Abstract
Agarwood oil and spikenard extract were examined for their sedative activity using a spontaneous vapor administration system. It was shown that inhalation of agarwood oil vapor sedated mice. The main volatile constituents of the oil were found to be benzylacetone [agarwood oil from a Hong Kong market (1)], or α-gurjunene and (+)-calarene [agarwood oil made in Vietnam (2)]. A hexane extract of spikenard contained a lot of calarene, and its vapor inhalation had a sedative effect on mice. Individual principles benzylacetone, calarene, and α-gurjunene were administered to mice, which reproduced the result of the corresponding oil or extract. However, the most effective dose of the compounds was lower than their original content in the oil and extract (benzylacetone 0.1%, calarene 0.17%, α-gurjunene 1.5%).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agarwood (in Japanese “Jinkoh”) and spikenard (“Kanshokoh”) have a long history as essential ingredients in oriental incense and sachet. Agarwood (or eaglewood in the United States) is the resinous portion of the wood of the Aquilaria species (Thymelaeaceae), of which the highest quality pieces are used for “Kho-doh,” a Japanese fragrance ceremony. In the Middle East and Europe, distilled oil of agarwood is more commonly used as a blending material for perfume and balm. Spikenard is the dried roots and rhizomes of Nardostachys chinensis (Valerianaceae) or N. jatamansi, often blended into a mixture for Japanese sachet.
A pharmacological effect on the central nervous system achieved by oral administration or abdominal injection of agarwood and spikenard extracts has been reported previously. Okugawa et al. reported that six sesquiterpenoids, namely, jinkoh-eremol, agarospirol, α- and β-santalols, dehydrocostus lactone, and costunolide, isolated from agarwood, inhibited acetic acid-induced writhing in mice [1]. Vidya et al. reported that there was a significant increase in γ-aminobutyric acid (GABA) level in rat brains following oral administration of an ethanol extract of N. jatamansi [2]. On the other hand, Itokawa et al. showed that the methanol extract of N. chinensis had a cytotoxic activity against P-388 cells [3], which means that oral and intravenous administration of the extract may not respond to the relevant activities so an administration method is required for their pharmaceutical use. However, aromatherapy has recently attracted much attention as an alternative medicine, especially for psychosomatic disease caused by stress. For example, lavender oil is used as a holistic relaxant and has a spasmolytic activity [4], while rosemary oil is used to treat depression [5]. In the United States, aromatherapy is generally allowed and prescribed for clinical use in syndromes such as Attention Deficit Disorder and Attention Deficit Hyperactivity Disorder instead of methylphenidate tablets, which are commonly given to such patients.
The clinical use of essential oils in aromatherapy is getting more attention nowadays, and the need for detailed study of the pharmacological activity of inhaled oil is increasing. In the present study, we present a newly developed experimental system for administering essential oil to mice by vapor inhalation. We investigated the sedative effects of essential oils and isolated compounds, using the results to identify the active components of agarwood and spikenard.
Materials and methods
Materials
Two types of agarwood oil (1: from a Hong Kong market, 2: made in Vietnam), dried roots of N. chinensis, and lavender oil were purchased from Mitsuboshi Pharmaceutical. Triethylcitrate (Merck), an odorless solvent, was used to dissolve the fragrance components. (+)-Calarene and α-gurjunene were from Fluka, and benzylacetone was from Tokyo Kasei. All other chemicals and reagents were of the highest grade available.
Animals
Male 4-week-old ddY mice were purchased from Japan SLC Shizuoka (Japan). They were housed in colony cages at an ambient temperature of 25 ± 2°C and relative humidity of 50 ± 10% with a light-dark cycle of 12 h each before being used for experiments. They were fed standard pellet chow and water ad libitum. All behavioral observations were conducted between 10:00 and 17:00 under the same temperature and humidity described above.
Evaluation of spontaneous motor activity
Fragrance components were evaluated for their sedative effect on spontaneous motor activity in an open-field test, which is an assay system for observing the behavior of animals placed in a foreign environment. Fragrance components were dissolved in triethylcitrate (400 μl total), and four filter-paper disks (8 cm diameter) were permeated with the solution. The disks were placed on the wall of a glass cage (W 60 cm × L 30 cm × H 34 cm) using Scotch tape, and the vapor of the solution pervaded the cage by natural diffusion. Sixty minutes after charging the solution, a mouse was placed into the center of the cage and was monitored by a video camera for another 60 min. The frequency of the mouse crossing lines drawn at the bottom of the cage at 10-cm intervals was counted every 5 min for 60 min. The area under the curve (AUC) indicating total locomotor activity for 60 min was calculated by the trapezoidal rule.
GC and GC–MS analysis
Qualitative analysis of the volatile component was conducted as follows. Adsorption and desorption of the volatile component using solid-phase micro extraction (SPME) are described in a previous report [6]. GC–MS analyses were performed on a G7000-M9000/3DQMS (Hitachi) under the following operating conditions: column: fused silica capillary column, TC-WAX (Hewlett Packard), 60 m × 0.25 mm, film thickness = 0.25 μm; column temperature: 80–220°C increasing at 5°C/min, 10 min holding at 220°C, 220–240°C increasing at 10°C/min, 3 min holding at 240°C; injector: 200°C; carrier gas: helium, 1 ml/min; ionization energy: 15 eV.
Quantitative analysis of the volatile component was conducted as follows. GC analyses were performed on a G5000 (Hitachi) with a flame ionization detector (FID) and operating conditions as follows: a fused silica capillary column, TC-WAX (Hewlett Packard), 60 m × 0.25 mm, film thickness = 0.25 μm; column temperature: 60–180°C increasing at 5°C/min, 60 min holding at 180°C; injector: 200°C; detector: 200°C; carrier gas: helium, 37 cm/min; injection volume: 1 μl. Relative concentrations of components were calculated based on GC peak areas.
Calarene isolation
Dried roots (700 g) of N. chinensis were extracted with n-hexane three times at room temperature. The hexane extract was concentrated under reduced pressure, and the concentrate (14.3 g) was subjected to silica gel column chromatography (Wakogel C-200). The column was first eluted with n-hexane to give fractions 1–5, and was then eluted with acetone to give fraction 6. Fraction 1 (721 mg) was applied to a Lobar RP-18 column (LiChroprep RP-18, 40–63 mm, Merck) and eluted with CH3CN:H2O = 5:1 (flow rate: 3.0 ml/min) to give fractions 1–1 through 1–3. Fraction 1–1 was extracted with hexane and applied to gel permeation chromatography (GPC) (LC-918 recycling HPLC system, column: JAIGEL-1H and -2H, 20 × 600 mm, Japan Analytical Industry) eluted with CHCl3 (flow rate: 3.0 ml/min) to give calarene (452 mg) isolated after 12 h of recycling.
Statistics
All values are expressed as mean ± SEM. Statistical analyses were carried out via Student’s t-test. Each assay was performed repeatedly, and the results were found to be quite reproducible. Therefore, six mice of each administration group were chosen at random for statistical analyses.
Results and discussion
Identification of the principal volatile compound in agarwood oils
Fragrant compounds of agarwood are known to be sesquiterpenoids and chromone derivatives, which are the main source of agarwood’s peculiar odor [7]. Agarwood oils vary in their composition; some oils contain a large amount of sesquiterpene compounds and others contain principally benzylacetone [8]. Analyses of the volatile components of agarwood oils used in this study were performed on SPME-GC-MS. SPME is suitable for analyzing volatile compounds, adsorbing compounds in the headspace of the sample vial, and desorbing them directly into a GC injection port. Measurement on GC with FID shows the compound’s mass in a liquid extract while that on SPME-GC shows the composition in the gas phase. As a result, agarwood oil (1) contained benzylacetone (47.1%) as the main volatile component, and agarwood oil (2) contained α-gurjunene (61.5%) and (+)-calarene (24.7%) (Fig. 1). GC analyses showed that benzylacetone accounted for only 0.96% of whole oil (1), and α-gurjunene and calarene for 15.1 and 17.3% of oil (2). A comparison of SPME-GC and GC analyses showed that agarwood oil (1) contained a large amount of nonvolatile compounds.
Effect of agarwood oil inhalation on mouse spontaneous motor activity
Evaluation of sedation and excitation activities by observing spontaneous motor activity of mice were previously reported for adenosine [9] and caffeine [10]; however, test compounds were administered by oral dose or by injection in those cases. Our experimental system was similar to those previous studies, but test samples were administered by vapor inhalation.
Prior to the experiments using agarwood oils, assays of lavender oil were done in order to ascertain the validity of the experimental system. Lavender oil is a common natural essential oil that was previously reported to have a sedative effect on animals and humans after inhalation [11]. Administration of 400 μl of lavender oil sedated mice within 30 min, and their spontaneous motor activities decreased with the time course (Fig. 2a). The AUC values of mice that inhaled lavender were found to be significantly smaller than those of the control. This showed that our system could reproduce the results of a different system (Fig. 2b).
Using this experimental system, 400 μl of agarwood oil was administered to mice. Subsequently, their spontaneous motor activities decreased as did those of the group administered lavender oil (Fig. 2a), and the AUC values were significantly smaller than those of the control (Fig. 2b).
Both agarwood oils (1) and (2) showed similar sedative activity, although their main volatile compounds were different.
Identification of the principal volatile compound in a hexane extract of spikenard
Fragrant compounds of spikenard were reported to be sesquiterpenoids and coumarins [7]. SPME-GC-MS analyses of a hexane extract of spikenard showed the extract contained calarene (82.3%) and aristolene (8.3%), both of which are sesquiterpene compounds with similar structures. Valerena-4,7(11)-diene (3.9%), a sesquiterpene compound previously isolated from Valeriana officinalis [12], was first isolated from spikenard (Fig. 3). The percentages of compounds in the whole extract were 26.1% (calarene), 5.0% (aristolene) and 3.4% (valerena-4,7(11)-diene), which were measured on GC with FID.
Effect of vapor inhalation of spikenard hexane extract on spontaneous motor activity
A hexane extract of spikenard was administered to mice at four different doses (1, 10, 100, 1,000 mg extract dissolved in 400 μl triethylcitrate). Mice in the 1, 10, and 100 mg administration groups calmed down within 30 min, and their spontaneous motor activities decreased with the time course (Fig. 4a). The AUCs of these three groups were significantly smaller than those of control, and the sedative effect was reinforced in a dose-dependent manner (Fig. 4b). A marked sedative effect was observed in the 100 mg administration group; however, mice that received the 1,000 mg dose were excited and jumped throughout the exposure time. This result suggests that the hexane extract of spikenard contains compounds which act as a sedative at lower doses, but as a stimulant at higher doses.
Isolation of calarene from the spikenard extract
Both agarwood oil (2) and the hexane extract of spikenard showed a sedative effect on mice, and they turned out to commonly contain calarene as the principal constituent; calarene was isolated from the hexane extract of spikenard during a single administration experiment. Separation and isolation were carried out using various column chromatographies on the extract to isolate calarene (452 mg). NMR spectra and the chemical shifts of the isolated compound agreed with the literature data [13].
Effect of benzylacetone, calarene, and α-gurjunene inhalation on spontaneous motor activity
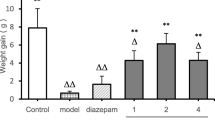
Principal volatile constituents isolated from agarwood oils or spikenard hexane extract, namely, benzylacetone, calarene, and α-gurjunene, were examined for their sedative activity on mice. The dose of each compound was determined on the basis of the content of the compound in agarwood oil (1) or (2). Agarwood oil (1) contained 0.96% benzylacetone, and agarwood oil (2) contained 17.3% calarene and 15.1% α-gurjunene. Results are shown in Figs. 5 (benzylacetone), 6 (calarene) and 7 (α-gurjunene).
Sedative effect of benzylacetone gradually strengthened in a dose-dependent manner in the dose range 0.001–0.1%, and the most effective dose was 0.1%, whereas 1.0 and 10% doses caused excited actions such as jumping and excess excretion. Especially at a dose of 1.0%, mice moved vigorously for the entire 60 min, and the AUC of this group was significantly larger than that of the control (Fig. 5). The sedative effect of calarene was the strongest at a dose of 0.17%, whereas 1.7 and 17% doses caused excited actions, and wobbling was observed after 30 min at a dose of 17% (Fig. 6). The AUC of the group administered 17% was small; however, this reduced activity is thought to be due to intoxication caused by an excess administration of calarene. α-Gurjunene had a highly sedative effect at a dose of 1.5%, but a 15% dose caused excited actions similar to the benzylacetone administration (Fig. 7).
An instance of intoxication caused by an overdose of an essential oil component has been reported for linalool. Lavender oil mainly includes linalool and linalyl acetate, and it has been reported that linalool has a sedative effect on humans by vapor inhalation at low doses [14]. It also has nerve toxicity, and the LC50 for mammals is 2,740 mg/m3 [15]. Compounds used in this study are also thought to be toxic at high doses.
It has been reported that pharmacological activities derived from inhalation of essential oils are mostly associated with the limbic system [16]. Fragrant components stimulate olfactory cells and signals are transferred into the brain, affecting the autonomic nervous system and/or hormone secretion. The mechanism of the sedative effect observed in this study may also be the same as the above.
Figure 8 shows a comparison of the AUC according to the calarene content in 400-μl samples administered to mice. The apparent effective dose range of calarene was shown to differ between a pure compound administration and a crude extract administration; it was between 0.1 and 1 mg when it was administered as a pure compound, while the calculated amount of calarene in the hexane extract of the effective dose was between 0.1 and 100 mg (Fig. 8). This shows that single doses of active ingredients have an instant and intense effect, whereas the hexane extract of spikenard has a wide range of effective doses. The extract contained many compounds other than calarene, so that various biological activities arising from these constituents are thought to have combined to cause a sedative effect within a wide range of doses in an in vivo experiment.
In this paper, we showed the effectiveness of our experimental system for investigating the sedative activity of volatile compounds by vapor inhalation. We also identified three active compounds from crude drugs.
It is expected that aromatherapy will become a popular treatment for treating diseases of the central nervous system. More trials must be carried out to establish and promote the health and healing benefits of aromatherapy.
References
Okugawa H, Ueda R, Matsumoto K, Kawanishi K, Kato K (2000) Effects of sesquiterpenoids from “Oriental incenses” on acetic acid-induced writhing and D2 and 5-HT2A receptors in rat brain. Phytomedicine 7(5):417–422
Prabhu V, Karanth KS, Rao A (1994) Effects of Nardostachys jatamansi on biogenic amines and inhibitory amino acids in the rat brain. Planta Med 60(2):114–117
Itokawa H, Masuyama K, Morita H, Takeya K (1993) Cytotoxic sesquiterpene from Nardostachys chinensis. Chem Pharm Bull 41(6):1183–1185
Lis-Balchin M, Hart S (1999) Studies on the mode of action of the essential oil of lavender (Lavandula angustifolia P. Miller). Phytother Res 13(6):540–542
Kovar KA, Gropper B, Friess D, Ammon HP (1987) Blood levels of 1,8-cineole and locomotor activity of mice after inhalation and oral administration of rosemary oil. Planta Med 53(4):315–318
Bais HP, Dattatreya BS, Ravishankar GA (2003) Production of volatile compounds by hairy root cultures of Cichorium intybus L. under the influence of fungal elicitors and their analysis using solid-phase micro extraction gas chromatography–mass spectrometry. J Sci Food Agric 83(8):769–774
Djerassi D, Connoly JD, Faulkner DJ, Mori K, Nakanishi K, Ourisson G, Raphael RA, Shamma M, Tamm CH (1993) Dictionary of natural products. Chapman and Hall, London
Yang JS, Wang YL, Su YL, He DH, Zheng QT, Yang J (1989) Studies on the chemical constituents of Aquilaria sinensis (Lour) Gilg. III. Elucidation of the structure of isobaimuxinol and isolation and identification of the constituents of lower boiling fraction of the volatile oil. Yao Xue Xue Bao 24(4):264–268
Dunwiddie TV, Worth T (1982) Sedative and anticonvulsant effects of adenosine analogs in mouse and rat. J Pharmacol Exp Ther 220(1):70–76
Lau CE, Falk JL (1994) Tolerance to oral and IP caffeine: locomotor activity and pharmacokinetics. Pharmacol Biochem Behav 48(2):337–344
Buchbauer G, Jirovetz L, Jager W, Dietrich H, Plank C (1991) Aromatherapy: evidence for sedative effects of the essential oil of lavender after inhalation. Z Naturforsch [C] 46(11–12):1067–1072
Paul C, Konig WA, Muhle H (2001) Pacifigorgianes and tamariscene as constituents of Frullania tamarisci and Valeriana officinalis. Phytochemistry 57(2):307–313
Abraham WR, Kieslich K, Stumpf B, Ernst L (1992) Microbial oxidation of tricyclic sesquiterpenoids containing a dimethylcyclopropane ring. Phytochemistry 31(11):3749–3755
Sugawara Y, Hara C, Tamura K, Fujii T, Nakamura T, Aoki T (1998) Sedative effect on humans of inhalation of essential oil of linalool: sensory evaluation and physiological measurements using optically active linalools. Anal Chim Acta 365(1):293–299
UNEP (2007) Screening information dataset for high volume chemicals. http://www.chem.unep.ch/irptc/sids/oecdsids/sidspub.html
Kagawa D, Jokura H, Ochiai R, Tokimitsu I, Tsubone H (2003) The sedative effects and mechanism of action of cedrol inhalation with behavioral pharmacological evaluation. Planta Med 69:637–641
Acknowledgements
This work was partially supported by a Grant-in-Aid for Scientific Research (No. 17406003 for M. Ito) provided by the Japan Society for the Promotion of Sciences. This work was also partly supported by the 21st Century COE program “Knowledge Information Infrastructure for Genome Science.”
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takemoto, H., Ito, M., Shiraki, T. et al. Sedative effects of vapor inhalation of agarwood oil and spikenard extract and identification of their active components. J Nat Med 62, 41–46 (2008). https://doi.org/10.1007/s11418-007-0177-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-007-0177-0