Abstract
Centella asiatica (L.) Urban has been traditionally used for the treatment of various disease and as a food for thousands of years in various parts of the world including eastern Asia, China and India. The goal of this study was to investigate the effects of Centella asiatica aqueous leaf extract on the induction of spermatogenic cell apoptosis in male rats. After lethal dose (LD50) assessment of plant extract, rats were divided in five groups. The experimental groups received orally 10, 50, 80 and 100 mg/kg aqueous leaf extract daily for 60 days and the control group received just water. After 60 days, body and testis weight were measured and blood samples were taken from the heart. To evaluate apoptosis and histological changes, tissue samples obtained from rat testes were stained by TUNEL assay and hematoxylin and eosin stain. Results showed that the sperm count, motility, and viability and the number of spermatogenic cells in the seminiferous tubules were significantly decreased compared with the control group. The number of apoptotic germ cells per seminiferous tubule cross-section was significantly increased in the experimental group (18.11 ± 3.5) compared with the control group (8.7 ± 0.81) (P < 0.05). Serum testosterone, follicle-stimulating hormone, and luteinizing hormone levels also showed significant decreases in the experimental groups (P < 0.05). There was also a significant decrease in testis weight in experimental groups compared with the control group (P < 0.05). It is concluded that Centella asiatica has toxicological effects on the reproductive system in male rats and, therefore, it is suggested that leaf extracts of Centella asiatica possess antifertility effects in the male rat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last few years, a marked decrease in the quality of human semen has been reported [1]. These changes in semen quality are more likely to be due to factors including chemicals, traditional healers and medicinal plants and misuse of drugs [2, 3]. Medicinal plants are commonly prescribed for a multitude of everyday conditions. Centella asiatica has been reported to be useful in the treatment of diarrhea, asthma, tuberculosis, inflammation, depression, lupus, and various skin lesions, and also to aid the circulatory system, soothe and minimize varicose veins, stimulate the sex drive, and improve the learning and memory processes. However, Centella asiatica has immunomodulatory, sedative, antidepressive, antimicrobial, antiviral, anticonvulsant and analgesic effects [4–7].
Centella asiatica (L.) Urban (syn. Hydrocotyle asiatica L.) belongs to the Apiaceae family (umbelliferae), and is native in India, northern Australia, Malaysia, Iran, Sri Lanka, Papua New Guinea, and other parts of Asia. Centella grows along ditches and in low wet areas; this aquatic plant is especially sensitive to pollutants in the water [8].
Centella asiatica contains triterpene glycosides such as centellasaponin, alkaloids, volatile and flavonoids compounds, steroids, asiaticoside, sceffoleoside, phenolic acid, and madecassic acid [9–12]. Asiaticoside is the most major triterpene glycoside in the water extract and it is transformed in vivo by hydrolysis into asiatic acid, which was shown to be effective in induction of apoptosis in different types of cancer [13–15].
Some studies have investigated Centella asiatica scientifically and it was found to possess a number of notable pharmacological effects including antipsoriatic [16], wound healing [17], hypoglycaemic [18], hepatoprotective [19], anti-gastric ulcer [20], anti-tumour, antimicrobial [21], antinociceptive and anti-inflammatory [22], antioxidant [23] and anxiolytic properties [24].
Male reproductive function is dependent on the normal status of the male reproductive organ and its accessory organs. The male reproductive organ is the testis, which is primarily responsible for the production of spermatozoa. Sperm production occurs in the seminiferous tubules of the testis, which is controlled by testosterone produced in Leydig cells [25]. Testosterone production is directly dependent on the concentration of luteinizing hormone (LH) secreted by the anterior pituitary gland. Follicle-stimulating hormone (FSH) released by the anterior pituitary stimulates the Sertoli cells which give support and nourishment to spermatozoa through their developmental steps. Therefore the quality and quantity of produced semen depend on normal function of the testicular structures and reproductive hormones [26]. Centella asiatica is thought to be medicinally useful; its traditional use in reproductive remedies has been reported: a decoction of plant leaves is widely used in India and eastern Asia as a male contraceptive. The aim of this study was to investigate the effect of a Centella asiatica aqueous extract, as an Iranian native plant, on spermatogenic cell apoptosis induction in the male rat.
Materials and methods
Plant collection and identification
The fresh leaves of Centella asiatica were collected in May 2007 from a local wetland at Bandar-Anzali, Iran. The accuracy of plant selection was proved and authenticated by Dr. Abrahmi, a senior botanist of the Department of Botany, University of Tehran, and a voucher specimen was deposited accordingly at the herbarium of the Department of Plant Sciences at the University of Tehran.
Extraction
The fresh leaves of the plant were air-dried at 40°C and ground to powder, which was then subjected to exhaustive extraction using ethanol in a Soxhlet apparatus. The dark green liquid extract was concentrated under vacuum and the resulting dried extract was lyophilized and preserved in a refrigerator at 4°C until use in the experiments.
Experimental animals
The animals used in this study were adult Wistar albino male rats, 8–10 weeks old and weighing 250 ± 10 g, obtained from animal facility of the Pasteur Institute (Tehran, Iran). Male rats were randomly distributed into cages and allowed to acclimatize for 10 days in a well-ventilated room at 25.0 ± 2.0°C with constant humidity of 40–70% and a 12-h light/12-h dark cycle prior to experimental protocols. All animals were treated in accordance to the principles of laboratory animal care [27]. Body weight and daily intake of food and water were determined several times per week throughout the study.
Acute toxicity test
The lethal dosage (LD50) values of Centella asiatica extract was evaluated in rats. In this method, rats were randomly divided in four groups (control and experimental groups) that received a single dose of Centella asiatica extract (100, 500, 1000, 2000 mg/kg) using intubation needles. All the animals were allowed access to feed and water. The mortality and manifestation rate was recorded during a 24-h period [28].
Experiment protocol
Forty adult male rats were equally divided in five groups: one control and four experimental groups. During the experiment, the crude extract was dissolved in distilled water and administered orally to rats at a concentration of 10, 50, 80, and 100 mg/kg body weight in 1 ml volume as single daily doses for 8 weeks using animal feeding gavage. Similarly, the controls received gavage of 1 ml of distilled water in the same schedule as the experimental rats. On the sixtieth day of treatment (end of the experiment), the rats were anesthetized using intraperitoneal pentobarbital sodium (50 mg/kg) and were then killed by decapitation according to the recommendation of the Institutional Ethical Committee [29]. Finally, the peritoneal cavity was opened through a lower transverse abdominal incision. The testes were carefully removed, washed in normal saline solution (0.9%), blotted, and weighed. Testes were fixed in Bouin’s fixative for assessing morphological changes and in formaldehyde fixative for TUNEL assays. Tissues were processed using standard procedures.
Measurement of semen parameters
The caudal epididymis was immediately dissected and an incision of about 1 mm made. A drop of seminal fluid containing sperm was loaded onto the microscope slide and 2 drops of normal saline were added to mobilize the sperm cells. Epididymal sperm motility was then assessed by calculating motile spermatozoa per microscope field and was expressed as a percentage. In order to count sperm, the epididymis was first homogenized in 5 ml of normal saline. The counting was then done using the counting chamber in the hemocytometer [30]. The sperm viability and morphology were also determined using eosin–nigrosin staining as described previously [31, 32].
Measurement of hormones
Blood samples were collected by heart puncture using a syringe into a lithium heparinized bottle, and centrifuged for 15 min at 3000 rpm. The serum obtained was stored at −20°C for hormone assay. Serum concentrations of FSH and LH were determined in duplicated samples by radioimmunoassay (RIA) with Rat FSH and LH kits (Biocode, Belgium) according to the protocol provided with each kit. The sensitivities of hormone detection per assay tube were 0.2 and 0.14 ng/nl for FSH and LH respectively. Serum concentration of total testosterone was measured by using a double antibody RIA kit (Beckman, USA). The sensitivity of hormone detection per assay tube was 0.025 ng/nl. [33].
Histopathological studies
As mentioned earlier, each testis was carefully isolated, weighed, washed in buffered saline and fixed in Bouin’s fixative for 1–2 days, and embedded in paraffin and then sectioned at 5 μm thickness. In accordance with standard histological techniques, the sections were stained with hematoxylin and eosin for microscopic evaluation to assess histopathological changes between control and experimental animals [34].
Assessment of apoptosis
The in situ detection of cells with DNA strand breaks was carried out by the TUNEL method. The TUNEL assay was performed the same as has been reported previously with some modifications. The choice of fixative was based on the results of previous studies, which showed that glutaraldehyde fixation significantly improved both TUNEL specificity and sensitivity while maintaining excellent morphological preservation [35–37].
In brief, after deparaffinization and rehydration, tissue sections were incubated with proteinase K for 15 min at room temperature, washed in distilled water, and then treated with 2% hydrogen peroxide in phosphate-buffered saline (PBS) for 5 min at room temperature to suppress endogenous peroxidase activity.
The sections were incubated with the TUNEL reaction mixture, fluorescein-dUTP (in situ Cell Death Detection, POD kit, Roche, Germany), for 60 min at 37°C. The slides were then rinsed three times with PBS and incubated with secondary anti-fluorescein-POD-conjugate for 30 min. After three times washing in PBS, diaminobenzidine-H2O2 (DAB, Roche, Germany) was added to sections and counterstained with hematoxylin. As a control for method specificity, the step using the TUNEL reaction mixture was omitted in negative control serial sections, and a nucleotide mixture in reaction buffer was used instead. Apoptotic germ cells were quantified by counting the number of TUNEL-stained nuclei per seminiferous tubule cross-section. Cross-sections of 100 tubules per specimen were assessed and the mean number of TUNEL-positive germ cells per tubule cross-section was calculated.
Statistical analysis
The data were statistically analyzed using SPSS software (version 13). The means of the experimental groups were compared with the control group using the ANOVA test and Student’s t test. Data were expressed as mean ± SEM and P < 0.05 was considered as a significant difference.
Results
Acute toxicity test
The oral LD50 of the extract of Centella asiatica was calculated with Pharm/Pcs probit scale versus log dose, value r = 0.96, standard deviation = 0.5 and regression equation, and found to be 200 mg/kg with a confidence of 1.9–2.2 in male rats. A dose of 100 mg/kg/day showed significant changes in the reproductive system.
Body and testes weight
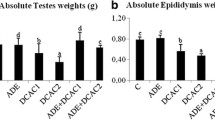
Table 1 shows that administration of extract after 60 days caused a significant increase in the body weight of treated males when compared with the control group. The weights of testes decreased significantly in experimental groups compared to the control group (P < 0.05).
Results of sperm count, viability and motility
Table 1 shows that administration of extract after 60 days significantly reduced sperm count and motility in all experimental groups relative to the control group. Moreover, sperm viability was significantly decreased in experimental groups relative to the control group.
Results of serum testosterone, LH and FSH hormones
Table 2 illustrates a significant reduction in serum levels of testosterone, FSH and LH relative to the control group.
Results of histopathology of testis
Histopathological studies of testis tissue demonstrated a significant decrease in the number of spermatogenic cells (spermatogonia, spermatocyte, spermatid and sperm) in the seminiferous tubules in all experimental groups relative to the control group. Intertubular spaces and venous congestion were increased in the treatment groups compared with those seen in the control group.
The loss in testicular weight likely corresponded to a dose-dependent decrease of mean spermatogenic cells in seminiferous tubules. Sperm density declined significantly in all experimental groups and seminiferous tubules showed degeneration (Fig. 1a–d).
Results of apoptotic germ cell assay
In this study, the TUNEL assay showed a number of positively stained spermatogenic cells in experimental groups. The TUNEL-positive spermatogonia and spermatocytes were the main germ cells undergoing apoptosis. The in situ detection method identified apoptotic cells by their darkly stained nuclei. We also observed a low incidence of spontaneous apoptosis in normal rat testis from the control group (Fig. 2a, b). The means of total apoptotic cells per seminiferous tubule cross-section (spermatogonia and spermatocytes) were 18.11 ± 3.5 and 8.7 ± 0.81 in the experimental and the control groups, respectively. Table 3 shows the descending trend of spermatogenic cell survival with increase in extract dose.
Discussion
Herbs have been used as important therapeutic agents for a long time. The knowledge of traditional medicine which is inherited from ancestors is very useful for daily life. Some of the herbs are more effective and valuable than those in modern medicine. Many of them have been claimed to be usable as drugs in traditional medicines. Medicinal plants are widely used in therapy because these plants have fewer side effects and lower price than modern drugs. However, there is little scientific data supporting most of their traditional uses, so studies are required to achieve more outcomes in humans and to introduce them into medical use.
The male reproductive system consists of the testis as the main reproductive organ and other accessory structures responsible for sperm production. Agents that alter testicular function will affect the quality and quantity of spermatozoa, which depends on several reproductive factors [38].
The animal model has previously been used to assess the effects of various extracts obtained from medicinal plants on male reproductive function. Since the spermatogenic process in rats lasts 60 days [39, 40], in this study aqueous leaf extracts of Centella asiatica were administrated for the same period. The present study showed that oral administration of Centella asiatica induces rat spermatogenic cell apoptosis and reduces fertility in male rats. As we know, the weight, size and secretory function of testis as reproductive organ is regulated by androgens [41]. Toxins and drugs may affect the pituitary gland and decrease the main process of spermatogenesis. Decreased androgen production is reflected by a decreased number of mature Leydig cells and their functional status. In the present study, the number of degenerating Leydig cells was significantly increased. This may manifest as a decrease in androgen levels. This decrease was further confirmed through a decline in spermatocytes and spermatids, a population which is completely androgen-dependent [42]. The results of this study show that the aqueous extract of Centella asiatica impairs reproductive function in the male rat. With regard to the ascending trend of adverse effects on semen parameters and testicular histology in conjunction with dose increase, it clearly appear that the effects of Centella asiatica may be mainly due to its direct deleterious impacts on the seminiferous tubules, according to the study by Mahanem and Norazalia [43].
In agreement with previous studies, exposure of the rats to a toxic compound was followed by a weight decrease in androgen-sensitive organs [44, 45]. Weight decrease of reproductive organs in the current study was further confirmed concurrently with the decrease in androgen levels. Researchers have demonstrated that physiological concentrations of testosterone, LH, and FSH play an important role in spermatogensis [46, 47], so the decrease in the concentration of these hormones in our study could reduce the number and function of somatic and germinal cells in the testis and consequently cause weight reduction of the testes.
The present study showed that administration of Centella asiatica for 60 consecutive days results in a marked reduction in sperm count, sperm motility and viability as compared to controls. This is in agreement with previous studies such as Yunianto et al. [48] who reported that Centella treatment for 42 days in mice caused in a marked reduction in sperm count and motility. In addition, it has been similarly shown that Centella asiatica treatment for four weeks in mice resulted in a significant decline in sperm count and did not affect sperm motility and morphology [49, 50]. The decrease in sperm count in this study is likely due to direct damages to the Leydig and Sertoli cells which are directly involved in the production of spermatozoa.
It has been reported that decrease in sperm count and motility are valid indices of male infertility in laboratory animals [51, 52]. However, sperm motility is often used as a marker of chemical-induced testicular toxicity [53]. It is also stated that the disruption of the seminiferous epithelium is indicative of male reproductive risk. Therefore, our experimental results suggest a gonadotoxic potential of Centella. One of the reasons for this effect could be Centella’s interaction with optimum energy metabolism for sperm vitality and motility. Apoptosis of germ cells occurs normally in the testis and is essential for the normal maintenance of spermatogenesis, whereas a relatively small increase in the percentage of germ cell apoptosis can result in defective spermatogenesis leading to infertility [45, 54]. Increases in the incidence of germ cell apoptosis are often observed as a result of various forms of physical or chemical injury to the testis [55]. The present study demonstrated that Centella asiatica can result in apoptosis in male rat germ cells. These results indicate that Centella asiatica, similar to other medicinal plants, may directly interfere in the process of spermatogenesis. The increase in germ cell apoptosis is possibly due to increased peroxide radical generation in the testis following Centella asiatica treatment. Therefore, Centella asiatica could also induce apoptotic pathways through activation of caspases. The results indicate that Centella asiatica has some effects in the male rat reproductive system. Furthermore, Centella asiatica can cause changes in the structure of the testicular histology, characterized by edema and reduced spermatogenesis.
As seen in Table 1, the body weight of the experimental group increased synchronously with decrease in testes weight when the dose changed from 10 to 100 mg/kg. Considering some studies on leptin receptors and leptin expression on germ cells within the testis [56, 57], there is a marked similarity between the outcomes obtained for leptin deficiency and for Centella asiatica experimental recommendation in male rat. As Bhat et al. [56] showed, body weights in the leptin-deficient cases are significantly increased while the weights of the testes are reduced as compared to control animals, the same as the present outcome using Centella asiatica. Regarding the above likenesses, and also similar inductive changes in endocrinological features such as LH and FSH reduction, and on the other hand the role of leptin changes in spermatogenic cell metabolism or even apoptosis [57], it appears that leptin could be a putative intermediate factor in the body weight increase in the experimental group. In addition, the relationship between sperm hypomotility with leptin deficiency as well as with Centella asiatica suggests a parallel regulatory role in reproduction at the level of the male gonad.
The results of the present study suggest that exposure to Centella asiatica through oral administration causes male reproductive toxicity in rats. Evidence for this toxicity included degenerative changes in the seminiferous tubules, absence of spermatozoa in the testes, and germ cell apoptosis, likely mediated via a direct deleterious action on the testis without strict disruption of the testicular endocrine function.
In conclusion, this study suggest that Centella asiatica has potential testicular toxicity as evidenced by decreased sperm count and motility, increased apoptotic cells, and structural damage to the testis in a rat model. It appears that this herbal remedy could be applicable to male contraception in humans. Although such herbal contraceptives would be useful if hormonal and surgical methods are contraindicated, many of these plants cannot be recommended for practical use in men due to a lack of comprehensive pharmacological data and knowledge. Therefore, further research is needed to achieve reliable contraceptives derived from these plants that do not induce any side effects.
References
Carlsen E, Giwercman A, Keiding N, Skakkebaek NE (1992) Evidence for decreasing quality of semen during past 50 years. Br Med J 305:609–613
Amann RP, Berndtson WE (1986) Assessment of procedures for screening agents for effects on male reproduction: effects of dibromochloropropane (DBCP) on the rat. Fundam Appl Toxicol 7:244–255
D’Cruz SC, Vaithinathan S, Jubendradass R, Mathur PP (2010) Effects of plants and plant products on the testis. Asian J Androl 12(4):468–479
Zheng CJ, Qin LP (2007) Chemical components of Centella asiatica and their bioactivities. Chin Integr Med 5(3):348–351
Kartnig T (1988) Clinical applications of Centella asiatica (L.). Herbs Spices Med Plants 3:145–173
Nalini K, Aroor AR, Karanth KS (1992) Effect of Centella asiatica fresh leaf aqueous extract on learning and memory and biogenic amine turnover in albino rats. Fitoterapia 63(3):232–237
Veerendra Kumar MH, Gupta YK (2002) Effect of different extracts of Centella asiatica on cognition and markers of oxidative stress in rats. J Ethnopharmacol 79:253–260
Vaidya ADB, Devasagayam TPA (2007) Current status of herbal drugs in India: an overview. J Clin Biochem Nutr 41:1–11
Brinkhaus B, Lindner M, Schuppan D, Hahn EG (2000) Chemical, pharmacological and clinical profile of the East Asian medical plant Centella asiatica. Phytomedicine 7(5):427–448
Bonfill M, Mangas S, Cusido RM, Osuna L, Pinol MT, Palazon J (2006) Indentification of friterpenoid compounds of Centella asiatica by thin layer chromatography and mass spectrometry. Biomed Chromatogr A 742:127–130
Inamdar PK, Yeole RD, Ghogare AB, De Souza NJ (1996) Determination of biologically active constituents in Centella asiatica. J Chromatogr A 742:127–130
Matsuda H, Morikawa T, Ueda H, Yoshikawa M (2001) Medicinal foodstuffs. XXVI. Saponin constituents of gotu kola (2): structures of new ursane- and oleanane-type triterpene oligoglycosides, centellasaponins B, C, and D, from Centella asiatica cultivated in Sri Lanka. Chem Pharm Bull 4:1368–1371
Coldren CD, Hashim P, Ali JM, Oh SK, Sinskey AJ, Rha G (2003) Gene expressing changes in the human fibroblast induced by Centella asiatica triterpenoids. Planta Med 69:725–732
Babu TD, Kutten G, Padikkala J (1995) Cytotoxic and anti-tumour properties of certain taxa of Umbelliferae with special reference to Centella asiatica (L.) Urban. J Ethnopharmacol 48:53–57
Lue YH, Sinha Hikim AP, Swerdloff RS, Im P, Khay ST, Bui T, Leung A, Wang C (1999) Single exposure to heat induces stage-specific germ cell apoptosis in rats: role of intratesticular testosterone on stage specificity. Endocrinology 140:1709–1717
Sampson JH, Raman A, Karlsen G, Navsaria H, Leigh IM (2001) In vitro keratinocyte antiproliferant effect of Centella asiatica extract and triterpenoid saponins. Phytomedicine 8:230–235
Shetty BS, Udupa SL, Udupa AL, Somayaji SN (2006) Effect of Centella asiatica L (Umbelliferae) on normal and dexamethasone-suppressed wound healing in Wistar Albino rats. Int J Low Extrem Wounds 5(3):137–143
Mutayabarwa CK, Sayi JG, Dande M (2003) Hypoglycaemic activity of Centella asiatica (L) Urb. East Cent Afr J Pharm Sci 6:30–35
Antony B, Santhakumari G, Merina B, Sheeba V, Mukkadan J (2006) Hepatoprotective effect of Centella asiatica (L) in carbon tetrachloride-induced liver injury in rats. Indian J Pharm Sci 68:772–776
Cheng CL, Guo JS, Luk J, Koo MW (2004) The healing effects of Centella extract and asiaticoside on acetic acid induced gastric ulcers in rats. Life Sci 74:2237–2249
Ullah MO, Sultana S, Haque A, Tasmin S (2009) Antimicrobial, cytotoxic and antioxidant activity of Centella asiatica. Eur J Sci Res 30:260–264
Somchit MN, Sulaiman MR, Zuraini A, Samsuddin L, Somchit N, Israf DA, Moin S (2004) Antinociceptive and antiinflammatory effects of Centella asiatica. Indian J Pharmacol 36:377–380
Hussin M, Abdul-Hamid A, Mohamad S, Saari N, Ismail M, Bejo MH (2007) Protective effect of Centella asiatica extract and powder on oxidative stress in rats. Food Chem 100:535–541
Wijeweera P, Arnason JT, Koszycki D, Merali Z (2006) Evaluation of anxiolytic properties of Gotukola—(Centella asiatica) extracts and asiaticoside in rat behavioral models. Phytomedicine 13:668–676
Mruk DD, Cheng CY (2004) Sertoli-sertoli and sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev 25(5):747–806
Roosen-Runge EC (1962) The process of spermatogenesis in mammals. Biol Rev Camb Philos Soc 37:343–377
Canadian council on Animal Care guidelines on the care and use of form animals in research, teaching and testing (2009) CCAC, Ottawa, pp 12–15
Miller LC, Tainter ML (1944) Estimation of ED50 and its error by means of log probit graph. Proc Soc Exp Biol Med 57:261–264
Erhardt W, Hebestedt A, Aschenbrenner G, Pichotka B, Blümel G (1984) A comparative study with various anesthetics in mice (pentobarbitone, ketamine-xylazine, carfentanyl-etomidate). Res Exp Med 184:3
Mishra SH, Padashetty SA (2007) Effect of terpenoidal fraction of Echinops echinatus roots on reproductive parameters of male rats. J Nat Med 61(4):452–457
Raji Y, Udoh US, Mewoyeka OO, Onoye FC, Bolarinwa AF (2003) Implication of reproductive endocrine malfunction in male antifertility efficacy of Azadirachta indica extract in rats. J Med Med Sci 32:159–165
World Health Organization (1999) Laboratory manual for the examination of human semen and sperm cervical mucus interation, 4th edn. Cambridge University Press, Cambridge, pp 3–104
Huang HF, Pogach LM, Nathan E, Giglio W, Seebode JJ (1991) Synergistic effects of follicle-stimulating hormone and testosterone on the maintenance of spermiogenesis in hypophysectomized rats: relationship with the androgen-binding protein status. Endocrinology 28(6):3152–3161
Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED (1990) Histological and histopathological evaluation of the testis. Cache River Press, Clearwater
Sinha Hikim AP, Rajavashisth TB, Sinha Hikim I, Lue YH, Bonavera JJ, Leung A, Wang C, Swerdloff RS (1997) Significance of apoptosis in the temporal and stage-specific loss of germ cells in the adult rat after gonadotropin deprivation. Biol Reprod 57:1193–1201
Sinha Hikim AP, Lue YH, Swerdloff RS (1997) Separation of germ cell apoptosis from toxin induced cell death by necrosis using in situ end-labeling histochemistry after glutaraldehyde fixation. Tissue Cell 29:487–493
Lue YH, Sinha Hikim AP, Wang C, Bonavera JJ, Baravarian S, Leung A, Swerdloff RS (1997) Early effect of vasectomy on testicular structure and on germ cell and macrophage apoptosis. J Androl 18:166–173
D’Cruz SC, Vaithinathan S, Jubendradass R, Mathur PP (2010) Effects of plants and plant products on the testis. Asian J Androl 12:468–479
Bataineh H, Nusier M (2005) Effect of cholesterol diet on reproductive functions in male albino rats. Saudi Med J 26:398–404
Bataineh H, Al-Hamood MH, Elbetieha AM (1998) Assessment of aggression, sexual behavior and fertility in adult male rat following long-term ingestion of four industrial metal salts. Hum Exp Toxicol 17:570–576
Agrawal S, Chauhan S, Mathur R (1986) Antifertility effects of embelin in male rats. Andrologia 18:125–131
Dym M, Raj HG, Lin YC, Chemes HE, Kotite NJ, Nayfeh SN, French FS (1979) Is FSH required for maintenance of spermatogenesis in adult rats? J Reprod Fertil 26:175–181
Mahanem MN, Norazalia MA (2004) Kesan In Vivo Ekstrak Daun Centella asiatica ke atas Histologi Testis dan Kualiti Sperma Mencit (In vivo effects of Centella asiatica leaf extract on the histology of testis and sperm quality in mice). Sains Malaysiana 33(2):97–103
Zitzmann M (2008) Effects of testosterone replacement and its pharmacogenetics on physical performance and metabolism. Asian J Androl 10(3):364–372
Gupta RS, Kumar P, Dixit VP, Dobhal MP (2000) Antifertility studies of the root extract of the Barleria prionitis Linn. in male albino rats with special reference to testicular cell population dynamics. J Ethnopharmacol 70(2):111–117
Choi SM, Lee BM (2004) An alternative mode of action of endocrine-disrupting chemicals and chemoprevention. J Toxicol Environ Health B Crit Rev 7(6):451–463
Huang HFS, Linsenmeyer TA, Li MT, Giglio W, Anesetti R, von Hagen J et al (1995) Acute effects of spinal cord injury on the pituitary-testicular hormone axis and Sertoli cell functions: a time course study. J Androl 16:148–157
Yunianto I, Das S, Mat Noor M (2010) Antispermatogenic and antifertility effect of Pegaga (Centella asiatica L) on the testis of male Sprague-Dawley rats. Clin Ter 161(3):235–239
Seed J, Chapin RE, Clegg ED, Dostal LA, Foote RH, Hurtt ME, Klinefelter GR, Makris SL, Perreault SD, Schrader S, Seyler D, Sprando R, Treinen KA, Veeramachaneni DN, Wise LD (1996) Methods for assessing sperm motility, morphology, and counts in the rat, rabbit, and dog: a consensus report. ILSI Risk Science Institute Expert Working Group on Sperm Evaluation. Reprod Toxicol 10(3):237–244
Huynh PN, Hikim AP, Wang C, Stefonovic K, Lue YH, Leung A, Atienza V, Baravarian S, Reutrakul V, Swerdloff RS (2000) Long-term effects of triptolide on spermatogenesis, epididymal sperm function, and fertility in male rats. J Androl 21(5):689–699
Lemasters GK, Selevan SG (1993) Toxic exposures and reproduction: a view of epidemiology and surveillance. In: Scialli AR, Zinaman MJ (eds) Reproductive toxicology and infertility. McGraw Hill, USA, pp 307–321
Working PK, Chellman GJ (1993) The testis, spermatogenesis and the excurrent duct system. In: Scialli AR, Zinaman MJ (eds) Reproductive toxicology and infertility. ISBN, McGraw Hill, USA, pp 55–76
Parveen S, Das S, Kundra cP, Pereira BMJ (2003) A comprehensive evaluation of the reproduction toxicity of Quassia amara in male rats. Reprod Toxicol 45(1):45–50
Moline JM, Golden AL, Bar-Chama N, Smith E, Rauch ME, Chapin RE et al (2000) Exposure to hazardous substances and male reproductive health: a research framework. Environ Health Perspect 108:803–813
Richburg JH (2000) The relevance of spontaneous-and chemically-induced alterations in testicular germ cell apoptosis to toxicology. Toxicol Lett 112–113:79–86
Bhat GK, Sea TL, Olatinwo MO, Simorangkir D, Ford GD, Ford BD, Mann DR (2006) Influence of a leptin deficiency on testicular morphology, germ cell apoptosis, and expression levels of apoptosis related genes in the mouse. J Androl 27(2):302–310
Lampiao F, Agarwal A, du Plessis SS (2009) The role of insulin and leptin in male reproduction. Arch Med Sci 5(1A):S48–S54
Acknowledgments
This study was financially supported by grant No. 775-11 from the Academic Center for Education, Culture and Research (ACECR), Iran. The authors had no conflict of interest in doing the research. The authors wish to express their gratitude to Dr M. Ghazi Khansari from Tehran University of Medical Sciences and Dr M. Ghaffari Novin from Shahid Beheshti University of Medical Sciences for their technical help. Thanks are also due to Dr S.M. Rezayat from Department of Pharmacology, Tehran University of Medical Sciences and Dr A.H. Jamshidi from Department of Food and Drug, Ministry of Health and Medical Education for the generous extract supply, and too Dr H. Zeraati for his assistance in the statistical analysis. We are very grateful for Mr K. Saliminejad helping in the Art Works.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heidari, M., Heidari-Vala, H., Sadeghi, M.R. et al. The inductive effects of Centella asiatica on rat spermatogenic cell apoptosis in vivo. J Nat Med 66, 271–278 (2012). https://doi.org/10.1007/s11418-011-0578-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-011-0578-y