Abstract
Extracts from 37 kinds of foods and foodstuffs were tested for inhibitory activity against recombinant human lanosterol synthase. Among them, extracts from five samples showed significant inhibition. Potent activity (55%) was found in 95% ethanol extract of Laurus nobilis L. Therefore, large-scale methanol extraction of the plant was carried out, and the constituents were separated by partition and fractionation by silica gel chromatography and HPLC. Four flavonoids, kaemperol 3-O-[2″,4″-O-di-E-p-coumaroyl-α-l-pyranorhamnoside] (1); 3,3′,4′,5,6,7,8-heptamethoxyflavone (2); 3′,4′,5,6,7,8-hexamethoxyflavone (nobiletin) (3); and 4′,5,6,7,8-pentamethoxyflavone (tangeretin) (4); and six sesquiterpens, eremanthine (5), dehydrocostus lactone (6), costunolide (7), zaluzanin C (8), zaluzanin D (9) and reynosin (10) were isolated. Eremanthine (5) showed the most potent activity, 70% inhibition, at the concentration of 500 μM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperlipemia is a risk factor for arteriosclerosis. Control of the cholesterol level in the body is effective for prevention and improvement of hyperlipemia. Currently, HMG-CoA reductase inhibitors are used clinically for the treatment of hyperlipemia. The HMG-CoA reductase inhibitors, however, might cause simultaneous reduction of the physiologically essential non-steroidal isoprenoid metabolites such as dolichol, ubiquinone and prenylated proteins because HMG-CoA reductase is located in the upstream of the cholesterol biosynthetic pathway. Therefore, the long-term administration of the inhibitors of the enzyme may cause unexpected side effects. Lanosterol synthase is considered to be a more selective target for suppression of cholesterol biosynthesis, since it is located in the middle stage of the biosynthetic pathway of the cholesterol in mammals. Lanosterol synthase-inhibiting compounds were isolated from microbial cultures using in vitro assay with recombinant human enzyme [1, 2].
In recent years, a tertiary function of foods contributing to healthy life by modulating human homeostasis has been recognized in addition to their nutritional and organoleptic functions. From the viewpoint of this function, we have investigated a new function of foods and foodstuffs, and found that apple-condensed tannins had an antiallergic effect on type I allergic symptoms [3]; extracts of cabbage, red cabbage, tomato, and watercress showed antiallergic effects [4]; and some constituents in watercress inhibited histamine release from RBL-2H3 cells [5].
In order to find the foods which function to reduce the cholesterol level, 130 kinds of vegetable extracts were examined for inhibition of human lanosterol synthase in our previous study. Among them, the extract of Colocasia esculenta (taro) exhibited the most potent activity. Monogalactosyl diacylglycerol (MGDG) and digalactosyl diacylglycerol (DGDG) were isolated as active components [6]. In this paper, we report inhibitory activities of another 37 kinds of foods and foodstuffs on human lanosterol synthase and isolation of the active compounds from the extract of Laurus nobilis L., which showed potent inhibitory activity among the tested samples.
Materials and methods
Materials
Dried powder of laurel (L. nobilis L.) was purchased from Yasuma (Tokyo, Japan). Other plant materials for screening were gifts from San-Ei Gen F.F.I., Inc. (Osaka, Japan).
Screening of human lanosterol synthase-inhibiting food and foodstuff
Thirty-seven foods and foodstuffs, Glycyrrhiza glabra L. (roots), Coriandrum sativum L. (fruits), Cistanche salsa G. Beck (whole plant), Cichorium intybus L. (roots), Dimocarpus longan Lour (fruits), Litchi chinensis Sonn. (fruits), Cymbopogon citratus Stapf. (leaves), Melissa officinalis L. (leaves), Uncaria gambir Roxburgh (leaves), Piper nigrum L. (fruits), L. nobilis L. (leaves), Pimpinella anisum L. (fruits), Acanthopanax senticosus Harms (bark), Diospyros kaki Thunberg (leaves), Urtica platyphylla Wedd. (aerial part), Elettaria cardamomum Maton (seeds), Citrus unshiu Markovich (fruits), Aloe arborescens Mill. (leaves), Lycium chinense Mill. (fruits), Syzygium aromaticum (L.) Merr. et Perry (buds), Gentiana lutea L. (roots), Jasminum grandiflorum L. (flowers), Illicium verum Hook. fil. (fruits), Mentha spicata L. (aerial part), Salvia officinalis L. (leaves), Curcuma longa L. (rhizomes), Myristica fragrans Houttuyn (seeds), Petroselium sativum L. (aerial part), Foeniculum vulgare Mill. (fruits), Brassica juncea (L.) Czerniak (seeds), Porphyra tenera Kjellman. (whole plant), Momordicae grosvenori Swingle (fruits), Achillea millefolium L. (buds), Zanthoxylum bungeanum Maxim. (fruits), Origanum majorana L. (leaves), Zizyphus jujuba Mill. var. inermis Rehd. (fruits), and Chrysanthemum morifolium Ramat. (flowers) were extracted with 95% ethanol, 50% ethanol or water on a water bath at 80°C for 2 h and adjusted to 5 mg/ml with ethanol to afford 64 sample solutions. Sixty μl (300 μg) of each sample solution was tested for inhibition of human lanosterol synthase as described in previous papers [1, 2].
Isolation of components from L. nobilis L.
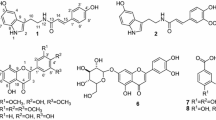
The laurel powder (800 g) was extracted by reflux with methanol (3.0 l×3). The solvent was removed under reduced pressure to yield 328.7 g of a methanol extract. The methanol extract (100 g) was suspended in water and partitioned with dichloromethane, ethyl acetate, and 1-butanol, successively, and resulted in three crude fractions, dichloromethane (8.6 g), ethyl acetate (16.1 g) and 1-butanol (20.1 g). The ethyl acetate soluble fraction was chromatographed over a silica gel column eluting with hexane and ethyl acetate, followed by MPLC and HPLC, to give kaemperol 3-O-[2″,4″-O-di-E-p-coumaroyl-α-l-pyranorhamnoside] (1; 13.3 mg) [7]. The dichloromethane soluble fraction was subjected to activated charcoal column chromatography (methanol, methanol-chloroform and chloroform) to afford four fractions (fractions 1–4). The third fraction was further separated by silica gel column chromatography using ethyl acetate-hexane, followed by HPLC, and yielded 3,3′,4′,5,6,7,8-heptamethoxyflavone (2, 24.3 mg) [8, 9]; 3′,4′,5,6,7,8-hexamethoxyflavone (nobiletin) (3, 2.9 mg) [8, 9]; 4′,5,6,7,8-pentamethoxyflavone (tangeretin) (4, 2.2 mg) [8, 9]. Fraction 1 was further separated by silica gel column chromatography using ether–hexane, followed by HPLC (80% MeOH) , and yielded eremanthine (5, 4.1 mg) [10], dehydrocostus lactone (6, 8.6 mg) [11] and costunolide (7, 8.1 mg) [12]. Fraction 2 was further separated by silica gel column chromatography using ether-hexane, followed by HPLC (80% MeOH), and yielded zaluzanin C (8, 31.7 mg) [13], zaluzanin D (9, 10.3 mg) [14], and reynosin (10, 5.1 mg) [15]. These compounds were identified by comparison of their spectral data with those published (Figs. 1, 2).
Results and discussion
Among 37 tested samples, five samples (L. nobilis, A. millefolium, U. gambri, P. higrum and C. unshiu) showed inhibitory activity (more than 5% inhibition) on human lanosterol synthase (Table 1). Among them, the active constituents of L. nobilis were investigated in this study. L. nobilis is an evergreen tree, family Lauraceae, native to Europe. The leaves of L. nobilis, bay leaf or laurel, are usually used as a spice and are known as a folk medicine for rheumatism in Europe [16, 17]. The constituents of L. nobilis have bioactivities such as nematocidal activity [18], ethanol-absorption inhibitory activity [19], trypanocidal activity [20] and inhibitory activity on nitric oxide production in lipopolysaccharide-activated mouse peritoneal macrophages [21].
The inhibitory activity of extracts of laurel with several solvents (95% aqueous ethanol, 50% aqueous ethanol and water) was 55, 22 and 0%, respectively, suggesting that the active components of L. nobilis were hydrophobic. Therefore, laurel was first extracted with methanol, and the methanol extract was partitioned with water and organic solvents (dichloromethane, ethyl acetate and 1-butanol, successively), and then the active compounds in the dichloromethane and ethyl acetate layers were further investigated. From the dichloromethane layer, we isolated three flavonoids (2–4) and six sesquiterpenes (5–10) which were identified to be 3,3′,4′,5, 6,7,8-heptamethoxyflavone (2); 3′,4′,5,6,7,8-hexamethoxyflavone (nobiletin) (3); 4′,5,6,7,8-pentamethoxyflavone (tangeretin) (4); eremanthine (5), dehydrocostus lactone (6); costunolide (7); zaluzanin C (8); zaluzanin D (9) and reynosin (10). Kaemperol 3-O-[2″,4″-O-di-E-p-coumaroyl-α-l-pyranorhamnoside] (1) was isolated from the ethyl acetate layer. These isolated non-glycoside flavonoids (2–4) were not new compounds, but were isolated from L. nobilis for the first time.
The inhibitory activity of compounds 1–10 is shown in Table 2. Among them, 5 showed the most potent inhibitory activity (70% at 500 μM) followed by 6 (63% at 500 μM). These compounds are bicyclic, guaiane-type sesquiterpenes, and the difference is in the geometry of the double bond at C10. Inhibitory activity was reduced in compounds 8 and 9, analogs of 6 with hydroxyl and acetoxyl at C3, suggesting that no substitution group at C3 is required for significant inhibitory activity. On the other hand, costunolide (7), a monocyclic, germacrane-type sesquiterpen, showed no enzyme inhibitory activity. Since the structure of 7 has a ten-membered ring, conformation of 7 is considerably flexible, compared to that of other sesquiterpenes listed in Fig. 2. These results suggested that a particular rigid conformation is a factor for high inhibitory activity.
Three flavonoids, 2–4, were isolated as active compounds (20–40% inhibitory activity) in addition to the sequiterpenes. Compound 1, a glycoside of flavonoid isolated from the ethyl acetate layer, however, showed no activity. The structures of 2–4 are characteristic in that all hydroxy groups are methylated. These results indicated that hydrophobicity of the molecule is indispensable for significant inhibitory activity. This is the first report to describe flavonoids as having lanosterol synthase inhibitory activity.
As shown in Table 1, the ethanol extract of A. millefolium L. and C. unshiu showed inhibitory activity. Since it has been reported that A. millefolium L. [22] and C. unshiu [23] contain non-glycosidic flavonoids, flavonoids are deduced to be the active components of A. millefolium L. and C. unshiu.
Five sesquiterpenes and three flavonoids were isolated from L. nobilis as lanosterol synthase inhibitors. The inhibitory activity of each compound was not as high as the known inhibitors, lauryldimethylamine N-oxide (LDAO), AMO 1618, lanopylins A1, B1, A2 and B2, and epohelmins A and B, for which the IC50 values were 0.84, 120, 15, 18, 33, 41, 10 and 6.0 μM, respectively, under the same assay conditions [1, 2]. However, the fact that laurel contains multiple compounds with moderate inhibitory activity and is ingested repeatedly may make laurel a functional food for reducing the amount of cholesterol in the human body by inhibition of its de novo biosynthesis, even though the activity of each compound is not so high.
References
Sakano Y, Shibuya M, Matsumoto A, Takahashi Y, Tomoda H, Ômura S, Ebizuka Y (2003) Lanopylins A1, B1, A2 and B2, novel lanosterol synthase inhibitors from Streptomyces sp. K99-5041. J Antibiot 56:817–826
Sakano Y, Shibuya M, Yamaguchi Y, Masuma R, Tomoda H, Ômura S, Ebizuka Y, (2004) Epohelmins A and B, novel lanosterol synthase inhibitors from a fungal strain FKI-0929. J Antibiot 57:564–568
Akiyama H, Sakushima J, Taniuchi S, Kanda T, Yanagida A, Kojima T, Teshima R, Kobayashi Y, Goda Y, Toyoda M (2000) Antiallergic effect of apple polyphenols on the allergic model mouse. Biol Pharm Bull 23:1370–1373
Hoshino K, Akiyama H, Goda Y, Tanimura A, Toyoda M (1998) Evaluation of antiallergic effects of extracts from ten kinds of vegetables using three in vitro assay systems. J Food Hyg Soc Japan 39:72–77
Goda Y, Hoshino K, Akiyama H, Ishikawa T, Abe Y, Nakamura T, Otsuka H, Takeda Y, Tanimura A, Toyoda M (1999) Constituents in watercress: inhibitors of histamine release from RBL-2H3 cells induced by antigen stimulation. Biol Pharm Bull 22:1319–1326
Sakano Y, Mutsuga M, Tanaka R, Suganuma H, Inakuma T, Toyoda M, Goda Y, Shibuya M, Ebizuka Y (2005) Inhibition of human lanosterol synthase by the constituents of Colocasia esculenta (taro). Biol Pharm Bull 28:299–304
Fiorini C, David B, Fouraste I, Vercauteren J (1998) Acylated kaempferol glycosides from Laurus nobilis leaves. Phytochemistry 47:821–824
Iinuma M, Matsuura S, Kusuda K (1980) 13C-nuclear magnetic resonance (NMR) spectral studies on polysubstituted flavonoids. I 13C-NMR spectra of flavones. Chem Pharm Bull 28:708–716
Machida K, Osawa K (1989) On the flavonoid constituents from the peels Citrus hassaku Hort ex Tanaka. Chem Pharm Bull 37:1092–1094
Yuuya S, Hagiwara H, Suzuki T, Ando M, Yamada A, Suda K, Kataoka T, Nagai K (1999) Guaianolides as immunomodulators. Synthesis and biological activities of dehydrocostus lactone, mokko lactone, eremanthin and their derivatives. J Nat Prod 62:22–30
Taniguchi M, Kataoka T, Suzuki H, Uramoto M, Ando M, Arao K, Magae J, Nishimura T, Otake N, Nagai K (1995) Costunolide and dehydrocostus lactone as inhibitors of killing function of cytotoxic T lymphocytes. Biosci Biotech Biochem 59:2064–2067
Chen W, Mayer R, Zimmermann H, Ruecker G (1989) A non-oxidized melampolide and other germacranolides from Aristrolochia yunnanensis. Phytochemistry 28:3233–3234
Romo de Vivar A, Cabrera A, Ortega A, Romo J (1967) Constituents of Zaluzania species. II. Structures of zaluzanin C and zaluzanin D. Tetrahedron 23:3903–3907
Ando M, Kusaka H, Ohara H, Takase K, Yamaoka H, Yanagi Y (1989) Studies on the synthesis of sesquiterpene lactones. 11 The synthesis of 3-epizaluzanin C, zaluzanin C, zaluzanin D, and related compounds 3-hydroxyguaia-1(10),4(15),11(13)-trieno-12,6-lactone and 3-hydroxyguaia-4(15),9,11(13)-trieno-12,6-lactone. J Org Chem 54:1952–1960
Navarro JJ, Caballero MC, Moran JR, Medarde M, Grande M, Anaya J (1990). Guaianolides and eudesmanolides from Centaurea ornat. J Nat Prod 53:573–578
Hokwerda H, Bos R, Tattje DHE, Malingre TM (1982) Composition of essential oils of Laurus nobilis, L nobilis var. angustifolia and Laurus azorica. Planta Med 44:116–119
Cuo J-X (1997) International collation of traditional and fork medicine, vol 4. World Scientific, Singapore
Kiuchi F, Nakamura N, Miyashita N, Nishizawa S, Tuda Y, Kondo K (1989) Nematocidal activity of some anthelmintics, traditional medicines, and spices by new assay method using larvae of Toxocara canis. Syoyakugaku Zasshi 43:279–287
Matsuda H, Shinoda H, Uemura T, Yoshikawa M (1999) Preventive effect of sesquiterpenes from bay leaf on blood ethanol elevation in ethanol-located rat: structure requirement and suppression of gastric emptying. Bioorg Med Chem Lett 9:2647–2652
Uchiyama N, Matsunaga K, Kiuchi F, Honda G, Tsubouchi A, Nakajima-Shimada J, Aoki T (2002) Trypanocidal terpenoids from Laurus nobilis. L Chem Pharm Bull 50:1514–1516
Matsuda H, Kagerura T, Toguchida I, Ueda H, Morikawa T, Yoshikawa M (2000) Inhibitory effects of sesquiterpenes from bay leaf on nitric oxide production in lipopolysaccharide-activated macrophages: structure requirement and role of heat shock protein induction. Life Sci 66:2151–2157
Falk AJ, Smolenski SJ, Bauer L, Bell CL (1975) Isolation and identification of three new flavones from Achillea millefolium L. J Pharm Sci 64:1838–1842
Matsuda H, Yano M, Kubo M, Iinuma M, Oyama M, Mizuno M (1991) Pharmacological study on citrus fruits. II. Anti-allergic effect of fruit of Citrus unshiu Markovich (2). On flavonoid components. Yakugaku Zasshi 111:193–198
Acknowledgements
This work was supported by a grant from the Japan Health Sciences Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanaka, R., Sakano, Y., Shimizu, K. et al. Constituents of Laurus nobilis L. inhibit recombinant human lanosterol synthase. J Nat Med 60, 78–81 (2006). https://doi.org/10.1007/s11418-005-0013-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-005-0013-3