Abstract
The antihyperglycemic and hypolipidemic activities of Helicteres isora Linn. (Sterculiaceae) root extracts were investigated in alloxan-induced diabetic rats and a possible mechanism of the blood glucose lowering action was studied. Alloxan-induced diabetic rats experienced 69.13 and 51.14%, 22.60 and 21.89%, 30.12 and 19.96%, and 50.05 and 34.29% reduction in blood glucose, total cholesterol, triglycerides, and urea levels following oral administration of butanol and aqueous ethanol extracts of H. isora root, respectively, at 250 mg/kg for 10 days. The beneficial effects of these extracts were supported by evidence from histological examinations of the liver, pancreas, and kidney. Following the treatment with both extracts, the degenerative changes caused by alloxan in pancreatic cells were restored, particularly with the butanol extract. Histological examination convincingly showed the restoration of pancreatic islets, kidney glomeruli, and liver to its normal size. These results suggest that H. isora roots possess antidiabetic principles and can be useful for treatment of diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is a chronic metabolic disorder, characterized by abnormalities in carbohydrate, protein, and lipid metabolism [1]. The management of diabetes mellitus is considered a global problem and successful treatment has yet to be discovered. Although insulin therapy and oral hypoglycemic agents are the mainstay of treatment of diabetes and are effective in controlling hyperglycemia, they have prominent side effects and fail to significantly alter the course of diabetic complications [2]. In India, indigenous remedies have been used in the treatment of diabetes mellitus since the time of Charaka and Shusrutha (sixth century). Available ethnobotanical information reports about 800 plants which may possess antidiabetic potential [3]. Several such herbs have shown antidiabetic activity when assessed using currently available experimental techniques [4–6]. However, the search for more effective and safer hypoglycemic agents continues to be an area of active research.
Helicteres isora Linn. (Sterculiaceae) is a sub-deciduous shrub or small tree, which occurs, often gregariously, throughout India, from Jamuna eastward to Bihar and Bengal and southward in central, western, and southern India and the Andaman Islands. In traditional medicine the roots and bark are used as an expectorant, demulcent, astringent, antigalactagogue, to lessen griping, and as a cure for scabies. Fruits of H. isora are demulcent, mildly astringent, and useful in alleviating griping and flatulence. Juice of the roots is claimed to be useful in treating empyema, stomach problems, and diabetes [7–9]. The presence of cucurbitacin B and isocucurbitacin B in the roots has been reported [10]. An ethanol extract of the root caused significant reduction in plasma glucose, triglyceride, and insulin levels at 300 mg/kg dose after 9 days of administration to insulin resistant and diabetic db/db mice [11]. An aqueous extract of the bark showed significant antidiabetic and hepatoprotective effects in streptozotocin-induced diabetic rats [12, 13]. Earlier, we reported that the aqueous ethanol and butanol extracts of H. isora root produced significant (30.35% and 48.86% protection, respectively) antihyperglycemic activity in alloxan-induced diabetic rats at a dose of 250 mg/kg after 3 h of treatment [14]. We also reported that the root extracts of H. isora showed significant oral hypoglycemic activity on glucose-loaded rats [15] and significant anti-inflammatory and antinociceptive activity at 250 mg/kg dose [16, 17].
Chakrabarti et al. [11] and Venkatesh et al. [14] independently showed the scientific basis for the antihyperglycemic activity of this plant root extract. Both groups demonstrated the antidiabetic activity of the extracts in various animal models including diabetic db/db mice. More recently, Kumar et al. [12] showed the antidiabetic activity of the bark extract. All these publications demonstrated the antidiabetic and hyperlipidemic activities of this plant based on the estimation of biochemical parameters and lowering of the blood glucose and lipid levels, but no focus was given to the histological level changes in the liver, pancreas, and kidney, which are essential to understanding the precise mechanism of action of this plant’s antihyperglycemic and hypolipidemic activities. Therefore, the present study was carried out to elucidate a possible mechanism of action of the blood glucose lowering effect of aqueous ethanol and butanol extracts of H. isora and other beneficial effects in alloxan-induced diabetes mellitus. In addition to biochemical estimations, histological examinations of the pancreas, liver, and kidney were carried out.
Materials and methods
Plant material
Helicteres isora roots were collected in September 2008 from Srisailam Forest, Andhra Pradesh (AP), India. Dr. S. T. Ramachandra Chari (a taxonomist from the Kama Reddy Degree College, Kama Reddy, AP, India) performed the botanical identification. A voucher specimen (HI/Rt/08) is maintained in the Phytochemistry Department of G. Pulla Reddy College of Pharmacy, Hyderabad, AP, India. The roots were cut, air-dried, and ground into powder.
Preparation of extracts
The shade-dried root powder (5 kg) was extracted with 80% aqueous ethanol by a cold maceration process for 3 days. The yield of crude aqueous ethanol extract was 2.26% (113 g). To the concentrated aqueous ethanol extract (ca. 110 g), 500 ml of water was added and fractionated with chloroform (4 × 500 ml), ethyl acetate (4 × 500 ml), and n-butanol (4 × 500 ml). The yields of chloroform, ethyl acetate, n-butanol, and left over aqueous extracts were 0.48, 0.25, 0.90, and 0.55% (w/w), respectively. The aqueous ethanol and n-butanol extracts were used for the present study.
Experimental animals
The animal experimental protocol was approved by the Institutional Animals Ethics Committee of G. Pulla Reddy College of Pharmacy, Hyderabad, India. Male Wistar rats (200–250 g) were used in the experiment and were maintained under standard environmental conditions of temperature, relative humidity, and dark/light cycle with free access to standard diet (Hindustan Lever, India) and water.
Instrumentation
High-performance liquid chromatography (HPLC) was carried out by using an Agilent 1110 series LC system (Agilent Technologies, Waldbronn, Germany) equipped with degasser, isopump, and fraction collector; 100-μl aliquots of extracts (dissolved in mobile phase) were injected onto a Symmetry Shield™ RP18 column (4.6 × 250 mm, 5 μm), which was kept at ambient room temperature. The isocratic mobile phase (acetonitrile/6% acetic acid in water 1:1, v/v) was delivered at a flow rate of 0.5 ml/min to resolve the phytoconstituents. Eluate was monitored by using a photodiode array detector (scan range 200–400 nm), and data integration was carried out by Chemstation software (version 4).
High-performance thin layer chromatography (HPTLC) was carried out on a CAMAG TLC Scanner 3 (Camag Laboratory, Muttenz) system by using precoated silica gel 60 F 254 HPTLC plates (4 × 10 cm, Merck KGaA, Germany). The extracts were dissolved in the mobile phase used in the HPLC and applied over the plate with a Linomat IV applicator. The resolved peaks were scanned at different wavelengths.
Effect of H. isora root extracts on alloxan-induced diabetic rats
Diabetes was induced in rats by a single intraperitoneal administration of alloxan monohydrate (120 mg/kg, Loba Chemie, India) in sterile normal saline [18]. Seven days after alloxan injection, rats with marked hyperglycemia (fasting blood glucose > 350 mg/dl) were separated and divided into four groups of eight animals each. Group I served as diabetic control and received distilled water. Groups II and III received aqueous ethanol and butanol extracts, orally (p.o.) as a fine suspension in 0.5% carboxymethyl cellulose, respectively, at a dose of 250 mg/kg. Group IV diabetic rats were treated with standard glibenclamide at a dose of 10 mg/kg p.o., as a fine aqueous suspension. Group V comprised normal rats that served as normal control, treated with distilled water. Treatment was continued for 10 consecutive days, once daily. Blood samples were collected through tail vein just prior to and on days 3, 5, 7, and 10 after administration of extracts. Serum samples were used to measure glucose, total cholesterol, triglyceride, and urea levels. Glucose levels were estimated by using a commercially available glucose kit (Autospan, Span Diagnostics, India) based on the glucose oxidase method [19]. Total cholesterol, triglyceride, and urea levels were measured by using commercially available kits based on quantitative colorimetric assays (Pointe Scientific, USA). On the 10th day of experiment, the animals were sacrificed under light ether anesthesia. Pancreas, kidney, and liver were separated and processed for histological studies.
Histological studies
The tissues of pancreas, kidney, and liver were fixed in 10% formalin and embedded in paraffin wax. Sections of 4–5 µm thickness were made by using a rotary microtome and stained with hematoxylin–eosin (H&E), and histological observations were made under a light microscope [20, 21].
Statistical analysis
All values were expressed as mean ± standard error of the mean (SEM). Results were analyzed statistically by using analysis of variance (ANOVA) followed by Dunnett’s test. Values of P < 0.05 were considered significant.
Results
Biochemical estimations
The effects of aqueous ethanol and butanol extracts of H. isora were studied on alloxan-induced diabetic rats by continuous treatment for 10 days. The serum glucose, total cholesterol, triglyceride, and urea levels were estimated. Both the extracts showed considerable effects on the levels of the four estimated parameters.
Effect on serum glucose
Administration of H. isora root extracts was found to reduce blood glucose levels in alloxan-induced diabetic rats from the 3rd day of their continuous treatment (Table 1). The serum glucose levels were markedly raised (up to 5.5 times) in the diabetic control as compared with normal control on the initial and 10th day of the experiment. The reduction in blood glucose levels of animals treated with extracts was found to be progressive. Treatment with aqueous ethanol and butanol extracts for 10 days significantly (P < 0.01) reduced the serum glucose levels by 51.14% and 69.13%, respectively, while glibenclamide produced a 30.10% reduction.
Effect on total cholesterol
The effect of H. isora extracts and glibenclamide on serum cholesterol levels is shown in Table 1. The total cholesterol levels were higher in the untreated diabetic rats (128.57 mg/dl) compared with those in normal rats (64.01 mg/dl). The aqueous ethanol and butanol extracts caused a considerable decrease in total cholesterol levels from the 3rd day onwards. However, the decrease in cholesterol level in butanol extract treated animals was significantly (P < 0.01) higher compared with the aqueous ethanol extract (P < 0.05) treated animals. The percentage reduction in total cholesterol levels caused by both extracts was comparable with glibenclamide activity (Table 1).
Effect on triglycerides
The effect of H. isora root extracts on serum triglyceride levels is presented in Table 2. In untreated diabetic rats a consistent increase in serum triglyceride levels was observed. Both extracts produced a gradual significant decrease in triglyceride levels. Intergroup comparison revealed that the butanol extract reduced the serum triglyceride level significantly (P < 0.01) on the 10th day, indicating its high antihypertriglyceridemic action compared with the aqueous ethanol extract. On the other hand the glibenclamide also exerted a significant antihypertriglyceridemic effect.
Effect on urea
Blood urea levels in diabetic animals can be used to assess kidney function. The changes in urea levels in all five groups of animals are given in Table 2. The urea levels were higher in the untreated diabetic rats compared with those in normal rats (27.7 mg/dl in normal vs. 95.13 mg/dl in diabetic control). The significant reduction in urea levels was observed from day 3 in the extract treated animals, while glibenclamide produced a significant reduction from day 7. The decrease of urea levels caused by the aqueous ethanol extract, butanol extract, and glibenclamide was 34.29%, 50.05%, and 25.05%, respectively, on 10th day.
Histological studies
Histological studies were carried out to further support the observed positive effects and to help postulate a possible mechanism of action for H. isora root extracts.
Pancreas
The pancreatic islets of untreated diabetic rats showed severe necrotic changes with pyknotic nuclei. Focal hyaline changes were observed (Fig. 1a), whereas in the aqueous ethanol extract treated animals, the pancreatic islets appeared normal with some focal hyaline changes (Fig. 1b). The restoration of pancreatic islets was observed in butanol extract treated rats with a prominent nucleus and nucleolus (Fig. 1c). In contrast the rats treated with glibenclamide showed focal hyaline changes with pyknotic nuclei (Fig. 1d) in comparison with normal rat pancreas (Fig. 1e).
a Microphotograph of pancreas from diabetic rat showing pyknotic nuclei, hyalinization of islets. H&E, ×500. b Microphotograph of pancreas from aqueous ethanol extract treated diabetic rat showing focal hyalinization of islets. H&E, ×310. c Microphotograph of pancreas from butanol extract treated diabetic rat showing prominent nucleus and nucleolus of pancreatic islets. H&E, ×500. d Microphotograph of pancreas from diabetic rat treated with glibenclamide showing focal hyaline with pyknotic nuclei. H&E, ×310. e Microphotograph of pancreas from normal rat. H&E, ×310
Liver
Liver tissue of diabetic rats showed (a) distortion in the arrangement of cells around the central vein, (b) enlargement and thickening of the walls of veins and capillaries, and (c) development of fibrosis on the degenerated cells. Furthermore, some hepatocytes showed necrotic changes with pyknotic nuclei (Fig. 2a). Aqueous ethanol extract treated rat liver showed mild congestion of central vein and intrahepatic hemorrhages (Fig. 2b). The butanol extract treatment restored the cellular arrangement around the central vein and reduced fibrosis, i.e., it restored the liver to its normal structure (Fig. 2c). The glibenclamide treated rat liver (Fig. 2d) showed mild to moderate intrahepatic hemorrhages and few erythrocytes in the central vein as compared with that of normal liver (Fig. 2e).
a Microphotograph of liver from diabetic rat showing congestion of central vein with necrotic changes in hepatocytes. H&E, ×500. b Microphotograph of liver from aqueous ethanol extract treated diabetic rat showing mild congestion of central vein and few erythrocytes. H&E, ×310. c Microphotograph of liver from diabetic rat treated with butanol extract resembling normal liver. H&E, ×160. d Microphotograph of liver from glibenclamide treated diabetic rat showing mild to moderate intrahepatic hemorrhages and few erythrocytes in the central vein. H&E, ×160. e Microphotograph of liver from normal rat. H&E, ×160
Kidney
Severe hemorrhages in glomeruli, mild intertubular hemorrhages, and mild dilatation were observed in untreated diabetic rats (Fig. 3a). The focal intertubular hemorrhages with dilated tubules and shrunken glomeruli were observed in aqueous ethanol extract treated rats (Fig. 3b). The kidney of butanol extract treated diabetic rats showed mild intertubular hemorrhages, degeneration of tubular epithelium, and restoration of glomeruli with few erythrocytes (Fig. 3c). The glibenclamide treated rat kidney showed mild degenerative changes of tubular epithelium with moderate hemorrhages in glomeruli (Fig. 3d) in comparison with normal rat kidney (Fig. 3e).
a Microphotograph of diabetic rat kidney showing hemorrhages in glomeruli, degeneration, and dilatation of tubules. H&E, ×250. b Microphotograph of diabetic rat treated with aqueous ethanol extract showing intertubular hemorrhages, cystic dilatation of tubules with shrunken glomeruli. H&E, ×200. c Microphotograph of kidney from butanol extract treated diabetic rat showing restoration of glomeruli with few erythrocytes and dilated tubules. H&E, ×310. d Microphotograph of kidney from diabetic rat with glibenclamide showing mild degenerated tubules and moderate hemorrhages in glomeruli. H&E, ×200. e Microphotograph of kidney from normal rat. H&E, ×200
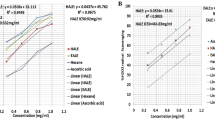
HPLC and HPTLC analysis of aqueous ethanol and butanol extracts
The aqueous ethanolic and butanolic extracts were analyzed by HPLC, using a diode array detector. Qualitatively both extracts gave a similar HPLC profile, although the peak intensity for some phytoconstitutents in the n-butanol extract was higher (because of their enrichment by the sequential extraction process used to obtain the butanolic extract, during which the nonpolar phytoconstituents are extracted by nonpolar solvents and hence only polar phytoconstituents are enriched). Both the extracts were then analyzed by HPTLC and similar observations were made.
Following confirmation of the presence of major phytoconstituent peaks in the HPLC profile of the butanol extract, we spiked several aliquots of the butanol extract and performed HPLC to allow fractional collection of major phytoconstituents based on their retention times. In total 5 major peaks fractions were collected (Fig. 4) and each fraction was dried carefully under a gentle stream of nitrogen. The residues of these 5 fractions were dissolved in aqueous ethanol and subjected to TLC analysis to help identify the nature of the phytoconstituent(s). All the phytoconstituents gave positive test for steroidal/triterpenoidal glycosides (Liberman Burchard test, Salkowski test for steroids/triterpenoids; Molisch test, Fehling’s test for carbohydrates) and negative test for the presence of alkaloids (Dragendorff’s test, Wagner’s test, Mayer’s test), flavonoids (Shinoda test, ferric chloride test), tannins (ferric chloride test), etc. [14].
Discussion
Helicteres isora root juice is claimed to be useful in treating diabetes. The results of biochemical estimations of serum glucose, total cholesterol, triglyceride, and urea levels indicate that both the aqueous ethanol and butanol extracts of H. isora root exert significant positive and beneficial effects on alloxan-induced diabetic rats. Alloxan monohydrate is one of the most widely used chemical diabetogens, and alloxan-induced diabetes in laboratory animals has become a valuable tool in diabetes research. Alloxan induces diabetes by partial destruction or massive necrosis of β-cells in the islets of Langerhans of the pancreas, leading to decreased levels of insulin that results in hyperglycemia. Alloxan is also known to produce structural and functional changes in kidney and liver [22–24].
The treatment with aqueous ethanol and butanol extracts (250 mg/kg) of H. isora root caused a significant fall in the blood glucose levels of diabetic rats. These results confirmed our earlier preliminary study on the antihyperglycemic activity of H. isora root [14]. The hyperglycemic condition (glucose level > 450 mg/dl) in untreated diabetic rats can be related to severe necrotic changes, decrease in size, and cell population of pancreatic insulin-secreting β-cells. These degenerative changes caused by alloxan in pancreatic cells were restored during treatment with the extracts, very prominently so in the butanol extract treated rats. This was evidenced by the decrease in glucose level to near normal (145.14 ± 4.74; P < 0.01) with a reduction of 69.13% on 10th day. This is probably due to stimulation and regeneration of β-cells and subsequent release of insulin and activation of the insulin receptors. Similar results have been reported for Pterocarpus marsupium [25], epicatechin [26], and Vinca rosea [27]. Treatment with glibenclamide (10 mg/kg) produced a 30.10% reduction in blood glucose. The significant antihyperglycemic activity caused by glibenclamide in alloxan-induced diabetic rats is an indication of the presence of some β-cells in them, as glibenclamide is known to stimulate insulin secretion from β-cells. Survival of some β-cells has been proved by several workers, who observed antihyperglycemic activity with oral hypoglycemic agents like glibenclamide and tolbutamide, in alloxan-induced diabetic rats [28–30]. Furthermore, the presence of pancreatic islets was evidenced by histological studies. Thus, the antihyperglycemic activity of H. isora root extracts could be due to its stimulating effect on β-cells, leading to insulin production and secretion, and it could also be due to its insulin-like activity.
Hypercholesterolemia and hypertriglyceridemia have been reported to occur in alloxan-induced diabetic rats [31, 32], and also observed in our experimental studies. This is mainly due to the increase in the mobilization of free fatty acids from the peripheral deposits, since insulin inhibits the hormone-sensitive lipase. On the other hand, glucagons, catecholamines, and other hormones enhance lipolysis [33]. Both the extracts caused a significant decrease in total cholesterol and triglyceride levels in diabetic rats; however, the butanol extract was found to be more potent than the aqueous ethanol extract. This is in agreement with the fact that the level of glycemic control is the major determinant of total and very low density lipoprotein (VLDL) triglyceride concentrations [34]. Hence the effect of these extract can be explained as a consequence of reduction in blood glucose via the stimulating effect on β-cells, leading to insulin production and secretion. Alloxan was also reported to increase urea levels [18, 35]. The present studies also indicate that H. isora can partially inhibit alloxan renal toxicity as seen from the decreased blood urea levels.
Histology of the liver during diabetes showed that there are structural alterations in the liver as a result of absence of insulin. The major alteration was thickening of the wall of the blood vessels and capillaries, which in turn affect the blood circulation in diabetes. The architecture of the liver in butanol extract treated diabetic rats was similar to that of normal liver, indicating that the degenerative changes initiated by diabetes are completely reversed by butanol extract administration, whereas this damage is partially reversed by aqueous ethanol extract treatment. Diuresis is a common feature associated with diabetes, which may be the reason for the structural changes observed with glomerulus. Histology of the kidney in diabetic rats showed severe hemorrhages in glomeruli, intertubular hemorrhages, and mild dilatation. The degenerative changes in the kidney brought about by alloxan administration were similar to earlier observations [36], and following treatment with butanol extract the architecture of diabetic rat kidney was completely reversed. Effects similar those of H. isora on liver and kidney were earlier reported for Gymnema sylvestre in diabetic rabbits [36].
In conclusion, we have demonstrated that the histological abnormalities seen in the pancreas, liver, and kidney of diabetic animals were completely reversed in butanol extract treated animals, giving almost normal tissue appearance compared with normal animals. Present efforts are directed to isolating the phytoconstituents from the butanol extract responsible for the antihyperglycemic and hypolipidemic activity of H. isora roots.
References
Cowie CC, Eberhardt MS (1996) Diabetes: vital statistics. American Diabetes Association, Alexandria
Rang HP, Dale MM (1991) The endocrine system pharmacology, 2nd edn. Longgman, UK, pp 504–508
Alarcon-Aguilara FJ, Roman-Ramas R, Perez-Gutierrez S, Aguilar-Contreras A, Contreras-Weber CC, Flores-Saenz JL (1998) Study of the anti-hyperglycemic effect of plants used as anti-diabetics. J Ethnopharmacol 61:101–110
Grover JK, Yadav S, Vats V (2002) Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol 81:81–100
Mukherjee SK, Saxena AM, Shukla G (2002) Progress of diabetes research in India during 20th century. National Institute of Science Communication (CSIR), New Delhi, India, pp 93–96
Shapiro K, William CG (2002) Natural products used for diabetes. J Am Pharm Assoc (Wash) 42:217–226
Anonymous (1959) The wealth of India, vol V. CSIR, New Delhi, India, pp 27–29
Chopra RN, Nayar SL, Chopra IC (1956) Glossary of Indian medicinal plants. CSIR, New Delhi, India, p 131
Kirthikar KR, Basu BD, An ICS (1981) Indian medicinal plants, vol 1. Lalit Mohan Basu, Allahabad, India, pp 370–372
Bean MF, Antoun M, Abrmson D, Chang CJ, Mc Laughlin JL, Cassady JM (1985) Cucurbitacin B and isocucurbitacin B: cytotoxic components of Helicteres isora. J Nat Prod 48:500
Chakrabarti R, Reeba KV, Ramesh M, Sharma VM, Jagadheshan H, Rao YN, Sairam P, Rajagopalan R (2002) Antidiabetic and hypolipidemic activity of Helicteres isora in animal models. J Ethnopharmacol 81:343–349
Kumar G, Banu SG, Murugugesan AG, Pandian MR (2006) Hypoglycaemic effect of Helicteres isora bark extract in rats. J Ethanopharmacol 107:304–307
Kumar G, Murugesan AG, Pandian MR (2006) Effect of Helicteres isora bark extract on blood glucose and hepatic enzymes in experimental diabetes. Pharmazie 61:353–355
Venkatesh S, Dayanand Reddy G, Madhava Reddy B (2003) Antihyperglycemic activity of Helicteres isora roots in alloxan-induced diabetic rats. Pharm Biol 41:347–350
Venkatesh S, Dayanand Reddy G, Reddy YSR, Sathyavathy D, Madhava Reddy B (2004) Effect of Helicteres isora root extracts on glucose tolerance in glucose-induced hyperglycemic rats. Fitoterapia 75:364–367
Sailakshmi K, Venkatesh S, Reddy BM, Mullangi R (2007) Anti-inflammatory and analgesic activity of Helicteres isora root. Indian Drugs 44:295–299
Venkatesh S, Lakxmi KS, Reddy BM, Ramesh M (2007) Antinociceptive activity of Helicteres isora. Fitoterapia 78:146–148
Joy KL, Kuttan R (1999) Anti-diabetic activity of Picrorrhiza kurroa extract. J Ethnopharmacol 67:143–148
Trinder P (1969) Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem 6:24–27
Dunn JS, Sheehan HL, Mc Letchie NGB (1943) Necrosis of Islets of Langerhans produced experimentally. Lancet 244:484–487
Luna GLHT (1968) Manual of histological and special staining methods of the Armed Forces Institute of Pathology, 3rd edn. McGraw-Hill, New York, pp 1–16
Cakici I, Conset H, Baha T, Nurettin AIK, Bilge S (1994) Hypoglycemic effect of Momordica charantia extracts in normoglycemic or cyprohepatidine induced hyperglycaemic mice. J Ethnopharmacol 44:117–121
Claus CR (1970) Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol Rev 22:485–518
Kameswara Rao B, Appa Rao CH (2001) Hypoglycemic and antihyperglycemic activity of Syzygium alternifolium Walp. Seed extracts in normal and diabetic rats. Phytomedicine 8:88–93
Chakravarthy BK, Gupta S, Gambhir SS, Gode KD (1980) Pancreatic β-cell regeneration—a novel antidiabetic mechanism of Pterocarpus marsupium Roxb. Indian J Pharmacol 12:123–127
Chakravarthy BK, Gupta S, Gode KD (1982) Functional beta cell regeneration in the islets of pancreas in alloxan induced diabetic rats by (−)-epicatechin. Life Sci 31:2693–2697
Ghosh S, Suryawanshi SA (2001) Effect of Vinca rosea extracts in treatment of alloxan diabetes in male albino rats. Indian J Exp Biol 39:748–759
Cherian S, Augusti KT (1993) Antidiabetic effect of a glycoside of leucopelargonidin isolated from Ficus bengalensis Linn. Indian J Exp Biol 31:26–29
Kumari K, Mathew BC (1995) Antidiabetic and hypolipidemic effects of S-methyl cysteine sulfoxide isolated from Allium cepa Linn. Indian J Biochem Biophys 32:49–54
Prince PSM, Menon VP, Gunasekharan G (1999) Hypolipidaemic action of Tinospora cordifolia roots in alloxan diabetic rats. J Ethnopharmacol 64:53–57
Kameswara Rao B, Kesavulu MM, Giri R, Appa Rao CH (1999) Antidiabetic and hypolipidemic effects of Momordica cymbalaria Hook fruit powder in alloxan diabetic rats. J Ethnopharmacol 67:103–109
Sharma SB, Nasir A, Prabhu KM, Murthy PS, Dev G (2003) Hypoglycaemic and hypolipidemic effect of ethanolic extract of seeds of Eugenia jambolana in alloxan-induced diabetic rabbits. J Ethnopharmacol 85:201–206
Al-Shamaony L, Al-Khazraji SM, Twaji HA (1994) Hypoglycaemic effect of Artemisia herbas alba 11. Effect of a valuable extract on some blood parameters in diabetic animals. J Ethnopharmacol 43:167–171
Laakso M (1995) Epidemiology of diabetic dyslipidemia. Diabetes Rev 3:408–412
Jimenez-Diaz C, Grande-Convian F, De Oya JC (1946) Alloxan diabetes and kidney function. Nature 158:589
Sahnmugasundaram KR, Pannerselvam SP, Samudram P, Shanmugasundaram ER (1983) Enzyme changes and glucose utilization in diabetic rabbits. The effect of Gymnema sylvestre R. Br J Ethnopharmacol 7:205–234
Acknowledgments
SV wishes to thank the All India Council of Technical Education, New Delhi, for providing financial support. The authors also wish to thank the management of the college for encouragement and providing facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Venkatesh, S., Madhava Reddy, B., Dayanand Reddy, G. et al. Antihyperglycemic and hypolipidemic effects of Helicteres isora roots in alloxan-induced diabetic rats: a possible mechanism of action. J Nat Med 64, 295–304 (2010). https://doi.org/10.1007/s11418-010-0406-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-010-0406-9