Abstract
Andrographis paniculata is a Malaysian traditional herb for treatment of fever-causing diseases and diabetes. The aim of the present study was to examine the antihyperglycemic effects of different extracts (95, 50, and 20% ethanol extracts and water extracts) of A. paniculata in normal and streptozotocin-induced diabetic rats. Only the treatment with 20% ethanol extract (at 1 g/kg for 7 h, p.o.) significantly (P < 0.01) reduced the rise in blood glucose levels in glucose-loaded rats. None of the different extracts (at 1 g/kg for 7 h, p.o.) significantly (P > 0.05) altered the blood glucose levels in normal and streptozotocin-induced diabetic rats as compared to the control group. However, administration of the 50 and 95% ethanol extracts (at 1 g/kg for 14 days, p.o.) significantly (P < 0.05) reduced fasting blood glucose and insulin levels in normal and diabetic rats compared to the pre-treatment level. HPLC profiles of the extracts were developed using andrographolide (AP) as a marker. The amounts of AP in 95, 50, and 20% ethanol extracts and water extracts were 25.8, 19.4, 2.0, and 0.8 mg/g of dry weight, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is a chronic metabolic disorder associated with abnormally high levels of glucose in the blood. Type 1 diabetes or insulin-dependent diabetes mellitus is associated with a specific and complete loss of pancreatic β-cells (insulin producing), leaving islets composed of an increased number of α-cells (glucagon producing), δ-cells (somatostatin producing), and PP-cells (pancreatic polypeptide). Type 2 diabetes or non-insulin-dependent diabetes mellitus arises as a consequence of failure of insulin action due to abnormalities at the cell surface (decreased affinity of the receptors for insulin) or within the cell (post-receptor defects) or deficiency in insulin secretion or a combination of these processes [1]. Diabetes results in disastrous clinical consequences including atherosclerosis, retinopathy, neuropathy, high blood pressure, kidney failure, and infection.
Herbal remedies have been practiced since ancient times for the treatment of diabetes mellitus [2]. The leaves of Andrographis paniculata are used traditionally for treatment of diabetes, usually taken orally in the form of a water concoction [3, 4]. Antihyperglycemic effects of the 80% ethanol (EtOH) extract of A. paniculata in normal and diabetic rats after oral administration have also been reported [5]. The main constituent in the leaves of the plant is andrographolide (AP), a bitter, colorless and crystalline diterpene lactone. In our previous study, high performance thin layer chromotography (HPTLC) and reversed-phase high performance liquid chromotography (RP-HPLC) with UV detection were used for quantitative analysis of the leaf extracts [6]. The aim of the present study was to determine insulin concentration and the antihyperglycemic effects of A. paniculata extracts in normal and streptozotocin-induced diabetic rats. HPLC profiles of the extract were also developed using AP as a marker.
Materials and methods
Chemicals and solvents
Reference drugs used as positive control were glibenclamide 10 mg/kg (Ho Yan Hor, Malaysia), metformin 500 mg/kg (UPHA, Selangor, Malaysia) and insulin 5 I.U./kg (Novo Nordisk, Malaysia). Streptozotocin 65 mg/kg (Sigma, Deisenhofen, Germany) injected intraperitoneally was used to induce diabetes in the rats. Ethanol was purchased from R&M Chemical, Essex, UK, as 95% v/v denatured ethanol. The 95% ethanol was reconstituted with distilled water to give 50 and 20% (v/v) ethanol. Distilled water was used as water solvent.
Plant material
The dried aerial part of the plant used in this study was authenticated and supplied by the Malaysian Research on Agriculture Development Institute (MARDI), Telong, Kota Bharu Kelantan, and was authenticated as A. paniculata Nees.
Preparation of extracts
The plant was harvested between 8 and 12 weeks after transplanting. The dried aerial part was ground to a powder using a milling machine. The dried powder was then divided equally into four parts, weighed, and extracted with 95, 50, and 20% ethanol (v/v) and distilled water, respectively, with reflux on a water bath at 40°C for 3 consecutive days. The extracts were filtered and evaporated under vacuum to dryness with a rotary evaporator and then placed in an oven at 60°C until constant weight was obtained. The final weights of all the extracts were determined. The yield was defined as the percentage of final extract per dried powder weight.
Animal
Male Sprague–Dawley rats (160–280 g) used in this study were obtained from the animal house of the School of Pharmaceutical Sciences, Universiti Sains Malaysia, Penang. The animals were kept in cages in the animal room (25 ± 2°C) under a 12-h light/dark cycle and maintained on a standard laboratory animal diet (Gold Coin, Penang, Malaysia) with water ad libitum. Animals were maintained and handled according to the recommendations of the local ethics committee, which approved the design of the animal experiments.
Collection of blood samples and measurement of glucose levels
A small sample (a drop) of blood was obtained by making a nick in the tail of each rat. The blood glucose levels were then determined using an Accutrend Advantage II clinical glucose meter (Roche, Kuala Lumpur, Malaysia). All subsequent blood samples for glucose measurements were obtained from the same nick.
Antihyperglycemic effect of acute administration of the extracts in normal and diabetic rats
Preliminary studies were performed to determine how antidiabetic drugs with different mechanisms of action (glibenclamide, metformin, and insulin) influence blood glucose levels in the different experimental animal models used in the study. The animal models used were the hypoglycemic test in normal rats, glucose tolerance test in normal rats, and hypoglycemic test in streptozotocin-induced diabetic rats.
Hypoglycemic test in normal rats
Male Sprague–Dawley rats (200–250 g) were divided into six groups of six animals in each group (n = 6) and fasted overnight.
-
Group I: Normal rats given oral saline 10 ml/kg
-
Group II: Normal rats given oral glibenclamide 10 mg/kg body weight
-
Group III: Normal rats treated with 95% ethanol extract
-
Group IV: Normal rats treated with 50% ethanol extract
-
Group V: Normal rats treated with 20% ethanol extract
-
Group VI: Normal rats treated with water extract
The extracts were administered orally at a concentration of 1 g/kg body weight with a 16-G oral needle. The blood glucose levels of the rats were measured before treatment and at 1, 2, 3, 5, and 7 h after treatment.
Intraperitoneal glucose tolerance test (IPGTT) for normal rats
For the IPGTT of normal rats, 36 male Sprague–Dawley rats (200–250 g) were divided into six groups of six animals in each group and fasted overnight.
-
Group I: Normal rats given oral saline 10 ml/kg served as negative control
-
Group II: Normal rats given oral metformin 500 mg/kg served as positive control
-
Group III: Normal rats treated with 95% ethanol extract
-
Group IV: Normal rats treated with 50% ethanol extract
-
Group V: Normal rats treated with 20% ethanol extract
-
Group VI: Normal rats treated with water extract
The extracts were administered orally at a concentration of 1 g/kg body weight with a 16-G oral needle. One hour later, all the rats were loaded with glucose 500 mg/kg i.p. Blood glucose levels of the rats were measured before the treatment and at 15, 30, 45, 60, 90, and 120 min after the glucose load.
Hypoglycemic test in diabetic rats
Diabetes was induced in male rats by injecting streptozotocin 65 mg/kg intraperitoneally. Three days after streptozotocin administration, fasting blood glucose level was measured and only rats with blood glucose above 15 mmol/l were considered diabetic and used for the study. The diabetic rats were divided into six groups with six animals in each group (n = 6).
-
Group I: Diabetic rats given oral saline 10 ml/kg served as negative control
-
Group II: Diabetic rats given oral insulin, 5 I.U./kg of body weight subcutaneously, served as positive control
-
Group III: Diabetic rats treated with 95% ethanol extract
-
Group IV: Diabetic rats treated with 50% ethanol extract
-
Group V: Diabetic rats treated with 20% ethanol extract
-
Group VI: Diabetic rats treated with water extract
The extracts were administered orally at a concentration of 1 g/kg body weight. All treatments were given orally with a 16-G oral needle. The blood glucose levels were determined before treatment and at 1, 2, 3, 5, and 7 h after treatment.
Antihyperglycemic effect of chronic administration of the extracts in diabetic rats
Male Sprague–Dawley rats (200–250 g) were treated with streptozotocin 65 mg/kg intraperitoneally. Three days after the inducement of diabetes, blood was obtained from the rat’s tail vein to determine the fasting blood glucose and insulin levels. Rats with blood glucose levels above 15 mmol/l were considered diabetic and used for the study. The diabetic rats were randomly divided into seven groups, with six rats (n = 6) in each group. The groups included those treated with (1) water as negative control, (2) metformin 500 mg/kg as positive control, and the test groups of (3) water extract, and (4) 20%, (5) 50%, and (6) 95% ethanol extracts of A. paniculata 500 mg/kg and (7) AP (Sigma) 40 mg/kg orally twice daily for 14 days. The fasting blood glucose and insulin levels were determined 3 days after the inducement of diabetes and were compared with results 14 days after chronic oral treatment.
Blood glucose assay
All the rats were fasted overnight before blood glucose level determination. Blood samples were obtained by making a small nick in the tail of the rat, and the blood glucose levels were determined using an Accutrend Advantage II clinical glucose meter (Roche).
Insulin assay
The method chosen for determining the insulin level was ELISA (enzyme-linked immunosorbent assay) instead of radioimmunoassay (RIA) because it is more sensitive and accurate than RIA for rat insulin assay [7, 8]. Blood obtained from the tail nick was collected in a capillary tube and centrifuged. The plasma was then collected and stored at −20°C until assayed. A commercial ELISA kit of rat insulin (Crystal Chem, IL, USA) was used to determine the level of insulin in the plasma.
HPLC analysis of the extracts
HPLC analysis was performed using an Agilent Technologies Series 1100 system equipped with an automatic injector, a column oven, and UV detector. A LiChrosorb RP-18 (250 × 4.6 i.d. mm2, 10-μm particle size) (Merck Darmstadt, Hohenbrumn, Germany) was used. AP was separated with the methanol-water (pH 2.8 with phosphoric acid) (6:4 v/v) mobile phase, using an injection volume of 20 μl, a flow rate of 1 ml/min and detected at 210 nm at room temperature. A stock solution of AP was prepared in methanol at 2.0 mg/ml, and standard solutions were prepared by dilution of the stock solution with methanol to give solutions containing AP in the concentration range of 1–2,000 μg/ml. The extracts were subjected to HPLC analysis and the concentrations of the markers determined by external standard method.
Statistical analysis of the data
All data are expressed as mean ± SEM. The means differences between the groups were statistically analyzed by one-way ANOVA, followed by Dunnett test as a post-hoc test. P < 0.05 was regarded as significant.
Results
Extracts and HPLC-UV analysis
The yields obtained from 95, 50, 20% ethanol, and water extracts were 8.70, 11.46, 13.29, and 10.03%, respectively. Figure 1a–d shows the HPLC-UV profiles of the 95, 50, 20% ethanol, and water extracts of A. paniculata. The marker AP was determined by using isocratic condition of methanol-water (pH 2.8 with phosphoric acid) (60:40 v/v) and eluted within 5 min. The peak of AP was identified by comparing the retention time of reference AP and extract under the same chromatographic conditions. The system suitability of the HPLC method was evaluated by using the intraday and interday precision and accuracy of replicates. The accuracy was evaluated through recovery studies by adding known amounts of the standards solution to the extract. Controls from samples were prepared and analyzed. The recovery experiment was performed at three concentrations of the standard and assessed by the external standard method. The relative standard deviations were <1.2 and 1.5% for intra- and interday variation studies. The recovery of AP (mean ± SD) was 98.6 ± 2.3%. The limit of detection (response = three times baseline noise in lowest standard prepared) was 0.03 μg/ml. The established HPLC-UV method was used for quantitative determination of AP in different A. paniculta extracts using the external standard technique. The amounts of AP in 95, 50, and 20% ethanol extracts, and water extracts were 25.8, 19.4, 2.0, and 0.8 mg/g dry weight, respectively.
Antihyperglycemic effect of acute administration of extracts in normal and diabetic rats
The hypoglycemic test showed that metformin did not significantly influence blood glucose levels in normal rats. There was no significant difference between the blood glucose levels in metformin-treated and saline-treated rats (Fig. 2a). However, the blood glucose levels in glibenclamide-treated rats were significantly reduced at 2, 3, 5, and 7 h after treatment (P < 0.05–0.01). In the IPGTT, glibenclamide and metformin significantly inhibited the rise in blood glucose levels at 30, 60, and 90 min (P < 0.05–0.01) after glucose load (Fig. 2b). The blood glucose levels in the glibenclamide-treated group were also significantly reduced below the fasting level at 120, 240, and 360 min after glucose load (P < 0.01). Figure 2c shows that, in diabetic rats, oral treatment with glibenclamide and metformin did not significantly lower the blood glucose levels when compared to the control group. However, the blood glucose levels in those treated subcutaneously with insulin were significantly reduced (P < 0.01) 1 h after treatment until the end of the experiment.
The effect of oral administration of a glibenclamide 10 mg/kg and metformin 500 mg/kg on blood glucose levels of normal rats, b glibenclamide 10 mg/kg and metformin 500 mg/kg on blood glucose levels of rats loaded with glucose 500 mg/kg intraperitoneally, c glibenclamide 10 mg/kg, metformin 500 mg/kg, and insulin 5.0 I.U./kg on blood glucose level of streptozotocin-induced diabetic rats. Values are the mean ± SEM of six animals (**P < 0.01, *P < 0.05)
Hypoglycemic test in normal rats
Figure 3a shows oral treatment with 95, 50, 20% ethanol, and water extracts of A. paniculata. The extracts at 1 g/kg did not significantly alter the blood glucose levels in normal rats (n = 6 in each group) as compared to the control group during the 7-h experiment. Similar to the results shown in Fig. 2a, the blood glucose levels in glibenclamide-treated rats were significantly reduced (**P < 0.01) from the first hour until the seventh hour after treatment.
The effect of oral administration of Andrographis paniculata extracts (1 g/kg), normal saline (control), and a glibenclamide (10 mg/kg) on fasting blood glucose levels of normal rats, b metformin (10 mg/kg) on blood glucose levels of rats loaded with glucose 500 mg/kg i.p., c subcutaneous administration of insulin (5.0 I.U./kg) on fasting blood glucose levels of streptozotocin-induced diabetic rats. Values are the mean ± SEM of six animals (**P < 0.01)
Intraperitoneal glucose tolerance test
Figure 3b shows that the rise in blood glucose levels in glucose-loaded rats was not significantly affected by 95 or 50% ethanol extracts or water extracts of A. paniculata 1 g/kg within 2 h after glucose loading. However, the blood glucose levels in rats treated with 20% ethanol extract of A. paniculata were significantly reduced (**P < 0.01) 90 min after glucose loading. Similar to the results shown in Fig. 2b, treatment with metformin lowered the glucose levels more rapidly than the control group 30–90 min after glucose loading (**P < 0.01).
Hypoglycemic test in diabetic rats
Treatment with 95, 50, and 20% ethanol extracts and water extracts of A. paniculata 1 g/kg did not lower the blood glucose levels in diabetic rats (Fig. 3c). The blood glucose levels of the extracts-treated rats were not significantly different from the control group. Similar to the results shown in Fig. 2c, the lowering of the blood glucose level of diabetic rats was only observed in the insulin-treated rats 1 h after treatment through the end of the experiment (P < 0.01).
Antihyperglycemic effect of chronic administration of the extracts in diabetic rats
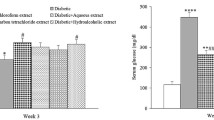
Figure 4a shows that the fasting blood glucose levels of rats treated with metformin, 50 and 95% ethanol extracts of A. paniculata were significantly reduced compared to the pre-treatment level (P < 0.05). However, the average glucose levels in all treated groups were not reduced to normal glucose fasting levels of normal rats. Only treatment with 50 and 95% ethanol extracts of the plant (at 1 g/kg for 14 days, p.o.) significantly lowered the insulin levels in diabetic rats compared to the pre-treatment levels (Fig. 4b).
The effect of oral treatment with 20, 50, and 95% ethanol and water extracts of A. paniculata 500 mg/kg, andrographolide 40 mg/kg, and metformin 500 mg/kg twice daily for 14 days on a fasting blood glucose levels of streptozotocin-induced diabetic rats and b insulin levels of streptozotocin-induced diabetic rats. Values are mean ± SEM, n = 6, *P < 0.05
Discussion and conclusions
The results of the preliminary studies showed that antidiabetic drugs glibenclamide and metformin produced different effects in the hypoglycemic tests performed on normal and diabetic rats and IPGTT in normal rats. Glibenclamide caused the lowering of blood glucose levels in normal (Fig. 2a) and glucose-loaded rats to below fasting levels (Fig. 2b). Glibenclamide stimulates β-cells to secrete insulin, which in turn stimulates the conversion of glucose to glycogen, triglyceride, and proteins in insulin’s major target tissues–liver, fat, and muscle [9]. Consequently, glibenclamide is capable of lowering blood glucose levels to below fasting levels in both hypoglycemic and IPGTT studies. Although metformin also inhibited the increase in blood glucose levels in glucose-loaded rats (Fig. 2b), it did not lower the blood glucose below the normal (fasting) level. Metformin acts mainly by impairing hepatic gluconeogenesis, directly stimulating glycolysis in adipose and muscle tissues while increasing glucose removal from blood, and slowing glucose absorption from the gastrointestinal tract [9]. Metformin’s blood glucose-lowering action does not depend on the presence of functioning pancreatic β-cells. Hence, metformin could only inhibit the rise of the blood glucose level in IPGTT but not reduce it below the normal level.
Streptozotocin-induced diabetes in rats in this study was analogous to type 1 diabetes mellitus in humans. The induction of streptozotocin presumably damaged some β-cells and produced hyperglycemia that was not high enough to cause mortality. In type 1 diabetes mellitus, β-cells do not produce enough insulin. Therefore, treatment of type 1 diabetes mellitus requires an addition of exogenous insulin, e.g., administered subcutaneously to stimulate reduction in blood glucose level. Although glibenclamide stimulates the production of endogenous insulin from β-cells, the amount produced is not large enough to lower blood glucose to normal levels as most of the β-cells in the pancreas have been destroyed by streptozotocin. Metformin failed to lower the blood glucose level in diabetic rats because the blood glucose was too high for the extrapancreatic action of metformin to bring it down to normal levels.
IPGTT was used instead of an oral glucose tolerance test (OGTT) to avoid false positive results due to the sticky nature of plant extracts such as the extract of A. paniculata. It was thought that when glucose was administered orally 1 h after oral administration of the extract, some of the glucose would be absorbed by the sticky extract and passed out with the stool instead of being absorbed in the gastrointestinal tract. This would reduce the amount of glucose entering the blood and inhibit the rise of blood glucose levels compared to saline-treated rats in the control group. The inhibition in the rise of the blood glucose level is used to determine whether an extract or drug possesses antihyperglycemic activity. The presence of splanchnic glucose retention phenomenon in OGTT has been reported [10]. The rise in plasma glucose following an oral glucose load is much less than would occur after intravenous administration. Factors that contribute include the extent of absorption and direct utilization of glucose by the gastrointestinal tract as well as liver uptake. The phenomenon causes an increase in the biological variation between rats and might also produce a false positive result.
None of the extracts of A. paniculata studied had hypoglycemic activity in normal and diabetic rats. In the IPGTT experiment, only the 20% ethanol extract of A. paniculata, at a dose of 1 g/kg, showed antihyperglycemic effect where the extract inhibited the rise of blood glucose levels in glucose-loaded rats. The effect of the 20% ethanol extract of A. paniculata was very similar to metformin, which has no hypoglycemic activity in normal and diabetic rats but has antihyperglycemic effect as it facilitated the decrease in blood glucose levels of glucose-loaded rats. The fact that the 20% ethanol extract did not have an antihyperglycemic effect in streptozotocin-induced diabetic rats further suggests that it did not act via the stimulation of β-cells. The antihyperglycemic effect was only measured 72 h after administration of streptozotocin [11] to ensure complete destruction of viable β-cells that could respond to hyperglycemia and produce insulin, an effect which subsequently could be potentiated with metformin or the extracts.
The nonpolar extract of 95% ethanol did not lower the blood glucose levels in normal rats in this study. Reports have indicated that the chloroform fraction of the methanol extract of A. paniculata caused hypoglycemic effect in normal rats [4]; however, the results of the present study contradict this observation. The contradiction may be attributed to the solvent used for the extraction.
Daily treatment with metformin, 50 and 95% ethanol extracts of A. paniculata for 2 weeks significantly reduced the fasting blood glucose levels of streptozotocin-induced diabetic rats. This shows that the 50 and 95% ethanol extracts of A. paniculata possess antihyperglycemic activity similar to metformin. The lowering of blood glucose levels after 14 days of treatment with 80% ethanol extract of A. paniculata has been reported previously by Zhang and Tan [5].
The antidiabetic compounds in A. paniculata are relatively nonpolar, as similar treatment with the more polar extracts of 20% ethanol and water extracts did not lower the blood glucose levels. This indicates that there might be two groups of active substances in A. paniculata that contribute to its antihyperglycemic properties: a group of polar compounds, which inhibited the rise of blood glucose levels in IPGTT, and relatively less polar compounds as shown in the 14-day experiment. The polar compounds are active when insulin secretion is normal and inactive in insulin-deprived situations, e.g., in streptozotocin-induced diabetic rats.
Chronic treatment of the diabetic rats with 50 and 95% ethanol extract significantly reduced the insulin levels, unlike those treated with metformin, AP, water, and 20% ethanol extracts. The result in the metformin-treated group was expected, as the antidiabetic drug metformin is known not to stimulate the release of insulin from the β-cells of the pancreas. This indicates that the antidiabetic activity of 50 and 95% ethanol extract is not via the mechanism of the stimulation of insulin release. The lowering of the insulin levels by these extracts was presumably a result of a physiological response to the lowering of the blood glucose levels. This may suggest that neither the active polar nor the less polar substances in the A. paniculata extract exert their antihyperglycemic property by stimulating insulin secretion from β-cells.
Our results showed that twice-daily treatment with AP for 14 days did not lower the blood glucose level of diabetic rats. The antihyperglycemic activity could not have been due to AP since pure AP did not show the activity. Moreover, HPLC analyses showed higher amounts of AP in the 50 and 95% ethanol extracts of A. paniculata, which did not show any antihyperglycemic activity.
In conclusion, hypoglycemic tests in normal and diabetic rats showed a reduction in fasting blood glucose and insulin levels in rats compared to the pre-treatment levels after oral administration of 50 and 95% ethanol extracts of A. paniculata at 1 g/kg for 14 days. The hypoglycemic test and the results can be utilized for screening antidiabetic activity and for further mechanistic studies to indicate the exact mechanism of action of the extracts. The HPLC method could be used for identification of the chemical components of botanicals containing A. paniculata. Further investigation is ongoing in our laboratory to determine the correlation between the HPLC profiles and antihyperglycemic activity.
References
Rodrigues B, Poucheret P, Battell ML, McNeill JH (1999) Streptozotocin-induced diabetes: induction, mechanism(s), and dose dependency. In: McNeill JH (ed) Experimental models of diabetes. CRC Press, Boca Raton, pp 3–17
Ajgaonkar SS (1979) Herbal drugs in the treatment of diabetes. A review. Int Diabetes Fed Bull 24:10–19
Perry LM, Metzger J (1980) Medicinal plants of East and Southeast Asia. MIT Press, Cambridge
Ahmad M, Asmawi MZ (1993) Some pharmacological effects of aqueous extract of Andrographis paniculata Nees. In: Abstracts of the International Conference on the Use of Traditional Medicine and Other Natural Products in Health Care, 8-11 June 1993, Penang, Malaysia. School of Pharmaceutical Sciences, Universiti Sains Malaysia, Penang
Zhang XF, Tan BK (2000) Antihyperglycaemic and anti-oxidant properties of Andrographis paniculata in normal and diabetic rats. Clin Exp Pharmacol Physiol 27:358–368
Akowuah AG, Zhari I, Norhayati I, Mariam A (2006) HPLC and HPTLC densitometric determination of andrographolides and antioxidant potential of Andrographis paniculata. J Food Compos Anal 19:118–126
Bank HL (1988) A quantitative enzyme-linked immunosorbent assay for rat insulin. J Immunoassay 9:135–158
MacDonald MJ, Gapinski JP (1989) A rapid ELISA for measuring insulin in a large number of research samples. Metabolism 38:450–452
Nolte MS, Karam JH (2001) Pancreatic hormones and antidiabetic drugs. In: Katzung BG (ed) Basic and clinical pharmacology, 8th edn. McGraw-Hill, New York, pp 721–723
Wigfull J, Bellamy M (2000) Physiology and pharmacology of the liver. Bull R Coll Anaesth 3:101–102
Aughsteen AA (2000) An ultrastructural study on the effect of streptozotocin on the islets of Langerhans in mice. J Electron Microsc 49:681–690
Acknowledgements
The study was funded by an Intensifying Research Priority Areas (IRPA) Grant from the Ministry of Science and Technology and Environment, Malaysia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmad, M., Razak, A., Akowuah, G.A. et al. HPLC profile and antihyperglycemic effect of ethanol extracts of Andrographis paniculata in normal and streptozotocin-induced diabetic rats. J Nat Med 61, 422–429 (2007). https://doi.org/10.1007/s11418-007-0157-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-007-0157-4