main active ingredients, it is known that the extract contains not only flavonoids such as kaempferol, quercetin and isorhamnetin but also terpene lactones such as bilobalide, ginkgolide A, B, C and J as specific components derived from Ginkgo biloba leaves [1, 4, 5].
Cells and viruses
Madin–Darby canine kidney (MDCK) cells were maintained in Eagle’s minimum essential medium (MEM) at 37 °C, in a 5 % CO2 atmosphere, supplemented with 10 % fetal bovine serum, 0.03 % l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin.
Influenza A/PR/8/34 (H1N1), A/Udorn/72 (H3N2), and B/Lee/40 viruses were grown at 35.5 °C for 48 h in allantoic sacs of 11-day-old embryonated eggs (Miyake Hatchery), and then the infected allantoic fluid was collected and stored at −80 °C until use.
Neutral red assay
The neutral red assay is based on incorporation of neutral red into lysosomes in living cells. To determine the effect of EGb on cell viability, MDCK cells (3.5 × 104 cells/well) were seeded into 24-well tissue culture plates and kept at 37 °C overnight. After removal of the culture medium, 0.4 ml of MEM containing various concentrations of EGb or DMSO was added to each well of the plates. After incubation for 24 h at 37 °C, 0.2 ml of neutral red solution (0.15 mg/ml) was added to each well. After incubation at 37 °C for 3 h, wells were washed with 0.2 ml of a fixative (1 % formalin and 1 % CaCl2). To extract the dye, 0.2 ml of 1 % acetic acid in 50 % ethanol was added to each well. After incubation at room temperature for 20 min, the amount of neutral red in each well was determined by measuring absorbance at 550 nm using a spectrometer. Results were represented as the cell number that was calculated from the standard curve of cell numbers. Furthermore, to determine the effect of EGb on the cell growth, MDCK cells (2.0 × 104 cells/well) were seeded into 24-well tissue culture plates and kept at 37 °C overnight. After removal of the medium, 0.4 ml of MEM containing 0, 10 and 100 μg/ml of EGb were added to each well. As control groups, DMSO was added to each well at final concentrations of 0.01 or 0.1 %. After incubation at 37 °C for 0, 24, 48 and 72 h, viable cells were determined with the neutral red assay as described above.
Treatment of viruses and cells by EGb
For pre-treatment of viruses by EGb, influenza A/PR/8/34 virus (500 pfu/ml) was mixed with EGb at several concentrations, incubated at room temperature for 10 min, and then subjected to the plaque formation assay. For post-treatment by EGb, MDCK cells infected with influenza viruses were overlaid with 0.8 % agarose containing EGb at several concentrations in the plaque formation assay. To investigate the direct effect of EGb on host cells, MDCK cells were exposed to EGb at several concentrations and incubated at 37 °C for 1 h. After removing the medium containing EGb, MDCK cells were infected with influenza viruses followed by the plaque formation assay.
Plaque formation assay
A confluent monolayer culture of MDCK cells in a 6-well tissue culture plates was washed with serum-free MEM and then infected with 0.5 ml of influenza virus solution [500 pfu/ml = multiplicity of infection (MOI) of 2.5 × 10−4] in serum-free MEM. After allowing 1 h at 37 °C for virus adsorption, the cells were washed with serum-free MEM and then overlaid with MEM containing 0.8 % agarose, 0.2 % BSA and 1 μg/ml l-1-tosylamide-2-phenylethyl chloromethyl ketone (TPCK)-treated trypsin (Sigma). After incubation at 37 °C for 2–3 days, plaques were visualized by staining cells with 0.5 % amido black. Results were represented as a ratio of the plaque number formed in the presence of EGb to that in the absence of EGb.
Hemagglutination assay
Influenza A/PR/8/34 virus (2 × 108 pfu/ml) was diluted nine times with phosphate buffered saline (PBS) (−) by twofold dilution each time, while 200 μg/ml of EGb was also diluted ten times with PBS (−) containing 0.2 % DMSO by twofold dilution each time. Fifty microliters of each diluted virus was mixed with 50 μl of each diluted EGb. These mixtures were then maintained at room temperature for 5 min. One hundred microliters of 0.5 % chicken erythrocyte suspension (Nippon Bio-Test Laboratories Inc., Japan) was added to each of these mixtures in 96-well round-bottom plates, and then the plate was incubated at room temperature for 30 min for hemagglutination. Results were represented as a plot where the x-axis and y-axis indicate concentrations of EGb and HA titer, respectively.
Statistical analysis
All of the data were represented as mean ± standard error of the mean (SEM). Comparisons for all pairs were performed by Student’s t test. A p value >0.05 was considered to be not significant. The calculations of 50 % cytotoxicity concentration (CC50) and inhibitory concentrations with 50 % plaque reduction (IC50) were performed by nonlinear regression using GraphPad Prism’s “log (inhibitor) versus response − variable slope” function (GraphPad Prism Version 5.01 for Windows, GraphPad Software Inc.).
Results
Effect of EGb on the viability and growth of MDCK cells
Before examining the anti-influenza virus activity of EGb, we investigated whether EGb affects the viability and growth of MDCK cells, which are routinely used as host cells for influenza viruses. We evaluated the cell viability and growth by counting the number of living cells as a function of time using the neutral red assay as described in “Materials and methods”. Cytotoxic effects of EGb were not observed at concentrations of <10 μg/ml (CC50 = 180 μg/ml) (Fig. 1a). Neither the growth rate nor the final cell density was affected by the presence of 10 μg/ml of EGb, whereas a marked decrease in the cell growth rate was observed at 100 μg/ml (Fig. 1b). Thus, EGb at a concentration of <10 μg/ml could be considered essentially nontoxic to MDCK cells. We confirmed that the solvent DMSO had no effect on the viability and growth of MDCK cells in the range of concentrations used in this study (data not shown).
Effect of EGb on the viability and the growth of MDCK cells. a MDCK cells (3.5 × 104) were seeded in 24-well tissue culture plates and incubated at 37 °C in the presence of various concentrations of EGb (closed circles) or solvent DMSO alone (open circles). After incubation for 24 h, the viable cell number was determined by the neutral red assay. b MDCK cells (2 × 104) were seeded in 24-well tissue culture plates and incubated at 37 °C in the absence (open circles) or presence of 10 μg/ml (closed square) and 100 μg/ml (closed triangles) of EGb, and 0.01 % (v/v) and 0.1 % (v/v) of DMSO alone (open square and open triangle, respectively). After incubation for the indicated periods, the viable cell number was determined by the neutral red assay
Inhibition of influenza virus infectivity by EGb
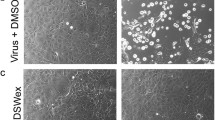
To examine whether EGb inhibits multiplication of influenza viruses, plaque assays were carried out as described in “Materials and methods”. Cells were infected with influenza A/PR/8/34 virus at 37 °C for 1 h. The cells were washed extensively with serum-free MEM and then overlaid with 0.8 % agarose in MEM containing EGb at various concentrations. The number of plaques and their sizes in the presence of EGb did not differ from those in the absence of EGb (Fig. 2a), indicating that EGb does not inhibit plaque formation by influenza virus infection. We further examined whether EGb is effective when mixed with viruses before exposure to cells. Influenza viruses were mixed with EGb at various concentrations at room temperature for 10 min and then exposed to MDCK cells. Under these conditions, EGb markedly inhibited viral infectivity in a dose-dependent manner (Fig. 2b). EGb at a concentration of 5 μg/ml almost completely inhibited the plaque-forming activity. These findings suggest that EGb inhibits the initial step of influenza virus infection before the virus enters the cytoplasm. Next, we examined whether the inhibitory effect of EGb against influenza virus was direct or indirect. Plaque assays were performed using MDCK cells treated with EGb at various concentrations for 1 h before infection with the influenza viruses. The number and sizes of the plaques of the tested groups in the presence of EGb did not differ significantly from those of the control group in the absence of EGb (Fig. 3), suggesting that EGb directly interacted with the influenza viruses and markedly reduced their infectivity.
Effect of EGb on plaque formation. Plaque assays were carried out as described in “Materials and methods”. a MDCK cells were infected with virus suspension (500 pfu/ml) and then overlaid with the overlay medium containing various concentrations of EGb. The profile of plaques is shown in the right panels. Panels 1, 2, 3, 4, 5 and 6 represent assays carried out in the presence of 0, 0.625, 1.25, 2.5, 5 and 10 μg/ml of EGb, respectively. b Influenza A virus (500 pfu/ml) was incubated with various concentrations of EGb prior to exposure to MDCK cells. The profile of plaques is shown in the right panels. Panels 1, 2, 3, 4, 5 and 6 represent assays in the presence of 0, 0.625, 1.25, 2.5, 5 and 10 μg/ml of EGb, respectively. Results are represented as the percentage of the plaque number formed in the absence of EGb
Effect of pre-treatment of host cells with EGb on influenza virus infection. MDCK cells were exposed to EGb at various concentrations and incubated at 37 °C for 1 h prior to virus infections. After removing EGb, MDCK cells were inoculated with influenza A/PR/8/34 viruses (500 pfu/ml), and plaque formation assays were carried out as described in “Materials and methods”. Results are represented as the percentage of the plaque number formed in the absence of EGb. All data are represented as mean ± SD, and the statistical analysis was performed using the t test to compare two groups
Inhibition of hemagglutination by EGb
Influenza virus infection is initiated by the interaction of hemagglutinin (HA) on the virion with sialic acids on the host cell surface. To understand how EGb prevents virus adsorption to cells, we examined whether EGb competitively inhibits influenza virus-mediated hemagglutination. As shown in Fig. 4, EGb inhibited hemagglutination in a dose-dependent manner, suggesting that EGb interferes with the interaction between HA and sialic acids.
HA titers of influenza A virus treated with various concentrations of EGb. Influenza A/PR/8/34 virus and EGb were diluted by twofold dilution each time and then mixed. After incubation at room temperature for 5 min, 0.5 % chicken erythrocyte suspension was added to each of these mixtures in a 96-well assay plate, and the plate was incubated at room temperature for 30 min for hemagglutination. Results are represented as a plot where the x-axis and y-axis indicate concentrations of EGb and HA titer, respectively. The result is representative of three independent experiments
Susceptibility of other influenza virus strains to EGb
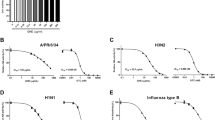
Our results suggest that EGb binds to HA and prevents virus adsorption to cells. We further examined whether the inhibitory effect of EGb is dependent on the type of influenza virus. EGb inhibited the infectivity of both influenza A/Udorn/72 (H3N2) and influenza B/Lee/40 viruses as well as of influenza A/PR/8/34 (H1N1) virus in an adsorption inhibition-dependent manner (compare Fig. 5a and b), albeit with slightly different sensitivities. To confirm the difference in infectivity inhibition, the 50 % inhibitory concentration (IC50) value of EGb was calculated for these three different strains of influenza viruses. Furthermore, the selectivity index (SI) was also calculated as the ratio of CC50 to IC50 (Table 1). The influenza A/PR/8/34 virus showed most sensitivity to EGb (Table 1). These findings suggest that the antiviral activity of EGb is not dependent on the type of influenza virus.
Effect of EGb on plaque formation by two different subtypes of influenza virus. Plaque assays were carried out as described in “Materials and methods”. a MDCK cells were infected with 0.5 ml of 500 pfu/ml of influenza A/Udorn/72 (H3N2) and B/Lee/40 viruses and then overlaid with the overlay medium containing various concentrations of EGb. b Each influenza virus strain was diluted to 500 pfu/ml and incubated with various concentrations of EGb prior to exposure to MDCK cells. One hour after virus inoculation, MDCK cells were washed with serum-free MEM and subsequently overlaid with the overlay medium without EGb. Results are represented as the percentage of the plaque number formed in the absence of EGb. a, b Results of A/Udorn/72 (H3N2) and B/Lee/40 are represented by gray bars and black bars, respectively
Discussion
In this study, we revealed the anti-influenza virus activity of Ginkgo biloba leaf extract (EGb). EGb acted directly on the influenza viruses and prevented their adsorption to the host cell surface, suggesting that EGb interfered with the interaction between the HA on the influenza virion and sialic acids on the host cell surface, although we could not exclude the possibility that EGb had a viricidal activity and directly inactivated the influenza virus.
The active constituents of EGb are standardized around the world; i.e., they contain 24 % flavonol glycosides (quercetin, kaempferol and isorhamnetin) and 6 % terpene lactones (ginkgolides and bilobalide). EGb also contains a class of condensed tannins, which are polymers composed primarily of flavan-3-ols (catechin and epicatechin) with a covalent bond between the individual flavonol units. Nakayama et al. [10] previously reported that two condensed tannins present in teas, (−)-epigallocatechin gallate (EGCG) and theaflavin digallate, bind to the HA of the influenza virus and inhibit its adsorption to MDCK cells. Furthermore, Song et al. [12] showed that catechin derivatives, including EGCG from green tea, inhibit not only the hemagglutination but also the NA activity of the influenza virus. The neuraminidase activity is thought to play a key role in the release of progeny virions from infected cells by cleavage of the sialic acid moieties of host cell receptors and in the prevention of self-aggregation of virions by cleavage of sialic acid still bound to the virus surface. These findings provide important insights into the molecular mechanisms of action of EGb.
Ginkgetin, a biflavone originally isolated from Ginkgo biloba leaves, has been found to inhibit the influenza virus sialidase [7]. However, our results showed that EGb prevented adsorption in the initial step of influenza virus infection. Therefore, in our study, a substance(s) in EGb other than ginkgetin may have been effective against influenza virus infection.
EGb was effective against the three different types of influenza viruses tested here, viz., the influenza A/PR/8/34 (H1N1), A/Udorn/72 (H3N2), and B/Lee/40 viruses, although the sensitivity towards EGb was slightly different among the three viruses. This finding suggests that EGb may be a potential wide-range inhibitor against influenza virus infection.
When plaque assays were performed with overlay medium containing EGb, neither the number of plaques nor their sizes were affected (Fig. 2a). Since our results suggest that EGb acts directly on the influenza virus and prevents the initial step in viral infection, we expected that the infectivity of the progenitor virions would be decreased owing to interaction with EGb present in the overlay medium and, consequently, that the size of individual plaques would be reduced in the plaque assay. This discrepancy between the predicted and the experimental results may be explained by our recent findings: we disclosed a novel transmission mode for influenza viruses, the so-called cell-to-cell transmission mode [8]. Influenza viruses have generally been believed to be capable of spreading via cell-free virions released from infected cells depending on the activity of NA. However, in cell-to-cell transmission, progeny virions are retained on the infected cell surface even after budding and transmitted from infected cells to adjacent uninfected cells without being released into the outer environment. The cell-to-cell transmission of the influenza virus is dependent on functional HA but independent of NA activity. The present study may demonstrate that EGb cannot inhibit the cell-to-cell transmission of influenza viruses but is highly effective in decreasing the infectivity of cell-free virions. This suggestion is in line with the findings of a previous study in which higher concentrations of anti-HA antibody were needed for inhibition of infection through cell-to-cell transmission than for that through cell-free viruses [8].
The plaque assay using drug-containing agarose gels is one of the most reliable methods for detecting anti-influenza virus activity and is frequently used as a screening method. However, our findings raise concerns that a particular anti-influenza virus activity, such as the inhibitory effect found here in EGb, may have been largely overlooked in past studies.
In conclusion, we have shown that EGb interacts directly with influenza viruses and markedly reduces the infectivity of the viruses by preventing their adsorption to host cells. Furthermore, the inhibitory effect of EGb seemed not to be restricted to a certain subtype of influenza virus. Taken together, these findings indicate the usefulness of EGb as an antiviral agent for influenza, although further studies are necessary to confirm its anti-influenza virus activity in vivo.
In addition to the finding of the anti-influenza virus activity of EGb, we demonstrated an interesting and important insight(s) into the screening system for anti-influenza virus activity. As was the case for the anti-influenza virus activity of EGb found in this study, some candidates for antiviral agents may have been overlooked in past studies because of the existence of the cell-to-cell transmission mode of influenza viruses. Therefore, our results signal a need for caution on the part of investigators trying to find anti-influenza virus compounds.
References
Chan PC, Xia Q, Fu PP (2007) Ginkgo biloba leave extract: biological, medicinal, and toxicological effects. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 25:211–244
DeFeudis FV, Drieu K (2000) Ginkgo biloba extract (EGb 761) and CNS functions: basic studies and clinical applications. Curr Drug Targets 1:25–58
Diamond BJ, Shiflett SC, Feiwel N, Matheis RJ, Noskin O, Richards JA, Schoenberger NE (2000) Ginkgo biloba extract: mechanisms and clinical indications. Arch Phys Med Rehabil 81:668–678
Drieu K (1986) Preparation and definition of Ginkgo biloba extract. Presse Med 15:1455–1457
Lichtblau D, Berger JM, Nakanishi K (2002) Efficient extraction of ginkgolides and bilobalide from Ginkgo biloba leaves. J Nat Prod 65:1501–1504
Long JK, Mossad SB, Goldman MP (2000) Antiviral agents for treating influenza. Cleve Clin J Med 67:92–95
Miki K, Nagai T, Suzuki K, Tsujimura R, Koyama K, Kinoshita K, Furuhata K, Yamada H, Takahashi K (2007) Anti-influenza virus activity of biflavonoids. Bioorg Med Chem Lett 17:772–775
Mori K, Haruyama T, Nagata K (2011) Tamiflu-resistant but HA-mediated cell-to-cell transmission through apical membranes of cell-associated influenza viruses. PLoS ONE 6(11):e28178
Nagata K, Sakagami H, Harada H, Nonoyama M, Ishihama A, Konno K (1990) Inhibition of influenza virus infection by pine cone antitumor substances. Antivir Res 13:11–21
Nakayama M, Suzuki K, Toda M, Okubo S, Hara Y, Shimamura T (1993) Inhibition of the infectivity of influenza virus by tea polyphenols. Antivir Res 21:289–299
Regoes RR, Bonhoeffer S (2006) Emergence of drug-resistant influenza virus: population dynamical considerations. Science 312:389–391
Song J, Lee K, Seong B (2005) Antiviral effect of catechins in green tea on influenza virus. Antivir Res 68:66–74
Turan K, Nagata K, Kuru A (1996) Antiviral effect of Sanicula europaea L. leaves extract on influenza virus-infected cells. Biochem Biophys Res Commun 225:22–26
Wang X, Jia W, Zhao A, Wang X (2006) Anti-influenza agents from plants and traditional Chinese medicine. Phytother Res 20:335–341
Watanabe K, Momose F, Handa H, Nagata K (1995) Interaction between influenza virus proteins and pine cone antitumor substance that inhibits the virus multiplication. Biochem Biophys Res Commun 214:318–323
Wiley DC, Skehel JJ (1987) The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem 56:365–394
Yoshikawa T, Naito Y, Kondo M (1999) Ginkgo biloba leaf extract: review of biological actions and clinical applications. Antioxid Redox Signal 1:469–480
Acknowledgments
We thank Katsushi Ogami and Yasuyuki Oku (Mitsubishi Paper Mills Ltd.) and Dr. Eri Nobusawa (Nagoya City University) for providing us with EGb and influenza B/Lee/40 viruses, respectively. We also thank Flaminia Miyamasu (Medical English Communication Center of Faculty of Medicine) for proofreading of this manuscript. This work was supported in part by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (KN) and a Grant for the project of Tsukuba Industrial Liaison and Cooperative Research (KN).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haruyama, T., Nagata, K. Anti-influenza virus activity of Ginkgo biloba leaf extracts. J Nat Med 67, 636–642 (2013). https://doi.org/10.1007/s11418-012-0725-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-012-0725-0