Abstract
In the present study, we investigated the inhibitory effect of the known oxycoumarins poncitrin (3), osthol (4), and xanthoxyletin (5), newly isolated from Clausena guillauminii (Rutaceae), together with the known carbazoles heptaphylline (1) and 7-methoxyheptaphylline (2) on inducible-nitric oxide synthase (iNOS) expression induced by lipopolysaccharide (LPS) and the NO generation in RAW 264.7 mouse macrophages. Isolation of active oxycoumarins was guided by Western blot analysis of iNOS protein expression. These oxycoumarins showed an inhibitory effect on iNOS protein expression at 10 μM. Further examination of the inhibitory effects of these compounds on inflammation mediators revealed that the synthesis of nitric oxide (NO) and the protein expression of tumor necrosis factor-α (TNF-α) and cyclooxygenase-2 (COX-2) were inhibited by 5. It was expected that these compounds show anti-inflammatory activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Almost all creatures have natural immunity as a defense mechanism, which is activated against infections such as bacteria and viruses and promptly eradicates them [1, 2]. When infection by a pathogenic organism is sensed, inflammatory cytokines, such as interleukin (IL)-1β and tumor necrosis factor (TNF)-α are generated as an initial immune response [1, 2]. Blood vessel permeation is accelerated by cytokines, macrophages migrate to the infected tissues, and inducible-nitric oxide synthase (iNOS) is heavily expressed in response to stimulation by toxins released from the pathogens; iNOS immediately produces NO using L-glutamine in tissues, which finally damages or destroys the pathogenic organisms [3]. However, although iNOS has an important role in excluding pathogenic organisms during infection, the large amount of NO produced simultaneously aggravates the tissues in the living body and causes transient inflammation. Furthermore, chronic inflammation conditions, such as atopic dermatitis, rheumatoid arthritis, and asthma may be induced by abnormal acceleration of this defense reaction [3, 4].
We have focused on the effect of naturally occurring products on lipopolysaccharide (LPS)-induced iNOS protein expression in RAW 264.7 cells, and found that some lignans are active components of the Thai plant Balanophora abbreviata Blume [5]. To enhance the development of Thai medicinal plants, we screened 50 extracts of Thai folk medicines and found that an extract of the root bark of C. guillauminii possessed significant inhibitory effects on iNOS protein expression induced by LPS in mouse macrophages. We isolated three known oxycoumarins together with two known carbazoles from the root bark of this plant, and the oxycoumarins showed potent inhibitory effects on protein expression of the inflammatory-related molecules iNOS, TNF-α, and cyclooxygenase-2 (COX-2). In this report we provide a detailed description of the screening of the plants, the isolation and identification of the active components of C. guillauminii, and an exploration of the pharmacological properties.
Materials and methods
Cell line, chemicals, and apparatus
Mouse macrophage RAW 264.7 cells were purchased from Riken Cell Bank (Osaka, Japan). The cells were cultured in RPMI-1640 medium with L-glutamine and NaHCO3 (Wako Pure Chemicals Co. Ltd., Osaka, Japan), containing 10% fetal bovine serum (Equitech-Bio Inc., Kerrville, TX, USA) and 1% antibiotics (Invitrogen Corp., Carlsbad, CA, USA). Macrophages were co-cultured in 24-well plates (5 × 105 cells/well) at 37°C in humidified 5% CO2/95% air. Cells were washed twice with fresh medium, and stimulated with 50 ng/ml LPS (Sigma, St. Louis, MO, USA) and respective concentrations of the samples for 16 h.
Melting points were measured on a Yanagimoto MP-SI. Nuclear magnetic resonance (NMR) spectra were obtained by using a JEOL JNM-GSX-400α (400 MHz) or a JNM-ECP-600 (600 MHz) spectrometer in CDCl3. Chemical shifts are expressed in δ values from tetramethylsilane (TMS) as the internal standard, and coupling constants are given in Hertz (Hz). Electron ionization mass spectroscopy (EI-MS) spectra were recorded on a JEOL GC Mate. Infrared (IR) spectra were measured by using a JASCO FT/IR-230 using an attenuated total reference (ATR) system. Thin-layer chromatography (TLC) was done on precoated silica gel 60 F254 plates (Merck; 0.25 mm thick). Column chromatography was carried out on a silica gel 60 (spherical).
Plant material
Clausena guillauminii TANAKA was collected at Srisaket Province, Thailand and was identified by comparison with voucher specimen (no. BKF37847) and was deposited at the Forest Herbarium, National Park, Wildlife and Plant Conservation Department, Ministry of Natural Resources and Environment, Bangkok, Thailand.
Extraction, isolation, and structure determination
Previously, extraction of 50 dried plant materials was carried out using Soxhlet’s extractor with hexane, AcOEt, chloroform or EtOH. Among the selected extracts that showed an inhibitory effect on iNOS expression, we focused on determining the active components in C. guillauminii. Dried crushed root bark was ground with a mixer. The powder (208.85 g) was extracted for 8 h each by Soxhlet’s extractor in reflux conditions with hexane (1.3 L), AcOEt (1.3 L), and EtOH (1.3 L), successively. The extracts of hexane, AcOEt, and EtOH were obtained by vacuum evaporation. After pharmacological evaluation, the hexane extract was separated by silica gel column chromatography (CC) with a hexane/AcOEt step-wise gradient (10/1, 5/1, 2/1, and 1/3), to give seven fractions (Fr.). A sample of Fr. 4 was purified repeatedly with silica gel CC (hexane to AcOEt ratio 10:1 to 4:1) and by washing with hexane to give heptaphylline (1) and 7-methoxyheptaphylline (2). Additionally, a sample of Fr. 5 was subjected to silica gel CC (benzene to AcOEt ratio 50:1) to give poncitrin (3). Finally, the whole of Fr. 6 was subjected to silica gel CC (benzene to Et2O ratio 100:1) to give two coumarins: osthol (4) and xanthoxyletin (5). These compounds were identified by comparison of their physical and spectral data with the literature.
Heptaphylline [2-hydroxy-1-(3-methyl-2-butenyl)carbazole-3-carboxyaldehyde] (1): yellow powder; m.p. 176.0–176.5°C; IR ν max cm−1 3290 (NH, OH), 1608 (C=O); 1H NMR (CDCl3, 400 MHz) δ: 1.77 (3H, s, H-14), 1.91 (3H, s, H-13), 3.65 (2H, d, J = 6.8 Hz, H-10), 5.33 (1H, brt, J = 6.8 Hz, H-11), 7.20 (1H, t, J = 7.5 Hz, H-6), 7.34 (1H, t, J = 7.7 Hz, H-7), 7.45 (1H, d, J = 8.0 Hz, H-8), 8.03 (1H, d, J = 7.8 Hz, H-5), 8.29 (1H, s, H-4), 9.93 (1H, s, CHO), 10.53 (1H, brs, NH), 11.73 (1H, s, OH); 13C NMR (CDCl3, 125 MHz) δ: 18.1 (C-14), 22.8 (C-10), 25.7 (C-13), 109.1 (C-1), 110.9 (C-8), 115.5 (C-3), 117.3 (C-4a), 119.8 (C-6), 120.9 (C-5), 121.2 (C-11), 123.7 (C-4b), 125.3 (C-7), 125.9 (C-4), 134.2 (C-12), 140.1 (C-8a), 145.1 (C-9a), 157.8 (C-2), 195.4 (CHO); EI-MS m/z 280 (MH+, 21.9), 279 (M+, 96.1), 264 (18.0), 236 (32.1), 224 (100), 167 (25.5).
7-Methoxyheptaphylline [2-hydroxy-7-methoxy-1-(3-methyl-2-butenyl)carbazole-3-carboxyaldehyde] (2): yellow powder; m.p. 146.0–152.0°C; IR ν max cm−1 3317 (OH), 1608 (C=O); 1H NMR (CDCl3, 600 MHz) δ: 1.76 (3H, s, H-14), 1.89 (3H, s, H-13), 3.60 (2H, d, J = 6.6 Hz, H-10), 3.89 (3H, s, OCH3), 5.30 (1H, brt, J = 6.9 Hz, H-11), 6.85 (1H, dd, J = 8.6, 2.0 Hz, H-6), 6.89 (1H, d, J = 2.0 Hz, H-8), 7.81 (1H, d, J = 8.6 Hz, H-5), 7.88 (1H, s, H-4), 8.14 (1H, brs, NH), 9.87 (1H, s, CHO), 11.63 (1H, s, OH); 13C NMR (CDCl3, 150 MHz) δ: 18.1 (C-14), 22.8 (C-10), 25.7 (C-13), 55.7 (OCH3), 95.6 (C-8), 108.9 (C-6), 109.1 (C-1), 115.4 (C-3), 117.2 (C-4a), 117.5 (C-5), 120.5 (C-4b), 121.3 (C-11), 124.0 (C-4), 134.1 (C-12), 141.5 (C-8a), 145.2 (C-9a), 157.3 (C-2), 159.0 (C-7), 195.4 (CHO); EI-MS m/z 309 (M+, 100), 294 (12.1), 266 (13.3), 254 (64), 210 (13.2), 149 (17.2), 84 (21.3).
Poncitrin [8,8-dimethyl-10-(1,1-dimethyl-2-propenyl)-5-methoxy-2H,8H-benzo[1,2-b:5,4-b’]dipyran-2-one] (3): colorless plates; m.p. 91.5–94.0°C; IR ν max cm−1 1725 (C=O); 1H-NMR (CDCl3, 400 MHz) δ: 1.45 (6H, s, H-16, H-17), 1.66 (6H, s, H-14, H-15), 3.83 (3H, s, OCH3), 4.86 (1H, dd, J = 10.5, 1.0 Hz, H-13a), 4.89 (1H, dd, J = 17.4, 1.0 Hz, H-13b), 5.70 (1H, d, J = 10.2 Hz, H-7), 6.19 (1H, d, J = 9.6 Hz, H-3), 6.30 (1H, dd, J = 17.4, 10.5 Hz, H-12), 6.57 (1H, d, J = 10.2 Hz, H-6), 7.87 (1H, d, J = 9.6 Hz, H-4); 13C NMR (CDCl3, 150 MHz) δ: 27.5 (C-16, C-17), 29.4 (C-14, C-15), 41.2 (C-11), 63.4 (OCH3), 77.3 (C-8), 107.5 (C-4a), 108.2 (C-13), 111.6 (C-3), 111.7 (C-5a), 116.3 (C-6), 119.2 (C-10), 130.8 (C-7), 138.9 (C-4), 149.8 (C-12), 151.2 (C-10a), 153.9 (C-5), 156.0 (C-9a), 160.8 (C-2); EI-MS m/z 327 (MH+, 20.8), 326 (M+, 92.0), 312 (68.0), 311 (100), 281 (34.9), 211 (60.4), 210 (35.5).
Osthol [7-methoxy-8-(3-methyl-2-butenyl)chromen-2-one] (4): Colorless powder; m.p. 81.5-83.0°C; IR ν max cm−1 1714 (C=O); 1H NMR (CDCl3, 400 MHz) δ: 1.67 (3H, s, H-13), 1.84 (3H, s, H-12), 3.54 (2H, d, J = 7.1 Hz, H-9), 3.92 (3H, s, OCH3), 5.22 (1H, dd-like, J = 5.9, 1.6 Hz, H-10), 6.24 (1H, d, J = 9.5 Hz, H-3), 6.83 (1H, d, J = 8.7 Hz, H-6), 7.29 (1H, d, J = 8.7 Hz, H-5), 7.61 (1H, d, J = 9.5 Hz, H-4); EI-MS m/z 244 (M+, 100), 229 (100), 213 (100), 201 (100), 189 (100), 187 (64.3), 159 (52.7), 131 (75.1).
Xanthoxyletin [8,8-dimethyl-5-methoxy-2H,8H-benzo[1,2-b:5,4-b′]dipyran-2-one] (5): colorless plates; m.p. 130.0–132.0°C; IR ν max cm−1 1720 (C=O); 1H NMR (CDCl3, 600 MHz) δ: 1.47 (6H, s, H-11, H-12), 3.89 (3H, s, OCH3), 5.72 (1H, d, J = 10.1 Hz, H-7), 6.20 (1H, d, J = 9.6 Hz, H-3), 6.54 (1H, s, H-10), 6.57 (1H, d, J = 10.1 Hz, H-6), 7.85 (1H, d, J = 9.6 Hz, H-4); 13C NMR (CDCl3, 150 MHz) δ: 28.2 (C-11, C-12), 63.5 (OCH3), 77.4 (C-8), 100.6 (C-10), 107.2 (C-4a), 111.2 (C-5a), 112.2 (C-3), 115.7 (C-6), 130.5 (C-7), 138.5 (C-4), 152.7 (C-5), 155.4 (C-10a), 157.4 (C-9a), 160.9 (C-2); EI-MS m/z 259 (MH+, 10.4), 258 (M+, 62.1), 244 (50.8), 243 (100), 228 (74.4), 200 (49.1), 91 (18.3).
Western blot analysis
Macrophages were incubated with or without LPS in the presence or absence of samples. After washing twice with ice-cold phosphate-buffered saline (PBS), the cells were lysed by three cycles of freezing and thawing in liquid nitrogen, and then resuspended in lysis buffer containing 50 mM Tris–HCl, 140 mM NaCl, 1% Triton X-100, 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM sodium orthovanadate, and 1 mM phenylmethylsulfonyl fluoride (PMSF). The cytosolic fraction was obtained from the supernatant after centrifugation at 15,000×g at 4°C for 20 min. Samples (50 μg of protein) were separated on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) at room temperature under voltage clamping at 100 V, and the proteins were transferred to nitrocellulose membranes on ice at a stationary current of 200 mA. The membranes were blocked with 5% nonfat milk in TBS-0.1% Tween (TBS-T) at room temperature for 1 h, and then incubated with antibodies for iNOS (Sigma, N7782), TNF-α (Sigma, T0938), COX-2 (Cell Signaling Technology Inc., #4842, Danvers, MA, USA) or β-actin (Sigma, A5316) in TBS-T containing 1% bovine serum albumin (BSA) and 0.5% fetal bovine serum (FBS) at 4°C for 16 h. After washing three times with TBS-T, the membrane was hybridized with secondary antibodies, goat anti-rabbit IgG (#12–348) and goat anti-mouse IgG (#12–349), horseradish peroxidase (HRP) conjugates (Upstate, Lake Placid, NY, USA) at room temperature for 1 h, and washed three times for 30 min with TBS-T. The membrane was incubated with enhanced chemiluminescence (ECL) reagent Immun-Star HRP (Bio-Rad Laboratories Inc., Hercules, CA, USA) for 3 min, and luminescent signals were detected by LAS-1000 Plus (Fuji Film, Tokyo, Japan).
Determination of NO concentration
RAW 264.7 cells (5 × 105 cells/well) were incubated on 24-well dishes for 16 h with 50 ng/ml of LPS and respective concentrations of samples. The presence of nitrite was determined as a stable oxidized product of NO in cell culture media by modification of the Griess method. Briefly, 100 μl of culture supernatant was removed and combined with 100 μl of Griess-Romijn nitrite reagent (Wako Pure Chemicals Co. Ltd.) at room temperature for 15 min in a 96-well plate, and absorbance was measured by a Multiscan JX microplate reader (Thermo Bio Analysis Japan, Tokyo, Japan) at 450 nm. Nitrite concentrations in the supernatants were determined by comparison with a sodium nitrite calibration curve.
Statistical analysis
Data are presented as means ± standard deviation (SD) of more than four separate experiments performed in duplicate, except for the results of screening in Fig. 2a (n = 1 in triplicate). Comparisons between multiple groups were performed with Dunnett’s multiple comparisons tests. Statistical significance was considered when P < 0.05.
Results and discussion
Previously, 50 extracts from Thai medicinal plants were screened for LPS-induced iNOS expression by Western blotting. In particular, the extract of Clausena excavata and C. guillauminii showed expression at a concentration of 50 μg/ml. Sunthitikawinsakul and co-workers reported the isolation of 5 and related oxycoumarins from C. excavata as antimycobacterial and antifungal compounds [6]. Thus, C. guillauminii, which did not have a research history until now, was chosen as the candidate for this research.
Crushed root bark of C. guillauminii was extracted with hexane, ethyl acetate (AcOEt), and ethanol (EtOH) using Soxhlet’s extractor. The extracts of hexane (2.30 g), AcOEt (5.00 g), and EtOH (4.17 g) were obtained by vacuum evaporation. Inhibitory activity on iNOS expression of these extracts at a concentration of 10 μg/ml were 61.0%, 55.0%, and 38.3% inhibition, respectively (Fig. 2a). The most potent extract, the hexane extract, was separated by silica gel CC to give seven fractions: Fr. 1 (5 mg), Fr. 2 (93 mg), Fr. 3 (191 mg), Fr. 4 (676 mg), Fr. 5 (228 mg), Fr. 6 (428 mg), and Fr. 7 (300 mg). Although Fr. 1-4 did not show any inhibitory effect on iNOS expression, Fr. 4 (559 mg) was purified repeatedly with silica gel CC and by washing with hexane to give heptaphylline (1, 28 mg, 0.0002%) and 7-methoxyheptaphylline (2, 13 mg, 0.00006%) [7]. The active fractions Fr. 5 and 6 showed significant effects of 37.7% and 82.0% inhibition at 2 and 5 μg/ml, respectively (Fig. 2b). Fr. 5 (210 mg) was subjected to silica gel CC to give poncitrin (3, 24 mg, 0.00001%) [8]. Finally, the whole of Fr. 6 was subjected to silica gel CC to give two coumarins, osthol (4, 264 mg, 0.001%) [9] and xanthoxyletin (5, 104 mg, 0.0005%) [10]. The structures of compounds 1–5 was identified by comparing the reported physical and spectral data (Fig. 1).
The effect of these compounds on iNOS expression was screened at the concentration of 5 μg/ml (approximately 18 μM) in duplicate, and 3, 4, and 5 showed relatively potent activities. Heptaphylline (1) was reported as a compound that exhibited antiplasmoidal activity isolated from Clausena harmandiana; however, the effect on iNOS expression was not potent [11]. Thus, pharmacological evaluation of the active components 3, 4, and 5 for their inhibitory effects on iNOS protein expression was performed as follows.
The inhibitory effect on iNOS expression was mediated by these oxycoumarins at concentrations from 5 to 50 μM (Fig. 3a). As mentioned above, these oxycoumarins showed potent effects at concentration of 5 μg/ml (approximately 18 μM) (Fig. 2a), thus the concentration range was set from 1/3 to 3× of 18 μM, approximately (Fig. 3b). Oxycoumarins 3 and 5 showed significant effects at 5 μM, and the inhibitory ratios were 48.4% (P < 0.05) and 47.4% (P < 0.01), respectively. Significant activity was shown by 4 at 10 μM (43.5% inhibition, P < 0.05), and 3 and 5 showed 70.2% (P < 0.001) and 81.2% (P < 0.001) inhibition at 50 μM, respectively. Curcumin was used as a positive control for inhibition of iNOS expression, and 10 μM of curcumin exhibited 74.3% inhibition (P < 0.001). In response to these results, we examined the influence on the activation of iNOS, i.e., the generation of NO, in addition to xanthoxyletin (5), which showed the strongest inhibitory effect on iNOS expression.
Isolation of active components from the extract of Clausena guillauminii, and their activities. a Activity-guided isolation scheme of hexane (Hx), AcOEt (Ac), and EtOH (Et) extracts. []: Relative expression of iNOS versus control (Cont) in duplicate (n = 1). b Pharmacological evaluation of Fr. 1 to 6. Data represent the mean ± SD (n = 4). **P < 0.01 and ***P < 0.001 versus control by Dunnett’s multiple comparison
Concentration dependency of the inhibitory effects of poncitrin (3), osthol (4), and xanthoxyletin (5) on LPS-induced iNOS protein expression. a Typical blotting of iNOS and β-actin. b Relative expression of iNOS versus control stimulated by various concentration of 3, 4, and 5. Data represents the mean ± SD (n = 4). *P < 0.05, **P < 0.01, and ***P < 0.001 versus control (Cont), by Dunnett’s multiple comparisons test. Curcumin (Cur, 10 μM) was used as a positive control
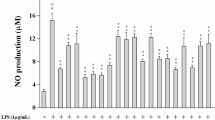
Macrophages play an important role in the regulation of the immune response and inflammation. NO is generated from macrophages by stimulus by a bacterial toxin, LPS. It is well known that an experimental model system similar to a transient inflammatory reaction can be constructed [12, 13]. NO production was inhibited by 5 in a concentration-dependent manner up to 100 μM (Fig. 4). In addition, iNOS protein expression was significantly inhibited by 5 at 5 μM, however, the production of NO was not inhibited at either 5 or 10 μM. It could be assumed that the difference in the active concentration range between protein expression and NO production is because the action mechanisms of 5 and other coumarins mainly participate in the inhibition of iNOS protein expression [14, 15]. On the other hand, the same concentration of curcumin (10 μM) showed equally potent activity on iNOS expression and NO production (74.3% and 76.9% inhibition, respectively), and it is suggested that these effects are augmented by the scavenging activity of NO and enzyme inhibition activities. This enhancing effect of curcumin is supported by a report by Onoda and co-workers [16].
Pretreatment of 3, 4, and 5 at concentrations up to 50 μM for 16 h did not show any significant cytotoxicity at a microscopic level.
Finally, the effects of 5 on the expression of COX-2 and TNF-α relating to NO generation in inflammatory conditions were evaluated (Fig. 5a). The pro-inflammatory cytokine TNF-α was investigated. The protein levels of TNF-α induced by LPS were significantly suppressed in a concentration-dependent manner by treatment with 5 (Fig. 5b). Also, COX-2 protein expression was significantly inhibited by 5 at 10 μM (32.1% inhibition, P < 0.001), and protein expression decreased in a concentration-dependent manner (Fig. 5c). These data indicated that the pro-inflammatory enzyme COX-2 and the pro-inflammatory cytokine TNF-α were also suppressed by treatment with 5.
The inhibitory effect of xanthoxyletin (5) on LPS-induced COX–2 and TNF-α protein expression. a Typical blotting of COX-2, TNF-α and β-actin. b, c Concentration dependency of the inhibitory effects on LPS-induced COX-2 and TNF-α protein expression, respectively. Data represents the mean ± SD (n = 4). ***P < 0.001 versus control (Cont) by Dunnett’s multiple comparison. Curcumin (Cur, 10 μM) was used as a positive control
The present study was undertaken to elucidate the pharmacological components of C. guillauminii, which demonstrated inhibitory activities on inflammatory mediators in macrophages. We first isolated three known oxycoumarins, poncitrin (3), osthol (4), and xanthoxyletin (5) from this plant, which suppressed the production of iNOS in LPS-stimulated RAW 264.7 cells. Murakami et al. reported that some coumarins isolated from Citrus hystrix DC are inhibitors of NO generation, and concluded that the reduction in NO level may be due to inhibition of the LPS-interferon (IFN)-γ-triggered iNOS expression pathways and/or iNOS enzyme activity, and not by the NO scavenging effect [17].
We used curcumin as a positive control in the evaluation of the inhibitory effects on the protein expression of iNOS, COX-2, and TNF-α, and in the generation of NO. Many researchers have demonstrated the inhibitory effects of curcumin on iNOS protein expression and enzymatic activity [16, 18]. Structurally, curcumin has two 4-hydroxy-3-methoxy-phenyl substituents, and these aromatic rings are connected by an unsaturated heptadiene structure. These conjugated double bonds scavenge free radicals such as NO, O2 ·− or ONOO− [16, 19, 20]. On the contrary, the conjugated double bonds in 5 are shorter than those on curcumin, and the inhibitory effect of 5 was weaker than that of curcumin at low concentration (10 μM). In particular, the inhibition of NO generation was not detected at 10 μM, although a potent effect was detected at 50 μM.
Although further research is required to elucidate the molecular mechanisms involved, it is suggested that these coumarins act at the transcription level on various inflammatory modulators such as iNOS, COX-2, TNF-α, and IL-1β.
There are some reports on the cytotoxicity of 4 in a carcinoma cell line [14, 15]. Ju and co-workers reported that 5 inhibited the incorporation of tritiated thymidine into human leukemia (HL-60) cells, which means that it inhibited DNA synthesis. They indicated that the 50% inhibition concentration (IC50) of 5 is 3.48 ppm (15.28 μM) [21], and this was one of a range of concentrations we examined. Although the cell line was certainly different, it is possible that xanthoxyletin could exert a certain action on the gene constructional system, and it is suggested that this could have caused the suppression of iNOS expression.
Kim and co-workers reported that cordyceptin (3′-deoxyadenosine), isolated from a medicinal mushroom Cordyceps militaris, inhibited LPS-induced iNOS expression and NO production. They concluded that the downregulation of iNOS, COX-2, and TNF-α occurred via suppression of the phosphorylation of Akt/protein kinase B (PKB) and p38 [22]. Inflammation is well known as an important factor in the development of diabetes; however, if coumarin suppresses the phosphorylation of Akt/PKB as a mechanism inhibiting inflammation, it may be hypothesized that this could be a factor in insulin resistance. On the other hand, Hu and co-workers demonstrated that benzopyran derivatives (oxycoumarin derivatives) activate peroxisome proliferator-activated receptor-γ (PPAR-γ) and exert a beneficial effect on insulin action on glucose uptake and lipid metabolism. It was shown that, as a mechanism accelerating insulin-stimulated glucose uptake, oxycoumarin increased the phosphorylation of Akt and p38 mitogen-activated protein kinase in skeletal muscle [23, 24]. In fact, we found that some oxycoumarin derivatives raised the expression level of LPS-induced iNOS in RAW 264.7 macrophages (data not shown). Thus, it is suggested that differences in the structures of these oxycoumarins are associated with these contradictions. Therefore, oxycoumarins used for anti-inflammatory treatment should be carefully selected. We next intend to clarify the structure-activity relationships of many oxycoumarins.
References
Akaike T, Maeda H (2000) Nitric oxide and virus infection. Immunology 101:300–308
Bruch-Gerharz D, Fehsel K, Suschek C, Michel G, Ruzicka T, Kolb-Bachofen V (1996) A proinflammatory activity of interleukin 8 in human skin: expression of the inducible nitric oxide synthase in psoriatic lesions and cultured keratinocytes. J Exp Med 184:2007–2012
Kolb H, Kolb-Bachofen V (1998) Nitric oxide in autoimmune disease: cytotoxic or regulatory mediator? Immunol Today 19:556–561
Suschek CV, Schnorr O, Kolb-Bachofen V (2004) The role of iNOS in chronic inflammatory processes in vivo: is it damage-promoting, protective, or active at all? Curr Mol Med 4:763–775
Hosokawa A, Sumino M, Nakamura T, Yano S, Sekine T, Ruangrungsi N, Watanabe K, Ikegami F (2004) A new lignan from Balanophora abbreviata and inhibition of lipopolysaccharide (LPS)-induced inducible nitric oxide synthase (iNOS) expression. Chem Pharm Bull (Tokyo) 52:1265–1267
Sunthitikawinsakul A, Kongkathip N, Kongkathip B, Phonnakhu S, Daly JW, Spande TF, Nimit Y, Rochanaruangrai S (2003) Coumarins and carbazoles from Clausena excavata exhibited antimycobacterial and antifungal activities. Planta Med 69:155–157
Nijsiri R, Jongjit A, Gordon LL, Michael GO (1990) Three new carbazole alkaloids isolated from Murraya siamensis. J Nat Prod 53:946–952
Tomimatsu T, Hashimoto M, Shingu T, Tori K (1972) Studies on the chemical components of Rutaceae plants VI: Components of the root of Poncitrus trifoliata Rafinesque (4) poncitrin, a new coumarin: Structure and nuclear Overhauser effects. Tetrahedron 28:2003–2010
Wei Y, Zhang T, Ito Y (2004) Preparative isolation of osthol and xanthotoxol from Common Cnidium Fruit (Chinese traditional herb) using stepwise elution by high-speed counter-current chromatography. J Chromatogr A 1033:373–377
Ju Y, Zhao Y, Still CC, Sacalis JN (2000) Cytotoxic components from Zanthoxylum americanum. Tsinghua Sci Tech 5:159–162
Yenjai C, Sripontan S, Sriprajun P, Kittakoop P, Jintasirikul A, Tanticharoen M, Thebtaranonth Y (2000) Coumarins and carbazoles with antiplasmodial activity from Clausena harmandiana. Planta Med 66:277–279
Deliconstantinos G, Villiotou V, Stavrides JC (1996) Nitric oxide and peroxynitrite released by ultraviolet B-irradiated human endothelial cells are possibly involved in skin erythema and inflammation. Exp Physiol 81:1021–1033
Deliconstantinos G, Villiotou V, Stavrides JC (1996) Alterations of nitric oxide synthase and xanthine oxidase activities of human keratinocytes by ultraviolet B radiation. Potential role for peroxynitrite in skin inflammation. Biochem Pharmacol 51:1727–1738
Chou SY, Hsu CS, Wang KT, Wang MC, Wang CC (2007) Antitumor effects of Osthol from Cnidium monnieri: an in vitro and in vivo study. Phytother Res 21:226–230
Yang LL, Wang MC, Chen LG, Wang CC (2003) Cytotoxic activity of coumarins from the fruits of Cnidium monnieri on leukemia cell lines. Planta Med 69:1091–1095
Onoda M, Inano H (2000) Effect of curcumin on the production of nitric oxide by cultured rat mammary gland. Nitric Oxide 4:505–515
Murakami A, Gao G, Kim OK, Omura M, Yano M, Ito C, Furukawa H, Jiwajinda S, Koshimizu K, Ohigashi H (1999) Identification of coumarins from the fruit of Citrus hystrix DC as inhibitors of nitric oxide generation in mouse macrophage RAW 264.7 cells. J Agric Food Chem 47:333–339
Kuo ML, Chau YP, Wang JH, Lin PJ (1997) The role of Src kinase in the potentiation by ethanol of cytokine- and endotoxin-mediated nitric oxide synthase expression in rat hepatocytes. Mol Pharmacol 52:535–541
Biswas SK, McClure D, Jimenez LA, Megson IL, Rahman I (2005) Curcumin induces glutathione biosynthesis and inhibits NF-kappaB activation and interleukin–8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxid Redox Signal 7:32–41
Toniolo R, Di Narda F, Susmel S, Martelli M, Martelli L, Bontempelli G (2002) Quenching of superoxide ions by curcumin. A mechanistic study in acetonitrile. Ann Chim 92:281–288
Ju Y, Still CC, Sacalis JN, Li J, Ho CT (2001) Cytotoxic coumarins and lignans from extracts of the northern prickly ash (Zanthoxylum americanum). Phytother Res 15:441–443
Kim HG, Shrestha B, Lim SY, Yoon DH, Chang WC, Shin DJ, Han SK, Park SM, Park JH, Park HI et al (2006) Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-kappaB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur J Pharmacol 545:192–199
Tang L, Yu J, Leng Y, Feng Y, Yang Y, Ji R (2003) Synthesis and insulin-sensitizing activity of a novel kind of benzopyran derivative. Bioorg Med Chem Lett 13:3437–3440
Hu X, Feng Y, Liu X, Zhao XF, Yu JH, Yang YS, Sydow-Backman M, Horling J, Zierath JR, Leng Y (2007) Effect of a novel non-thiazolidinedione peroxisome proliferator-activated receptor alpha/gamma agonist on glucose uptake. Diabetologia 50:1048–1057
Acknowledgments
The authors would like to thank the staff of the Analytical Center of Chiba University for MS spectra.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakamura, T., Kodama, N., Arai, Y. et al. Inhibitory effect of oxycoumarins isolated from the Thai medicinal plant Clausena guillauminii on the inflammation mediators, iNOS, TNF-α, and COX-2 expression in mouse macrophage RAW 264.7. J Nat Med 63, 21–27 (2009). https://doi.org/10.1007/s11418-008-0277-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-008-0277-5