Abstract
Excessive receptor activator of NF-κB ligand (RANKL) signaling causes enhanced osteoclast formation and bone resorption. The downregulation of RANKL expression and its downstream signals may be an effective therapeutic approach to the treatment of bone loss diseases such as osteoporosis. Here, we found that coptisine, one of the isoquinoline alkaloids from Coptidis Rhizoma, exhibited inhibitory effects on osteoclastogenesis in vitro. Although coptisine has been studied for its antipyretic, antiphotooxidative, dampness dispelling, antidote, antinociceptive, and anti-inflammatory activities in vitro and in vivo, its effects on osteoclastogenesis have not been investigated. Therefore, we evaluated the effects of coptisine on osteoblastic cells as well as osteoclast precursors for osteoclastogenesis in vitro. The addition of coptisine to cocultures of mouse bone marrow cells and primary osteoblastic cells with 10−8 M 1α,25(OH)2D3 caused significant inhibition of osteoclast formation in a dose-dependent manner. Reverse transcriptase polymerase chain reaction (RT-PCR) analyses revealed that coptisine inhibited RANKL gene expression and stimulated the osteoprotegerin gene expression induced by 1α,25(OH)2D3 in osteoblastic cells. Coptisine strongly inhibited RANKL-induced osteoclast formation when added during the early stage of bone marrow macrophage (BMM) cultures, suggesting that it acts on osteoclast precursors to inhibit RANKL/RANK signaling. Among the RANK signaling pathways, coptisine inhibited NF-κB p65 phosphorylations, which are regulated in response to RANKL in BMMs. Coptisine also inhibited the RANKL-induced expression of NFATc1, which is a key transcription factor. In addition, 10 μM coptisine significantly inhibited both the survival of mature osteoclasts and their pit-forming activity in cocultures. Thus, coptisine has potential for the treatment or prevention of several bone diseases characterized by excessive bone destruction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoclasts are hematopoietic lineage cells derived from bone marrow myeloid precursors and circulating monocytes [1]. The first key step in osteoclastogenesis is the generation of osteoclast precursors that express high levels of receptor activator of NF-κB (RANK), which mediates differentiation in response to the major osteoclastogenic factor RANK ligand (RANKL). The key event in subsequent osteoclastogenesis is the activation of RANK by RANKL, which is expressed by osteoblast lineage cells under physiological conditions [2].
RANKL is expressed by osteoblasts as a membrane-associated cytokine [3, 4]. Osteoclast precursors express RANK, which recognizes RANKL expressed by osteoblasts through cell–cell interactions, and subsequently differentiate into osteoclasts in the presence of macrophage colony-stimulating factor (M-CSF). Osteoprotegerin (OPG), which is mainly produced by osteoblasts, is a soluble decoy receptor for RANKL [5]. OPG blocks osteoclastogenesis by inhibiting the RANKL–RANK interaction. Bone resorption-stimulating hormones and cytokines enhance the expression of RANKL in osteoblasts. Mature osteoclasts also express RANK, and RANKL supports the survival and stimulates the bone-resorbing activity of osteoclasts [3, 4]. Excessive RANKL signaling causes enhanced osteoclast formation and bone resorption. Therefore, the downregulation of RANKL expression or its downstream signals may be a therapeutic approach to the treatment of pathological bone loss.
To discover new types of antiresorptive agents, we screened for natural compounds that regulate osteoclast differentiation. In our screening, we found that coptisine had strong inhibitory effects on osteoclast formation in vitro. Coptisine (Fig. 1), a bioactive constituent of the Berberis and Coptis species, possesses the same tetracyclic structure but differs in the nature of the substituents on the benzo ring, comprising methylene dioxy and/or dimethoxy substituents, relative to berberine and palmatine, respectively. Regardless of the similarities in their structures, coptisine has been reported to exhibit a limited number of biological activities compared with berberine and palmatine. In the present study, we investigated the effects of coptisine on osteoclast differentiation and function, as well as its mechanism of action. We found that coptisine had not only inhibitory effects on RANKL-induced osteoclast differentiation, but also suppressive effects on 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3)-induced RANKL and OPG gene expression in osteoblasts.
Materials and methods
Materials
Male Std ddY mice (6–9 weeks of age) were purchased from Japan SLC Co. (Hamamatsu, Japan). All animal protocols and procedures used in this study were approved by the Institutional Animal Care and Use Committee of Chubu University. Recombinant soluble RANKL was purchased from Oriental Yeast Co. Ltd. (Tokyo, Japan). Recombinant human M-CSF (Leukoprol) was purchased from PeproTech EC Ltd. (London, UK). Coptisine chloride, 1α,25-(OH)2D3, and prostaglandin E2 (PGE2) were purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan). Specific polymerase chain reaction (PCR) primers for mouse RANKL, OPG, M-CSF, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were synthesized by Life Technologies Inc. (Tokyo, Japan). Anti-c-Jun N-terminal kinase (JNK), anti-phospho-JNK, anti-p38 MAPK, anti-phospho-p38 MAPK, anti-NF-κB p65, anti-phospho-NF-κB p65, and anti-NFATc1 mouse polyclonal antibodies were purchased from Cell Signaling Technology Inc. (Beverly, MA, USA). Phospho-NF-κB p65 detects NF-κB p65 only when phosphorylated at serine 536. It does not cross-react with the p50 subunit or other related protein. An anti-β-actin mouse polyclonal antibody was purchased from Sigma Chemical Co. (St. Louis, MO, USA). Type I collagen gel solution was obtained from Nitta Gelatin Co. (Osaka, Japan). Alexa-phalloidin was purchased from Invitrogen (Carlsbad, CA, USA). Mouse clonal stromal cells from bone marrow (ST2) were obtained from the RIKEN Cell Bank (Tsukuba, Japan). All other chemicals and reagents were of analytical grade.
Mouse bone marrow cells and cocultures
Bone marrow cells (BMCs) were obtained from the tibiae of 4- to 6-week-old male ddY mice. In the coculture system, BMCs were cocultured with ST2 cells in 24-well plates in the presence of 10−8 M 1α,25-(OH)2D3 for 5 days. After the coculture, some of the cells were fixed and stained for tartrate-resistant acid phosphatase (TRAP), a marker enzyme for osteoclasts. To obtain mature osteoclasts, BMCs (1 × 107 cells) and ST2 cells (1 × 106 cells) were cocultured in collagen gel-coated 100-mm plates for 5–6 days in α-MEM containing 10% fetal bovine serum (FBS), 10−8 M 1α,25(OH)2D3, and 10−6 M PGE2. The plates were treated with collagenase and whole cells were harvested for use in subsequent experiments. ST2 cells were examined for the ALP activity induced by BMP2 to identify the osteoblastic cells.
PCR amplification of reverse-transcribed mRNA
For reverse transcriptase PCR (RT-PCR) analyses, ST2 cells were cultured in α-MEM containing 10% FBS and 10−8 M 1α,25(OH)2D3 in 60-mm dishes. After culture for 48 h, total cellular RNA was extracted from the cells using TRIzol solution (Life Technologies Inc.). First-strand cDNA was synthesized from the total RNA with random primers and subjected to PCR amplification with Ex Taq polymerase (Takara Biochemicals, Shiga, Japan) using the following specific PCR primers: mouse RANKL, 5′-CGC TCT GTT CCT GTA CTT TCG AGC G-3′ (forward) and 5′-TCG TGC TCC CTC CTT TCA TCA GGT T-3′ (reverse); mouse OPG, 5′-TGG AGA TCG AAT TCT GCT TG-3′ (forward) and 5′-TCA AGT GCT TGA GGG CAT AC-3′ (reverse); mouse M-CSF, 5′-GAG AAG ACT GAT GGT ACA TCC-3′ (forward) and 5′-CTA TAC TGG CAG TTC CAC C-3′ (reverse); mouse GAPDH, 5′-ACC ACA GTC CAT GCC ATC AC-3′ (forward) and 5′-TCC ACC ACC CTG TTG CTG TA-3′ (reverse). The PCR products were separated by electrophoresis in 2% agarose gels and visualized by ethidium bromide staining under UV light illumination. The sizes of the PCR products for mouse RANKL, OPG, M-CSF, and GAPDH were 587, 720, 516, and 452 bp, respectively.
Cell viability assay
Cell viability was measured by the MTT assay. Bone marrow macrophages (BMMs) were cultured under the same conditions used for the osteoclastogenesis experiments, and the MTT reagent was added at 3 h before the end of the culture. The supernatants were carefully removed and dissolved in DMSO before their absorbances were measured at 550 nm using a microplate reader.
Osteoclast differentiation from mouse BMMs
To obtain BMMs, BMCs were cultured in α-MEM containing 10% FBS and M-CSF (50 ng/mL) in 60-mm dishes. After culture for 1 day, non-attached cells in the culture plates were collected and used as BMMs. BMMs were cultured in 96-well plates in the presence of M-CSF (50 ng/mL) for 3 days, treated with RANKL (100 ng/mL), and cultured for a further 3 days. Next, the cells were sequentially fixed with 10% formalin for 10 min and ethanol for 1 min, and dried. Staining for TRAP was performed. TRAP-positive multinucleated cells containing more than five nuclei were counted.
Western blot analysis
Cells were lysed with RIPA buffer (20 mM Tris–HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 50 mM β-glycerophosphate, 1% NP-40, 1 mM Na3VO4, and 1× protease inhibitor cocktail). The extracted proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred onto PVDF membranes. The membranes were incubated with primary antibodies against c-JNK, phospho-JNK, p38 MAPK, phospho-p38 MAPK, phospho-NF-κB p65, NF-κB p65, NFATc1, and GAPDH, followed by secondary antibodies conjugated with horseradish peroxidase. Immunocomplexes were visualized by a chemiluminescence reaction using ECL reagents (Amersham Pharmacia Biotech, Buckinghamshire, UK).
Pit formation and TRAP staining on dentin slices
Mature osteoclasts were obtained from mouse cocultures on collagen gel-coated dishes as described above. The pit formation assay on dentin slices is a commonly used method for investigating the function of mature osteoclasts [6]. For resorption pit assays, aliquots of the crude mature osteoclast preparations were placed on dentin slices in 96-well plates. After preincubation for 2 h, the dentin slices were transferred to 48-well plates (1 slice/well) containing 0.3 mL/well of α-MEM supplemented with 10% FBS, and further cultured with or without coptisine for 48 h. At the end of the incubation, the osteoclasts were stained for TRAP activity and the numbers of TRAP-positive cells were counted. The resorption pits on the dentin slices were visualized by staining with hematoxylin. The number of resorption pits on each slice was counted.
Statistical analysis
The data were expressed as mean ± standard deviation (SD). Statistical analyses were performed by an unpaired two-tailed Student’s t-test assuming unequal variances. Values of p < 0.01 were considered to indicate statistical significance.
Results
Coptisine inhibits 1α,25(OH)2D3-induced osteoclast formation by regulating the gene expression of RANKL and OPG in osteoblasts
To clarify the effects of coptisine on osteoclast formation, BMCs were cocultured with ST2 cells derived from mouse stromal in the presence of 10−8 M 1α,25(OH)2D3. Many TRAP-positive multinucleated osteoclasts were formed in the cocultures within 5 days in response to 1α,25(OH)2D3 (Fig. 2a). We found that treatment with coptisine dose-dependently inhibited the osteoclast formation in the cocultures. Complete inhibition of osteoclast formation was observed after treatment with 10 μM coptisine (Fig. 2b). Next, we investigated the effects of coptisine on osteoblastic ST2 cells to support osteoclast formation in the presence of 1α,25(OH)2D3. ST2 cells were cultured with increasing concentrations of coptisine and their proliferation was examined by MTT assays (Fig. 3a). The cell viability was not affected by treatment with coptisine, even at 40 μM. In addition, we examined the effects of coptisine on the expression of RANKL, OPG, and M-CSF in ST2 cells treated with 10−8 M 1α,25(OH)2D3. The treatment of ST2 cells with 1α,25(OH)2D3 enhanced the expression of RANKL, suppressed the expression of OPG, and sustained the expression of M-CSF. At concentrations of 10–40 μM, coptisine had a suppressive effect on the upregulation of RANKL mRNA expression in a dose-dependent manner, as well as an inductive effect on the downregulation of OPG mRNA expression (Fig. 3b). These findings suggest that coptisine suppresses osteoclast formation by regulating the RANKL and OPG gene expression in osteoblastic cells.
Effects of coptisine on viability and gene expression in mouse cocultures. a BMCs and stromal cells were cocultured in 24-well plates for 5 days in the presence of 10−8 M 1α,25(OH)2D3. After 5 days, the cells were fixed and stained for TRAP. b TRAP-positive multinucleated cells containing five or more nuclei were counted as osteoclasts. The results are expressed as the mean ± SD of three cultures. *p < 0.05
Effects of coptisine on 1α,25(OH)2D3-induced osteoclastogenesis in ST2 cells. a Cell viability was determined by MTT assays. ST2 cells were cultured with coptisine at 1, 10, 20, or 40 μM. After 2 days, the MTT reagent was added to each well. The absorbance was measured at 550 nm, and the cell viability was calculated. b ST2 cells were cultured for 48 h in the presence of 1α,25(OH)2D3 (10−8 M) and coptisine (1, 10, 20, or 40 μM). Total RNA was extracted from the cells, and the expression levels of RANKL, OPG, and M-CSF mRNAs were analyzed by RT-PCR. The results are expressed as the mean ± SD of three cultures. *p < 0.05
Coptisine inhibits RANKL-induced osteoclast formation in BMM cultures
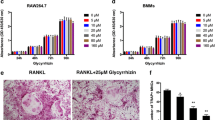
To determine the effects of coptisine on osteoclast formation from osteoclast progenitor cells in the absence of osteoblasts, mouse BMMs were incubated with coptisine in the presence of RANKL and M-CSF, and allowed to proliferate and differentiate into osteoclasts. As shown in Fig. 4b, RANKL dramatically induced osteoclast formation in BMM cultures. At concentrations of 10–20 μM, coptisine significantly inhibited the osteoclast formation induced by RANKL. MTT assays revealed that the BMM viability was slightly decreased by 20 μM coptisine throughout the culture period (Fig. 4a). To explore the effects of coptisine on osteoclast formation in more detail, we added coptisine to BMM cultures with RANKL and M-CSF at three different time points (Fig. 4d). After exposure to coptisine for 24 h, the culture media containing coptisine were removed and exchanged for coptisine-free culture media (D 0–1) (Fig. 4e). As a result, we confirmed that even 1 μM coptisine had an inhibitory effect to osteoclast formation when the cells were exposed at D 0–1 after RANKL treatment without cytotoxicity (data not shown). However, coptisine only showed an inhibitory effect to osteoclast formation at the early culture stage, D 0–1(Fig. 4f, left panel). The similar inhibitory effect on osteoclast formation was observed when coptisine was added at D 1–2 (data not shown). However, the inhibitory effect was attenuated when coptisine was added at D 2–3 (Fig. 4e, right panel). These findings suggest that coptisine directly influences RANKL-induced osteoclastogenesis from osteoclast precursors to early signaling events.
Effects of coptisine on RANKL-induced osteoclast formation in mouse BMMs. BMMs were cultured with coptisine for 72 h in 96-well plates. a Cell viability was determined by MTT assays. b Mouse BMMs were cultured with RANKL (100 ng/mL) and M-CSF (50 ng/mL). After the culture, the cells were fixed and stained for TRAP. TRAP-positive multinucleated cells containing more than five nuclei were counted as osteoclasts. c TRAP staining of osteoclasts in 96-well plates is also shown. d Durations of exposure to coptisine treatment. e TRAP-positive multinucleated cells were counted as osteoclasts (durations: D 0–1, left panel; D 2–3, right panel). The results are expressed as the mean ± SD of three cultures. *p < 0.05
Coptisine inhibits osteoclast differentiation via the suppression of NF-κB phosphorylation
To elucidate the inhibitory mechanism and pathway influenced by coptisine, BMMs were treated with RANKL with or without coptisine for 0–30 min. Analyses of phospho-NF-κB p65 clearly indicated that coptisine eliminated the RANKL-induced phosphorylation (Fig. 5a). On the other hand, the RANKL-induced phosphorylation of JNK and p38 MAPK was not affected by treatment with coptisine. The NFATc1 pathways play critical and fundamental roles in osteoclast development, and a lack of this transcription factor arrests osteoclastogenesis [7]. Therefore, we examined the effects of coptisine on the expression of NFATc1 (Fig. 5b). We found that coptisine impaired the RANKL-stimulated expression of NFATc1. Taken together, these findings suggest that the inhibitory effects of coptisine on osteoclast formation result from the downregulation of NF-κB signaling.
Effects of coptisine on RANKL-induced signaling pathways. Mouse BMMs were prepared from BMC cultures treated with M-CSF. a BMMs were treated with coptisine and RANKL (100 ng/mL) for the indicated times. Cell lysates were collected and separated by 10% SDS-PAGE. The levels of phosphorylated and non-phosphorylated p38 MAPK, JNK, and NF-κB p65 were determined by Western blotting analyses. b Effects of coptisine on the expression level of NFATc1 during osteoclastogenesis. The cells were incubated in the presence or absence of coptisine and stimulated with RANKL (100 ng/mL) for the indicated days. Cytoplasmic extracts were prepared, separated by SDS-PAGE, transferred onto PVDF membranes, and immunoblotted with specific antibodies. GAPDH was evaluated as an internal control
Coptisine suppresses the survival of mature osteoclasts
To confirm the effects of coptisine on the bone-resorbing function, we examined the effects of coptisine on the pit-forming activity of osteoclasts induced by 1α,25(OH)2D3 and PGE2 in the mouse coculture system. Osteoclasts formed by cocultures of BMCs and ST2 cells readily created resorption pits on dentine slices. Coptisine inhibited the pit formation on dentin slices in a dose-dependent manner. At a concentration of 10 μM, coptisine inhibited the pit formation by approximately 90% (Fig. 6a, upper panel; b, left panel). Before the pit formation, the dentin slices were stained for TRAP and the numbers of TRAP-positive osteoclasts on the dentin slices were counted. Similar to the pit formation findings, the number of TRAP-positive cells observed on the slices treated with 10 μM coptisine was markedly decreased compared with the control slices (Fig. 6a, lower panel; b, right panel). These findings suggest that the inhibition of the pit-forming activity of mature osteoclasts by coptisine is mainly caused by a reduction in the number of mature osteoclasts.
Effects of coptisine on the function of mature osteoclasts. Mature osteoclasts were cultured on dentin slices with increasing concentrations of coptisine. a After culture for 48 h, the cells were fixed with paraformaldehyde and the slices were fixed with paraformaldehyde and stained for TRAP. After removal of the cells, the dentin slices were stained with Mayer’s hematoxylin to identify the resorption pits. b TRAP-positive multinucleated cells containing more than five nuclei were counted as mature osteoclasts. The numbers of pits were counted on the dentin slices. The results are expressed as the mean ± SD of three cultures. *p < 0.05
Discussion
Plants used in traditional medicines have been recognized as one of the main sources for drug discovery and development. Since natural products of plant origin still form a major part of traditional medicine systems, a resurgence of interest in herbal medicines has occurred in Western countries as an alternative source of drugs, often for intractable diseases such as rheumatoid arthritis [8]. Li et al. [9] reported that Kampo formulae (Tsu-kan-gan; Phellodendri cortex, Anemarrhenae rhizome, and Cinnamomi cortex) showed inhibitory activity on bone resorption stimulated by parathyroid hormone in vitro and prevented the decrease in bone mineral density in ovariectomized mice in vivo. In the present study, we clarified the effects of coptisine, a bioactive constituent of Phellodendri cortex and Coptidis Rhizoma, on osteoclast differentiation and function in vitro. The effectiveness of this crude extract on osteoclastogenesis has potential for application to therapeutic strategies by targeting the differentiation of osteoclasts as well as their functions.
Coptisine is one of the isoquinoline alkaloids, which include berberine, palmatine, epiberberine, and magnoflorine [10], that are known to exert a variety of activities, including antidiabetic [11], anti-inflammatory [12], and antioxidant [13] effects. Pharmacokinetic studies of coptisine have shown that coptisine has the potential for oral bioavailability [14, 15]. In particular, berberine, the major medically important isoquinoline alkaloid, has been extensively studied and verified to be a major active constituent in the suppression of bone resorption [16]. However, its structural relative, coptisine, has been reported to exhibit a limited number of biological activities compared with berberine and palmatine.
Here, we found that coptisine effectively inhibited osteoclast formation in a mouse coculture system. Furthermore, RANKL-induced osteoclast formation from mouse BMMs was inhibited by coptisine. RT-PCR analyses showed that coptisine significantly affected the expression levels of RANKL and OPG mRNAs in osteoblasts with 1α,25(OH)2D3 treatment. The inhibitory effect of coptisine on pit formation on dentin slices was coincident with the number of remaining mature osteoclasts, suggesting that coptisine inhibits the survival of mature osteoclasts. Taken together, these findings suggest that coptisine acts on osteoclast precursors, osteoblasts, and mature osteoclasts, and, consequently, inhibits osteoclast differentiation and function.
Osteoclast formation is a multistep process that involves cell proliferation, commitment, fusion, and activation [3, 17]. Under pathophysiological conditions, signaling of the RANKL/RANK/TRAF6 axis in cooperation with costimulatory immunoreceptors leads to the robust induction of NFATc1, which is a necessary and sufficient factor for osteoclast differentiation [18, 19]. For this pathway, activation of the NF-κB pathway is a prerequisite for osteoclast differentiation [20, 21]. The addition of coptisine to osteoclast precursors attenuated the RANKL-induced phosphorylation of NF-κB. According to Hu et al. [22], berberine attenuates the RANKL-induced activation of NF-κB by inhibiting the nuclear translocation of NF-κB. In fact, berberine showed very similar effects on the signaling pathways to the present results for coptisine, although there are structural differences between coptisine and berberine. Moreover, it also inhibited RANKL-induced NFATc1, which is a necessary and sufficient factor for osteoclast differentiation. NFATc1 is a key transcription factor for the expression of TRAP and other osteoclastogenesis-associated genes. The introduction of an NFATc1 siRNA converts TRAP-positive cells into TRAP-negative cells [23]. Therefore, the inhibitory mechanism of coptisine appears to be related to this pathway in osteoclast precursor signaling.
On the other hand, osteoclasts have unique properties for resorbing bone. The most characteristic features of osteoclasts are the presence of ruffled borders and sealing zones [24–27]. It has been reported that disruption of the sealing zones suppresses the bone-resorbing activities of osteoclasts [28–30]. Therefore, the identification of drugs that can disturb the integrity of this bone-resorbing activity could be a useful approach for therapies aimed at slowing bone resorption. At a concentration of 10 μM, coptisine inhibited osteoclastic bone resorption by approximately 90%. In addition, the number of TRAP-positive cells observed on the dentine slices treated with 10 μM coptisine was markedly decreased compared with the control slices. Consistent with these findings, the inhibitory effect of coptisine on RANKL-induced osteoclast formation was attenuated in the late stage of culture, suggesting that coptisine acts more strongly on osteoclast precursors. Taken together, the anti-bone-resorbing activity may be not a direct effect on mature osteoclasts. Hu et al. [22] reported that berberine induces apoptosis by increasing the active form of caspase-3 in osteoclasts. Therefore, we cannot exclude the possibility that the inhibitory effect of coptisine on bone resorption is partially caused by apoptosis of mature osteoclasts.
Taken together, the present data suggest that coptisine has the potential to inhibit osteoclast differentiation and function in vitro. Accordingly, continued and advanced studies on the alterations in gene expression in bone cells and coptisine will provide a basis for understanding the observed bone cell responses to various pharmacological interventions.
References
Asagiri M, Takayanagi H (2007) The molecular understanding of osteoclast differentiation. Bone 40:251–264
Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, Miyata T, Anderson DM, Suda T (1999) Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor κB (RANK) receptors. J Exp Med 190:1741–1754
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423:337–342
Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL (2000) The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res 15:2–12
Udagawa N, Takahashi N, Yasuda H, Mizuno A, Itoh K, Ueno Y, Shinki T, Gillespie MT, Martin TJ, Higashio K, Suda T (2000) Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology 141:3478–3484
Takahashi N, Udagawa N, Kobayashi Y, Suda T (2007) Generation of osteoclasts in vitro, and assay of osteoclast activity. Methods Mol Med 135:285–301
Teitelbaum SL (2004) RANKing c-Jun in osteoclast development. J Clin Invest 114:463–465
Phillipson JD, Anderson LA (1989) Ethnopharmacology and Western medicine. J Ethnopharmacol 25:61–72
Li H, Miyahara T, Tezuka Y, Namba T, Nemoto N, Tonami S, Seto H, Tada T, Kadota S (1998) The effect of Kampo formulae on bone resorption in vitro and in vivo. I. Active constituents of Tsu-kan-gan. Biol Pharm Bull 21:1322–1326
Sun J, Ma JS, Jin J, Wang HS, Wen QH, Zhang HG, Zhou QL (2006) Qualitative and quantitative determination of the main components of huanglianjiedu decoction by HPLC-UV/MS. Yao Xue Xue Bao 41:380–384
Tang LQ, Wei W, Chen LM, Liu S (2006) Effects of berberine on diabetes induced by alloxan and a high-fat/high-cholesterol diet in rats. J Ethnopharmacol 108:109–115
Kuo CL, Chi CW, Liu TY (2004) The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett 203:127–137
Yokozawa T, Satoh A, Cho EJ, Kashiwada Y, Ikeshiro Y (2005) Protective role of Coptidis Rhizoma alkaloids against peroxynitrite-induced damage to renal tubular epithelial cells. J Pharm Pharmacol 57:367–374
Tan B, Ma Y, Shi R, Wang T (2007) Simultaneous quantification of three alkaloids of Coptidis Rhizoma in rat urine by high-performance liquid chromatography: application to pharmacokinetic study. Biopharm Drug Dispos 28:511–516
Li HL, Zhang WD, Liu RH, Zhang C, Han T, Wang XW, Wang XL, Zhu JB, Chen CL (2006) Simultaneous determination of four active alkaloids from a traditional Chinese medicine Corydalis saxicola Bunting. (Yanhuanglian) in plasma and urine samples by LC-MS-MS. J Chromatogr B Analyt Technol Biomed Life Sci 831:140–146
Qin L, Han T, Zhang Q, Cao D, Nian H, Rahman K, Zheng H (2008) Antiosteoporotic chemical constituents from Er-Xian Decoction, a traditional Chinese herbal formula. J Ethnopharmacol 23:271–279
Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ (1999) Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev 20:345–357
Takayanagi H (2007) Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol 7:292–304
Walsh MC, Kim N, Kadono Y, Rho J, Lee SY, Lorenzo J, Choi Y (2006) Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol 24:33–63
Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, Leonardi A, Tran T, Boyce BF, Siebenlist U (1997) Requirement for NF-κB in osteoclast and B-cell development. Genes Dev 11:3482–3496
Iotsova V, Caamaño J, Loy J, Yang Y, Lewin A, Bravo R (1997) Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nat Med 3:1285–1289
Hu JP, Nishishita K, Sakai E, Yoshida H, Kato Y, Tsukuba T, Okamoto K (2008) Berberine inhibits RANKL-induced osteoclast formation and survival through suppressing the NF-κB and Akt pathways. Eur J Pharmacol 580:70–79
Chang EJ, Kim HJ, Ha J, Kim HJ, Ryu J, Park KH, Kim UH, Lee ZH, Kim HM, Fisher DE, Kim HH (2007) Hyaluronan inhibits osteoclast differentiation via Toll-like receptor 4. J Cell Sci 120:166–176
Chambers TJ, Magnus CJ (1982) Calcitonin alters behaviour of isolated osteoclasts. J Pathol 136:27–39
Väänänen HK, Zhao H, Mulari M, Halleen JM (2000) The cell biology of osteoclast function. J Cell Sci 113:377–381
Destaing O, Saltel F, Géminard JC, Jurdic P, Bard F (2003) Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol Biol Cell 14:407–416
Saltel F, Destaing O, Bard F, Eichert D, Jurdic P (2004) Apatite-mediated actin dynamics in resorbing osteoclasts. Mol Biol Cell 15:5231–5241
Lakkakorpi PT, Väänänen HK (1990) Calcitonin, prostaglandin E2, and dibutyryl cyclic adenosine 3′,5′-monophosphate disperse the specific microfilament structure in resorbing osteoclasts. J Histochem Cytochem 38:1487–1493
Nakamura I, Takahashi N, Sasaki T, Tanaka S, Udagawa N, Murakami H, Kimura K, Kabuyama Y, Kurokawa T, Suda T, Yasuhisa F (1995) Wortmannin, a specific inhibitor of phosphatidylinositol-3 kinase, blocks osteoclastic bone resorption. FEBS Lett 361:79–84
Suzuki H, Nakamura I, Takahashi N, Ikuhara T, Matsuzaki K, Isogai Y, Hori M, Suda T (1996) Calcitonin-induced changes in the cytoskeleton are mediated by a signal pathway associated with protein kinase A in osteoclasts. Endocrinology 137:4685–4690
Acknowledgments
This study was supported by a grant for Bio-Defense Programs from the Ministry of Education, Science and Technology (MEST) of the Republic of Korea.
Conflict of interest
The authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, JW., Iwahashi, A., Hasegawa, Si. et al. Coptisine inhibits RANKL-induced NF-κB phosphorylation in osteoclast precursors and suppresses function through the regulation of RANKL and OPG gene expression in osteoblastic cells. J Nat Med 66, 8–16 (2012). https://doi.org/10.1007/s11418-011-0537-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-011-0537-7