Abstract
Purpose
In this study it is investigated whether typical pollution sources cause differences of heavy metal (Cd, Cu, Pb, and Zn) pollution in the soil-leafy vegetable systems.
Materials and methods
Soil and leafy vegetable samples were collected in the fields contaminated by industrial operation sources (IOS) and organic fertilizer sources (OFS), respectively, in the Yangtze River Delta (YRD) areas. The differences of heavy metal pollution were compared in soil-vegetable systems contaminated by IOS and OFS (IOS- and OFS-systems). Those mainly included (1) Cd, Cu, Pb, and Zn contents in soils and vegetables; (2) heavy metal fractions distribution in soils and their connection with the heavy metal in leafy vegetables; (3) the key influential factor of heavy metal accumulation of leafy vegetable; and (4) the ecological risk of heavy metal pollution in soil-vegetable systems.
Results and discussion
The mean pollution index (PI) of Cd, Pb, Cu, and Zn was 5.05, 1.89, 0.72, and 1.21 in IOS-soils (soils contaminated by IOS), and 2.51, 0.25, 0.64, and 0.71 in OFS-soils (soils contaminated by OFS), respectively. For PIS > 1, the percentages of Cd were 100% in both sources. The IOS caused more significant Cd and Pb pollution in vegetables, but OFS induced higher Cu and Zn accumulation. The Cd in soils had a relatively higher bioavailable fraction proportion (the sum of water-soluble, exchangeable and carbonate-bound fraction), mobility, and transferability in IOS-soils. Nevertheless, such indicators for Cu and Zn were higher in OFS-soils. Compared with IOS-soils, OFS-soils were characterized by a higher proportion of metals bound to humic acid and organic matter. Redundancy analysis (RDA) results showed that the accumulation of Cd, Pb, Cu, and Zn in IOS-vegetables (vegetables contaminated by IOS) was mainly controlled by F3-Cd, F5-Pb, F5-Cu, and F3-Zn in soils, respectively. Moreover, F1-Cd, F4-Pb, F2-Cu, and F2-Zn could be the dominant influential factors of Cd, Pb, Cu, and Zn absorption by OFS-vegetables (vegetables contaminated by OFS), respectively. Risk assessment results revealed that the IOS-systems had higher heavy metal pollution level than OFS-systems.
Conclusions
The source of heavy metals is one of the main factors for the mobility and transfer of heavy metals in the soil–plant systems. There are obvious differences in pollution characteristics between IOS- and OFS-soils. Compared with OFS, IOS caused the more serious ecological risk of heavy metal pollution to the soil-vegetable systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heavy metals have attracted much attention in recent years due to high bioavailability, long-term toxicity, and non-degradability (Meng et al. 2019). Various pollution sources, such as smelters, steel mills, mining, and even organic fertilizer, could contain heavy metals which can enter agricultural products mainly through the soil–plant system (Yi et al. 2018). Crops with high heavy metal content pose a serious threat to human health through the food chain (Zhang et al. 2018). In China, the great demand for vegetables consumption results in a massive cultivation, which might lead to heavy metals pollution (Li et al. 2018c). Thus, the quality and security of the vegetables are of great concern.
The discharge of industrial wastes is an important source of soil heavy metal pollution. In fact, in some soils polluted by the discharge of industrial wastes, heavy metal contents obviously exceeded the environmental quality standards (Liao et al. 2016; Meng et al. 2019; Wu et al. 2012). For example, Pb and Hg content of leafy vegetable samples from industrial operation sources exceeded food safety standards by 47% and 7%, respectively (Li et al. 2018c). Livestock manure has been used as the main raw material for organic fertilizer production to increase plant yields and improve soil quality for a long time (Li et al. 2018a; Shan et al. 2016). However, the long-term use of organic fertilizer has become one of the major sources of heavy metals in agricultural soil (Guo et al. 2018). The mean contents of Cd, Cu, and Zn of greenhouse soils with organic fertilizer application for an average of 8 years were 263%, 40%, and 25% higher than the control soil value, respectively (Bai et al. 2015). Extreme differences in types, contents, and fractions of heavy metals have been reported, deriving from different sources. Acid-soluble fraction proportion of Cd, Cu, and Zn in soils polluted by electroplating wastewater were high, because of their high contents present in the wastewater (Xiao et al. 2017). Huang et al. (2017) reported that the average contents of Cu and Zn in the commercial chicken manure samples were very high, and even 10.3% of those samples exceeded the limit set for Cd. The fractions of Cu and Zn in pig manure were mainly concentrated in the acid-soluble and reducible fractions (Zeng et al. 2018). Thus, the different pollution source may result in a larger difference in the concentration and chemical speciation of heavy metals in soils. High contents of Cd, Pb, and Zn were found to be effective in industrial operation sources (IOS) soils, while Cd, Cu, and Zn were the main contaminants in soils under long-term organic fertilizer application (OFS) (Dong et al. 2019; Yi et al. 2018). Organic fertilizer could immobilize heavy metals by adsorption or reduction of available fractions in polluted vegetable fields (Hao et al. 2007). Application of pig manure increased significantly the contents of organic-bound Cu and Cd but declined the amounts of these metals in available forms in soils (Mohamed et al. 2010). Previous studies have assessed the occurrence and fraction distribution of heavy metals in vegetable soils but focused only on the areas polluted by a single pollution source. However, little attention has been given on whether typical pollution sources cause differences of heavy metal fractions’ distribution in soils.
The mobility, bioavailability, and toxicity of heavy metals in soils depend on their specific chemical forms and their binding state. Many studies have reported that the water-soluble, exchangeable, and carbonate-bound fractions are labile and bioavailable and may be directly absorbed by plants (Zhao et al. 2011; Zeng et al. 2011). There was a significant positive correlation between Cd, Cu, and Zn contents of edible parts of crops (including rice and leafy vegetables) and the exchangeable Cd and Zn contents in IOS-soils, respectively (Liao et al. 2016). Non-labile parts (humic acid-bound, Fe and Mn oxide-bound, and organic matter-bound) of heavy metals in soils are considered as slowly-available heavy metals which could be again released under suitable conditions and absorbed by plants (Zhang et al. 2018; Zhao et al. 2011). Speciation of heavy metals input by pollution sources is mainly affected by heavy metals aging in soil and soil properties. The bioavailability in most of the heavy metals is high in acidic soil but gets lower in alkaline soil (Zheng et al. 2020). Organic matter and cation exchange capacity (CEC) have been indicated to bear the effect on the bioavailability of heavy metals in soils (Qian et al. 2018; Yu et al. 2018). Most previous studies focused on the influence of the soil properties and labile fraction of heavy metals in soil on mobility or bioavailability (Hao et al. 2007; Liao et al. 2016; Wan et al. 2020; Zheng et al. 2020). However, there are few studies that have discussed the contribution and respective effect of different metal fractions and soil properties on heavy metal accumulation in soil-vegetable system, especially humic acid-bound, Fe and Mn oxide-bound, and organic matter-bound fractions.
Vegetables are more susceptible to different pollution sources (Yi et al. 2018). Actually, there are only a few studies on the heavy metal pollution features in soil-vegetable systems polluted by different sources, which were the main objective of the current study. Soil-vegetable systems polluted by IOS and OFS in the YRD were chosen to fulfill the objective using the following steps: (i) to determine the heavy metal contents in soils and vegetables polluted by IOS and OFS; (ii) to clarify the distribution characteristics of heavy metal fractions in IOS- and OFS-soil and to reveal its relationship with the heavy metal in the edible vegetable parts; (iii) to explore the major influential factor of heavy metal accumulation on leafy vegetables; and (iv) to evaluate the ecological risk of heavy metals in soil-vegetable systems polluted by IOS and OFS. The results are essential for improving the accuracy of heavy metal source analysis and the effective treatment of heavy metals in the future.
2 Materials and methods
2.1 Sampling areas
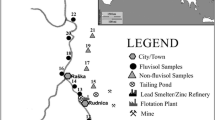
Leafy vegetable fields contaminated by IOS and OFS were chosen in the areas of the YRD (Fig. 1). Sampling sites of IOS in fields were selected nearby of a Pb–Zn mine in Qixia (QX), a steel factory in Banqiao (BQ), a smelter in Xushe (XS), a Cu-mine in Tongling (TL), and from OFS fields located in Tongjiangji (TJJ), Zhuzhuang (ZZ), Dongyue (DY), and Huaiyuan (HY), respectively (Fig. 1). These industrial zones had operated for more than 30 years. OFS-samples were collected from organic farms where organic fertilizer was applied for more than 5 years. Table S1 describes the detailed addresses of the sampling sites.
2.2 Sample collection and chemical analysis
2.2.1 Sample collection
Samples were collected from December 2018 to April 2019. Three plants for the same species were taken and combined into one sample. The species of leafy vegetables in sampling sites are listed in Table S1. Rhizospheric soils were collected by light shaking plant root with hand (Phillips and Fahey 2006), air-dried at room temperature, and sieved by 10-mesh and 100-mesh, respectively. Plant tissue including root and aboveground part was washed many times with tap water, three times with ultrapure water, and weighed after drying at room temperature, and dried at 105 °C for 30 min to deactivate enzymes, then dried at 65 °C until constant dry weight. The leafy vegetable samples were ground (< 2 mm) in a grinder.
2.2.2 Sample analysis methods
Soil pH was determined with a pH meter (Mettler Toledo, Switzerland) in a 1:2.5 soil:water suspension. The SOM content was measured through the K2Cr2O7–H2SO4 digestion method. Soil CEC content was determined by the ammonium acetate exchange method (Lu 2000). Particle size distributions (clay < 0.002 mm, silt = 0.002–0.05 mm and sand = 0.05–2.00 mm) was measured by the hydrometer method (Bouyoucos 1951). The soil basic properties are shown in Table S2.
Total heavy metal concentrations (Cd, Pb, Cu, and Zn) of soil and vegetables were detected with the methods described by Cui et al. (2011). The chemical fractionation of metals was further measured by a sequential extraction method (CGS 2005). The seven sequential extraction steps were carried out as follows. Water-soluble fraction (F1) – 2.5-g air-dry soil sample by ultrapure water in ratio 1:10 (w/v), ultrasonic digestion for 30 min; exchangeable fraction (F2) – 25 ml 1 M MgCl2 at pH 7.0, ultrasonic digestion for 30 min; carbonate-bound fraction (F3) – 50 ml 1 M NaOAc/HOAc, pH 5.0 for 6 h; humic acid-bound fraction (F4) – 25 ml 1 M Na4P2O7/HNO3 at pH 10.0 for 4 h; Fe and Mn oxide-bound fraction (F5) – 50 ml 0.25 M HONH3Cl and 0.25 M HCl, ultrasonic digestion for 1 h; organic matter-bound fraction (F6) – H2O2/HNO3 at pH 2 for 10 h; and residual fraction (F7) – digested with a mixture of HCl, HNO3, HClO4 and HF for 4 h for Cd, Pb, Cu and Zn.
2.2.3 Sample detection and quality control
The determination of Pb and Cd was determined by the graphite furnace atomic absorption spectrometry (GFAAS, SectrAA 220Z, Varian, USA); Cu and Zn were measured by atomic absorption spectrophotometer (FAAS, Beijing Puxin TAS 986). The analytical quality was controlled by using the national standard material (GSS-1 and GSV-2), and the recovery rates ranged from 90.1 to 104.3%.
2.3 Data analysis
Data analyses were performed by SPSS 20.0 (IBM, Armonk, NY, USA), and the graphics were drawn using Origin 2018 (OriginLab, USA) and Canoco 4.5 (Microcomputer Power, USA). The T-tests were used for data analysis at the significant level of P < 0.05. Canoco 4.5 was used for redundancy analysis (RDA) to further assess the relationship between soil heavy metal fractions and plant metal concentrations and explore the contribution of different heavy metal fractions on heavy metal accumulation in leafy vegetables. The metal concentrations in the edible part of leafy vegetables were set as the response variable, while all 7 heavy metal fractions and soil properties, including pH, SOM, CEC, clay, silt, and sand, were selected as explanatory variables. Detrended correspondence analysis (DCA) proved that the first axis’s length for the gradient of the Cd, Pb, Cu, and Zn contents in leafy vegetables was 0.736, 1.060, 0.693, and 0.716, respectively, showing that the data could continue further RDA analysis. After running the Monte Carlo test 999 times (P < 0.05), the main variables were selected in RDA analysis.
2.3.1 Heavy metal pollution assessment method
The pollution index (PIi) is calculated using the following equation:
where Ci is the metal (i) content in soil or crop. Si is the reference value of heavy metal content in soils and vegetables. The Si in soils is chosen based on the soil environmental quality risk control standard for soil contamination of agricultural land (GB 15,618–2018) (MEPPRC 2018) or the background values of heavy metal in the YRD (Wei and Chen 1991). The Si in vegetables is chosen based on Food Safety National Standard (GB2762-2017) (MHPRC 2017) except for Cu and Zn which are based on Food Safety National Standard (GB15199-1994) (MHPRC 1994) and (GB13106-1991) (MHPRC 1991).
2.3.2 Mobility risk assessments
The Risk Assessment Code (RAC) of mobility and bioavailability of heavy metals in soil is defined as follows (Perin et al. 1985):
where Cwat, i, Cex, i, and Ccarb, i are the concentration of the water-soluble bound, exchangeable bound, and carbonate bound of element i, respectively; Ct, i is the total concentration of metal i. RACi is the RAC of metal i value. The risk level classifications of the RAC are shown in Table S3.
2.3.3 Transfer factor
TFavail is soil-to-plant transfer factors based on bioavailable (Cavail) concentrations of heavy metals in soils, which is defined as follows (Zhang et al. 2018):
where Cplant is the concentration of heavy metals in plants (in fresh weight) and Cavail is equivalent to the sum of the F1, F2, and F3 concentrations in soils.
3 Results
3.1 Heavy metal concentrations and fractions in soils
Soil heavy metal concentrations are shown in Table 1. The results revealed that heavy metal concentrations in IOS-soils were significantly higher than that in OFS-soils. The mean contents of Pb, Cd, Zn, and Cu in soils contaminated by IOS were 8.25, 2.01, 1.70, and 1.31 times higher than that of OFS-soil, respectively. Soil heavy metal concentrations were significantly increased by 1.04 ~ 19.16 times compared with soil background value (Wei and Chen 1991). The value of PIB was in an order of Cd > Pb > Zn > Cu in IOS-soils and Cd > Zn > Cu > Pb in OFS-soils. Based on the soil environmental quality risk control standard (GB 15,618–2018) (MEPPRC 2018), PIS-Cd, -Zn, and -Pb in IOS-soils increased by 101.2%, 70.4%, and 656% compared to OFS-soils, respectively.
Soil heavy metal contents and distributions in different sequential extraction fractions are shown in Table S4 and Fig. 2. The contents of F2-Cd, -Pb, and -Zn in IOS-soils were 7.40, 19.38, and 2.70 times higher than that in OFS-soils. On the contrary, F2-Cu in OFS-soils was 2.86 times higher than that in IOS-soils (Table S4). The percentage of F2-Cd in soils ranged from 6.24 to 34.17% in which F2-Cd in IOS-soils was 3.18 times higher than that in OFS-soils. In contrast, the percentage of F2-Cu (ranged from 0.20 to 3.95%) and F3-Zn (3.92 to 27.51%) in OFS-soils was significantly higher than that in IOS-soils (0.07 to 0.75% and 1.74 to 19.36%, respectively). The percentage of F4-Cd, -Cu, -Pb, and -Zn ranged from 9.98 to 41.99% in OFS-soils was 2.38, 2.15, 1.17, and 2.78 times higher than that (1.05 to 39.18%) in IOS-soils. The percentage of F5 and F7 ranged from 15.89 to 40.60% and 25.94 to 55.31%, respectively. The percentage of the water-soluble fraction (F1) was very low, ranging from 0.07 to 0.84%.
The Risk Assessment Code (RAC) is shown in Table 2. Compared to other metals, the mobility of Cd in soil was rather strong with RAC of 35.64% and 17.74% in IOS- and OFS-soils, respectively. The RACCd and RACPb in IOS-soils were 2.01 and 1.30 times higher than those in OFS-soils, while RACCu and RACZn were 1.25 and 1.38 times higher in OFS-soils than those in IOS-soils, respectively. Based on the levels of potential metal release risk by RAC values (Table S3), Cd and Pb in IOS-soils had high and medium mobility risk, respectively. The Cd and Zn in the OFS-soils had medium mobility risk, and the other heavy metals had low mobility risk. The RACCu in OFS-soils was significantly higher than that in IOS-soils though Cu had low mobility risk in sampling soils.
3.2 Transfer characteristics of heavy metals in soil-leafy vegetables
Heavy metal concentrations and pollution index (PI) of the edible part of vegetables are shown in Table 3. Compared with OFS, Cd and Pb concentration in vegetable samples from IOS increased 6.07 and 1.70 times, respectively. In contrast, Cu concentrations in the vegetable from OFS were 1.59 times higher than that from IOS. There was no significant difference in Zn concentration in the vegetable. The PI of metals in edible parts contaminated by IOS and OFS were all below 1 except for Cd and Pb from IOS which were 1.30 and 1.42, respectively.
TFavail of heavy metals is showed in Fig. 3. TFavail-Cd and -Cu were higher, and TFavail-Pb was the lowest. TFavail-Cd in IOS was 2.93 times those in OFS. In contrast, TFavail-Cu and-Zn in OFS was increased by 61% and 72%, respectively, compared with IOS. There were no differences for TFavail-Pb between IOS and OFS.
3.3 Major influential factors of heavy metal accumulation in leafy vegetables
To explore the major influential factors for heavy metal accumulation in vegetables contaminated by IOS and OFS, the concentration of heavy metal fractions (F1 to F7), soil pH, SOM, CEC, clay, silt, and sand was combined in RDA analysis (Fig. 4). Results of RDA showed that the interpretation of the two sorting axes reached 74.0%, 82.5%, 50.3%, and 67.4% in RDA-Cd, Pb, Cu, and Zn, respectively. The reserved variables could explain 61.7%, 46.7%, 43.2%, and 50.7% of the response variables which were shown in the first (horizontal axis) sorting axes in RDA-Cd, Pb, Cu, and Zn, respectively. The results presented that F3-Cd and F1-Cd were the main influencing factor for Cd uptake in IOS- and OFS-vegetables, respectively. The influential factors of Cu uptake were F5-Cu and F2-Cu in IOS- and OFS-vegetables, respectively. The F3-Zn and F2-Zn were the major influential factor of Zn accumulation in IOS- and OFS-vegetables, respectively. Interestingly, the Pb concentration of vegetables was a positive and negative correlation with F4 and F6, respectively, in OFS, and a positive correlation with F5 in IOS. A negative correlation was shown between OFS-CEC and OFS-Pb, OFS-pH and OFS-Zn, IOS-SOM, and IOS-Cd.
Contributions of heavy metal fractions and soil properties to metals accumulation of vegetables. IOS- or OFS-metal represents the metal content in the edible part of leafy vegetables contaminated by IOS or OFS. IOS- or OFS-FN represents the metal fraction in IOS- or OFS-soils. IOS- or OFS-properties represent the soil properties in IOS- or OFS-soils
4 Discussion
4.1 Heavy metals contamination in IOS and OFS soils
The results showed that heavy metal contents in soils were significantly increased by 1.31 ~ 8.25 times compared with the control soil value. This may be related to heavy metal inputs from industrial pollution sources. The average contents of Cd and Pb in the industrial sewage sludge samples were as high as 4.71 mg·kg−1 and 123.4 mg·kg−1 (Baran and Antonkiewicz 2017). The concentration of Cd and Pb in vegetable soils contaminated by mining activity in the Dabaoshan mine was 4.9 to 9.8 and 1.4 to 1.9 times higher than China’s environmental quality standard (Liao et al. 2016). Furthermore, our results demonstrated that the Cd contents in OFS-soils were all exceeded the reference value. Li et al. (2018a) showed that soil Cd concentrations were significantly increased by 2.75 times under organic fertilizer application for more than 10 years. Excessive Cd in feeds can be excreted from the animal’s body with the livestock manure (Wang et al. 2013). In addition, our results revealed that Cu and Zn in OFS-soils showed also significant accumulation with a relatively low exceeding rate compared to IOS-soils. Four years of pig manure application resulted in the increase of Cu and Zn in soil by 61.55% and 86.38% (Qian et al. 2018). This was mainly due to the high concentration of Cu and Zn in manure caused by the abuse of those elements as a feed additive in the livestock industry (Qian et al. 2016). Huang et al. (2017) found that the average contents of Cu and Zn in the commercial chicken manure samples were as high as 143 mg·kg−1 and 331 mg·kg−1.
4.2 Heavy metals accumulation in vegetables
The speciation of soil heavy metals varies with the source of pollution. Results indicated that the higher percentage of F2-Cd and F3-Cd in soil induced high mobility risk compared to other elements, and the risk of IOS is relatively high (Fig. 2, Table 2). It has been reported that the percentage of the exchangeable fraction of Cd in industrial areas could be up to 30% (Meng et al. 2019). The bioavailable fractionation of Cd in soil was over 30% in IOS-soils in Huaibei industrial areas (Cheng et al. 2018). Wu et al. (2012) also demonstrated that the percentage of the acid extractable fraction of Cd reached 12.1–16.2% in agriculture soils with pig manure application for 17 years. This could be related to the higher content of bioavailable Cd present in industrial and agricultural waste. The previous study has shown that the percentage of the bioavailable fraction of Cd in industry solid wastes was about 40–50% (Moturi et al. 2004). Irrigation water polluted by IOS was an important source of Cd at the rate of input flux 8.42 g/hg/y (Hou et al. 2014). Moreover, Zeng et al. (2018) found that the percentage of the acid-soluble fraction of Cd in livestock manure was about 26.2–29.0%. However, the mobility risk of Cu and Zn in OFS-soils was significantly higher than that in IOS-soils (Fig. 2, Table 2). It had been reported that the percentage and content of the bioavailable Cu and Zn fraction increased in soil with organic fertilizer application (Qian et al. 2016). The contents of exchangeable and adsorbed fractions of Cu and Zn in soil with pig slurry application for 11 years were almost 6.5 and 30 times higher than that of control treatment, respectively, and the proportions of available Cu and Zn fraction increased by 4.9% and 10.7%, respectively (Formentini et al. 2015). Zeng et al. (2018) showed that the percentage of the acid-soluble fraction of Cu and Zn in livestock manure was 31.2% and 27.5%, respectively. Compared with soils without livestock manure application (only 5% and 10%), the percentage of the acid-soluble fraction of Cu and Zn in livestock manure was up to 31.2% and 27.5%, respectively (Xiao et al. 2017; Zeng et al. 2018). In addition, the decomposition of livestock manure could also increase the contents of DOMs and low-molecular-weight organic acids, which could increase the contents of F1 and F2 fractions of Cd, Cu, and Zn, and consequently promote the accumulation of Cd, Cu, and Zn by vegetables (Almaroai et al. 2012). DOM can form complexes with metals, especially with Cu and Zn, to increase their solubility and improve their bioavailability (Li et al. 2013; Michel et al. 2007).
Furthermore, the percentage of humic acid- and organic matter-bound fractions for heavy metals in OFS-soils was higher than that of IOS. Since SOM is a heavy metal sink in soils, it is an environment that can immobilize heavy metals in stable organomineral formations (Shaheen and Rinklebe. 2015). Taghipour and Jalali (2020) indicated that the contents and fractionations of Cd, Pb, Cu, and Zn increased in organic-bound fractions after the addition of organic wastes. Decomposition of livestock manure by microbial activity could increase the contents of soil humic substances and organic matter and improve the formation of F4- and F6-metals (Shan et al. 2016). Higher organic matter in OFS-soil could immobilize the Cd by transformation or adsorption and thus reduced the Cd mobility risk (Wan et al. 2020). For Cu and Zn, the content and percentage of residual bound fraction for heavy metals in IOS-soils were higher than that of OFS. The Cu and Zn in IOS-soils were mainly combined with iron-bearing minerals and were likely to enter the mineral lattice through lattice replacement, which were not absorbed by plants (Mamindy-Pajany et al. 2014; Yang et al. 2011).
Based on the GB2762-2017, Cd and Pb concentrations in vegetable samples from IOS were 1.30 and 1.42 times compared with the food safety standards, respectively (Table 3). A previous study proved that 75% and 47% of leafy vegetables exceeded the Cd and Pb standards in IOS-soils, respectively (Li et al. 2018c). The concentrations of Pb and Zn were significantly higher than the food safety standards in a part of the OFS-leafy vegetables, respectively (Table 3). The Zn content exceeded the local food safety standards in lettuce using organic fertilizer (Tandi et al. 2004). Furthermore, the TFavail-Cd was significantly higher in IOS than that in OFS, but the TFavail-Cu and TFavail-Zn were significantly higher in OFS than in IOS (Fig. 3). These results showed that Cd had relatively higher transfer ability in IOS-systems, while Cu and Zn in OFS-systems. Since IOS and OFS could increase the bioavailable fraction content and distribution of Cd, Cu, and Zn, they determine the heavy metal transfer in the soil–plant system (Cheng et al. 2018; Formentini et al. 2015; Meng et al. 2019; Qian et al. 2016). These outcomes were consistent with the previous results of this study (Fig. 2, Table 2).
The RDA results demonstrated that the major influential factors for heavy metal uptake in vegetables changed with pollution sources and soil basic properties (Fig. 4). Overall, the content of available heavy metal (F1, F2, and F3) was the dominant influential factors for vegetable uptake. The bioavailable fraction of heavy metals in soil was a better index to assess the transfer capacity of heavy metals in soil-crop systems (Khan et al. 2008). Plants could directly absorb the water-soluble, exchangeable, and carbonate-bound fractions of metals in soils, because they were labile and easy to transfer in the soil–plant system (Haider et al. 2021; Zhao et al. 2011). Absorption of Cd and Zn in edible parts of leafy vegetables was mainly determined by F2-Cd and F2-Zn in soil, since they showed a significantly positive correlation (Liao et al. 2016). Zhang et al. (2018) also found the contents of Cu and Pb in edible parts of lettuce were mainly controlled by the bioavailable fraction (the sum of F1, F2, and F3) of Cu and Pb in soils. F4-Pb and F6-Pb showed a promoting and inhibiting effect on Pb accumulation in vegetables from OFS, respectively. F4 and F6 were part of the metals combined with humic acid and higher molecular weight organic matter, respectively (Zhao et al. 2011). Humic acid could be decomposed and utilized by microorganisms because of its smaller molecular size (Plaza et al. 2005), which led to humic acid-bound heavy metals entering soil solution, and then had the potential bioavailability (Tang et al. 2015). The organic matter with a larger molecular size could form a stable complex or chelate with heavy metals and reduce their bioavailability (Warne et al. 2008). A previous study found that Cd and Zn contents of edible parts of leafy vegetables had a negative correlation with F6-Cd and F6-Zn in soils, respectively (Fan et al. 2017). Furthermore, F5-Pb and Cu were the main controlling factors to promote the absorption of lead and cadmium in vegetables from IOS (Fig. 4). Heavy metal bound by Fe–Mn oxides was considered to be the potential source of metal released, which could be uptaken by plants in acidic environments, even though they usually form coprecipitate (Zhao et al. 2011). Previous studies also observed that the fraction bound to Fe–Mn oxides of metals could become bioavailable because these metals could be released from Fe–Mn oxides under certain circumstances (Agrawal et al. 2016). There was a positive correlation between the Pb contents of edible parts of lettuces and F5-Pb in soils (Zhang et al. 2018). Fan et al. (2017) also revealed that Cu and Pb contents of edible parts of leafy vegetables had a positive correlation with F5-Cu and F5-Pb in soils, respectively.
Furthermore, the IOS-SOM, OFS-CEC, and OFS-pH could have certain disincentive effects on the absorption of Cd, Pb, and Zn by vegetables, respectively. At low pH, the binding competition of protons with the free positively charged metal ions (such as zinc) to the soil particles increases, leading to higher metal concentrations in pore water (Yu et al. 2016). Soil with higher organic matter content (SOM) and high cation exchange capacity (CEC) can improve the adsorption or chelation capacity of heavy metals and therefore decrease plant uptake (Laborda et al. 2008). The content of Cd and Pb in crops decreased significantly with the increase of SOM or CEC content in soils (Zheng et al. 2020). Li et al. (2018b) demonstrated that there was a negative correlation between the SOM content and the Cd content in edible parts of vegetables because SOM antagonized the Cd availability for plants’ absorption through adsorption and formation of stable organomineral formations. It had been found that humic substances could immobilize Cd through the formation of complexes with Cd ions (Yu et al. 2018). Due to the alkalizing effect and high CEC content of organic fertilizer, soil pH and CEC would increase with the addition of organic fertilizer, and high soil pH and CEC could reduce the absorption of metal elements by vegetables (Qian et al. 2018; Yu et al. 2016).
4.3 Ecological risk of heavy metal pollution in IOS and OFS systems
In this study, PI and RAC were used to evaluate the ecological risk of heavy metal pollution. The pollution degree of heavy metals in IOS-systems was significantly higher than that in OFS-systems, especially Cd and Pb (Tables 1 and 3). The pollution duration by IOS was completely longer than that by OFS (Bai et al. 2015; Guo et al. 2018; Li et al. 2018c; Liao et al. 2016). This led to much higher heavy metal inputs in IOS-soils than that in OFS-soils. Results revealed that Cd and Pb in IOS-soils had extremely higher metal release risk potential than that in OFS-soils, respectively (Table 2). The Cd and Pb in industrial wastes mainly existed in available fractions (Baran and Antonkiewicz 2017; Moturi et al. 2004). However, the soil organic matter inputs with OFS could improve adsorption or chelation capacity of heavy metals and therefore decrease metal release and plant uptake, especially of highly toxic metals such as Cd and Pb, and alleviate the ecological risk of heavy metal pollution to a certain extent (Hao et al. 2007; Wan et al. 2020). Thus, the ecological risk of heavy metal pollution caused by IOS was much higher than that caused by OFS.
5 Conclusion
This study provided the analysis of new data based on the comparison of the pollution characteristics of heavy metals contaminated by IOS and OFS in the YRD. The Cd was the main metal pollutant in both IOS- and OFS-soils. IOS-soils were more severely contaminated by Cd, Cu, Pb, and Zn. The source of heavy metals is one of the major factors for the fraction distribution of heavy metals in soils. Heavy metal concentrations in vegetables were collectively determined by the fractions of soil heavy metals and soil properties, but they were mainly determined by the fractions of soil heavy metals, which could be further characterized by the pollution sources. For the mobility and transfer of heavy metals in the soil-vegetable systems, the source of heavy metals is one of the key factors. In general, IOS caused the severe ecological risk of heavy metal pollution to the soil-vegetable systems, as compared to OFS.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Agrawal R, Kumar B, Priyanka K, Narayan C, Shukla K, Sarkar J (2016) Micronutrient fractionation in coal mine-affected agricultural soils. India Bull Environ Contam Toxicol 96(4):449–457. https://doi.org/10.1007/s00128-016-1745-3
Almaroai YA, Usman AR, Ahmad M, Kim K, Moon DH, Lee SS, Ok YS (2012) Effects of synthetic chelators and low-molecular-weight organic acids on chromium, copper, and arsenic uptake and translocation in maize (Zea mays L.). Soil Sci 177(11):655–663. https://doi.org/10.1097/SS.0b013e31827ba23f
Bai LY, Zeng XB, Su SM, Duan R, Wang YN, Gao X (2015) Heavy metal accumulation and source analysis in greenhouse soils of Wuwei District, Gansu Province, China. Environ Sci Pollut Res 22:5359–5369. https://doi.org/10.1007/s11356-014-3763-1
Baran A, Antonkiewicz J (2017) Phytotoxicity and extractability of heavy metals from industrial wastes. Environ Prot Eng 43(2):143–155. https://doi.org/10.5277/epe170212
Bouyoucos GJ (1951) A recalibration of the hydrometer method for making mechanical analysis of soils 1. Agron J 43(9):434–438
Cheng SW, Liu GJ, Zhou CC, Sun RY (2018) Chemical speciation and risk assessment of cadmium in soils around a typical coal mining area of China. Ecotox Environ Safe 160:67–74. https://doi.org/10.1016/j.ecoenv.2018.05.022
CGS (2005) Geological survey technical standard. China Geological Survey
Cui LQ, Li LQ, Zhang AF, Pan GX, Bao DD, Chang A (2011) Biochar amendment greatly reduces rice Cd uptake in a contaminated paddy soil: a two-year field experiment. BioResources 6(3):2605–2618
Dong B, Zhang RZ, Gan YD, Cai LQ, Freidenreich A, Wang KP, Guo TW, Wang HB (2019) Multiple methods for the identification of heavy metal sources in cropland soils from a resource-based region. Sci Total Environ 651:3127–3138. https://doi.org/10.1016/j.scitotenv.2018.10.130
Fan Y, Li H, Xue ZJ, Zhang Q, Cheng FQ (2017) Accumulation characteristics and potential risk of heavy metals in soil-vegetable system under greenhouse cultivation condition in Northern China. Ecol Eng 102:367–373. https://doi.org/10.1016/j.ecoleng.2017.02.032
Formentini TA, Mallmann FJK, Pinheiro A, Fernandes CVS, Bender MA, Da Veiga M, Dos Santos DR, Doelsch E (2015) Copper and zinc accumulation and fractionation in a clayey Hapludox soil subject to long-term pig slurry application. Sci Total Environ 536:831–839. https://doi.org/10.1016/j.scitotenv.2015.07.110
Guo T, Lou CL, Zhai WW, Tang XJ, Hashmi MZ, Murtaza R, Li Y, Liu XM, Xu JM (2018) Increased occurrence of heavy metals, antibiotics and resistance genes in surface soil after long-term application of manure. Sci Total Environ 635:995–1003. https://doi.org/10.1016/j.scitotenv.2018.04.194
Haider FU, Liqun C, Coulter JA, Cheema SA, Wu J, Zhang R, Wenjun M, Farooq M (2021) Cadmium toxicity in plants: impacts and remediation strategies. Ecotox Enivron Safe 211:111887. https://doi.org/10.1016/j.ecoenv.2020.111887
Hao XZ, Zhou DM, Huang DQ, Zhang HL, Wang YJ (2007) The growth and Cu and Zn uptake of pakchois (Brassica chinesis L.) in an acidic soil as affected by chicken or pig manure. J Environ Sci Heal B 42(8): 905–912. https://doi.org/10.1080/03601230701623571
Hou QY, Yang ZF, Ji JF, Yu T, Chen GG, Li J, Xia XQ, Zhang M, Yuan XY (2014) Annual net input fluxes of heavy metals of the agro-ecosystem in the Yangtze River delta, China. J Geochem Explor 139:68–84. https://doi.org/10.1016/j.gexplo.2013.08.007
Huang SW, Tang JW, Li CH (2017) Status of heavy metals, nutrients, and total salts in commercial organic fertilizers and organic wastes in China(in Chinese). J Plant Nutr Fertil 23(1):162–173
Khan S, Cao Q, Zheng YM, Huang YZ, Zhu YG (2008) Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing. China Environ Pollut 152(3):686–692. https://doi.org/10.1016/j.envpol.2007.06.056
Laborda F, Bolea E, Gorriz MP, Martin-Ruiz MP, Ruiz-Begueria S, Castillo JR (2008) A speciation methodology to study the contributions of humic-like and fulvic-like acids to the mobilization of metals from compost using size exclusion chromatography-ultraviolet absorption-inductively coupled plasma mass spectrometry and deconvolution analysis. Anal Chim Acta 606:1–8. https://doi.org/10.1016/j.aca.2007.10.048
Li J, Zhu L, Tong LH, Lv YZ, Li J (2018a) Risk assessment of heavy metals accumulation in soils under long-term greenhouse vegetable cultivation conditions(in Chinese). Journal of Agro-Environment Science 37(05):2159–2165
Li T, Tao Q, Liang C, Shohag MJI, Yang X, Sparks DL (2013) Complexation with dissolved organic matter and mobility control of heavy metals in the rhizosphere of hyperaccumulator Sedum alfredii. Environ Pollut 182:248–255. https://doi.org/10.1016/j.envpol.2013.07.025
Li G, Khan S, Ibrahim M, Sun T, Tang J, Cotner JB, Xu Y (2018b) Biochars induced modification of dissolved organic matter (DOM) in soil and its impact on mobility and bioaccumulation of arsenic and cadmium. J Hazard Mater 348:100–108. https://doi.org/10.1016/j.jhazmat.2018.01.031
Li XY, Li ZG, Lin CJ, Bi XY, Liu JL, Feng X, Zhang H, Chen J, Wu TT (2018c) Health risks of heavy metal exposure through vegetable consumption near a large-scale Pb/Zn smelter in central China. Ecotox Environ Safe 161:99–110. https://doi.org/10.1016/j.ecoenv.2018.05.080
Liao JB, Wen ZW, Ru X, Chen JD, Wu HZ, Wei CH (2016) Distribution and migration of heavy metals in soil and crops affected by acid mine drainage: Public health implications in Guangdong Province, China. Ecotox Environ Safe 124:460–469. https://doi.org/10.1016/j.ecoenv.2015.11.023
Lu RK (2000) Analytical methods for soil and agrochemistry. Agricultural Science and Technology Press, Beijing
Mamindy-Pajany Y, Sayen S, Mosselmans JFW, Guillon E (2014) Copper, nickel and zinc speciation in a biosolid-amended soil: pH adsorption edge, μ-XRF and μ-XANES investigations. Environ Sci Technol 48:7237–7244. https://doi.org/10.1021/es5005522
Meng L, Huang TH, Shi JC, Chen J, Zhong FL, Wu LS, Xu JM (2019) Decreasing cadmium uptake of rice (Oryza sativa L.) in the cadmium-contaminated paddy field through different cultivars coupling with appropriate soil amendments. J Soil Sediment 19(4):1788–1798. https://doi.org/10.1007/s11368-018-2186-x
MEPPRC (2018) Soil environmental quality: risk control standard for soil contamination of agricultural land, GB15618, Beijing
MHPRC (1991) Maximum levels of contaminants in food, GB13106, Beijing
MHPRC (1994) Maximum levels of contaminants in food, GB15199, Beijing
MHPRC (2017) Maximum levels of contaminants in food, GB2762, Beijing
Michel K, Roose M, Ludwig B (2007) Comparison of different approaches for modelling heavy metal transport in acidic soils. Geoderma 140:207–214. https://doi.org/10.1016/j.geoderma.2007.04.005
Mohamed I, Ahamadou B, Li M, Gong CX, Cai P, Liang W, Huang QY (2010) Fractionation of copper and cadmium and their binding with soil organic matter in a contaminated soil amended with organic materials. J Soil Sediment 10(6):973–982. https://doi.org/10.1007/s11368-010-0199-1
Moturi MCZ, Rawat M, Subramanian V (2004) Distribution and fractionation of heavy metals in solid waste from selected sites in the industrial belt of Delhi, India. Environ Monit Assess 95:183–199. https://doi.org/10.1023/B:EMAS.0000029900.86810.85
Perin G, Craboledda L, Lucchese M, Cirillo R, Dotta L, Zanette ML, Orio AA (1985) Heavy metal speciation in the sediments of northern Adriatic Sea. A new approach for environmental toxicity determination. Heavy metals in the environment 2(1):454–456
Phillips RP, Fahey TJ (2006) Tree species and mycorrhizal associations influence the magnitude of rhizosphere effects. Ecology 87:1302–1313. https://doi.org/10.1890/0012-9658(2006)87[1302:TSAMAI]2.0.CO;2
Plaza C, Senesi N, García-Gil JC, Polo A (2005) Copper(II) complexation by humic and fulvic acids from pig slurry and amended and non-amended soils. Chemosphere 61:711–716. https://doi.org/10.1016/j.chemosphere.2005.03.046
Qian M, Wu H, Wang J, Zhang H, Zhang Z, Zhang Y, Lin H, Ma J (2016) Occurrence of trace elements and antibiotics in manure-based fertilizers from the Zhejiang Province of China. Sci Total Environ 559:174–181. https://doi.org/10.1016/j.scitotenv.2016.03.123
Qian XY, Wang ZQ, Shen GX, Chen XH, Tang ZZ, Guo CX, Gu HR, Fu K (2018) Heavy metals accumulation in soil after 4 years of continuous land application of swine manure: a field-scale monitoring and modeling estimation. Chemosphere 210:1029–1034. https://doi.org/10.1016/j.chemosphere.2018.07.107
Shaheen SM, Rinklebe J (2015) Impact of emerging and low cost alternative amendments on the (im)mobilization and phytoavailability of Cd and Pb in a contaminated floodplain soil. Ecol Eng 74:319–326. https://doi.org/10.1016/j.ecoleng.2014.10.024
Shan H, Su SM, Liu RL, Li ST (2016) Cadmium availability and uptake by radish (Raphanus sativus) grown in soils applied with wheat straw or composted pig manure. Environ Sci Pollut R 23(15):15208–15217. https://doi.org/10.1007/s11356-016-6464-0
Taghipour M, Jalali M (2020) Effects of some industrial and organic wastes application on growth and heavy metal uptake by tomato (Lycopersicum esculentum) grown in a greenhouse condition. Environ Sci Pollut R 27(5):5353–5366. https://doi.org/10.1007/s11356-019-07017-6
Tandi NK, Nyamangara J, Bangira C (2004) Environmental and potential health effects of growing leafy vegetables on soil irrigated using sewage sludge and effluent: a case of Zn and Cu. J Environ Sci Heal B 39(3):461–471. https://doi.org/10.1081/PFC-120035930
Tang X, Li X, Liu X, Hashmi MZ, Xu J, Brookes PC (2015) Effects of inorganic and organic amendments on the uptake of lead and trace elements by Brassica chinensis grown in an acidic red soil. Chemosphere 119:177–183. https://doi.org/10.1016/j.chemosphere.2014.05.081
Wan YN, Huang QQ, Wang Q, Yu Y, Su DC, Qiao YH, Li HF (2020) Accumulation and bioavailability of heavy metals in an acid soil and their uptake by paddy rice under continuous application of chicken and swine manure. J Hazard Mater 384:121293. https://doi.org/10.1016/j.jhazmat.2019.121293
Wang H, Dong YH, Yang YY, Toor GS, Zhang XM (2013) Changes in heavy metal contents in animal feeds and manures in an intensive animal production region of China. J Environ Sci-China 25(12):2435–2442. https://doi.org/10.1016/S1001-0742(13)60473-8
Warne MSJ, Heemsbergen D, Mclaughlin M, Bell M, Broos K, Whatmuff M, Barry G, Nash D, Pritchard D, Penney N (2008) Models for the field-based toxicity of copper and zinc salts to wheat in 11 Australian soils and comparison to laboratory-based models. Environ Pollut 156:707–714. https://doi.org/10.1016/j.envpol.2008.06.012
Wei FS, Chen JS (1991) Study on the background value of soil environment in China(in Chinese). Environmental Science 4:12–19
Wu LH, Tan CY, Liu L, Zhu P, Peng C, Luo YM, Christie P (2012) Cadmium bioavailability in surface soils receiving long-term applications of inorganic fertilizers and pig manure. Geoderma 173:224–230. https://doi.org/10.1016/j.geoderma.2011.12.003
Xiao L, Guan D, Peart MR, Chen Y, Li Q (2017) The respective effects of soil heavy metal fractions by sequential extraction procedure and soil properties on the accumulation of heavy metals in rice grains and brassicas. Environ Sci Pollut R 24:2558–2571. https://doi.org/10.1007/s11356-016-8028-8
Yang JJ, Regier T, Dynes JJ, Wang J, Shi JY, Peak D, Zhao YD, Hu TD, Chen YX, Tse JS (2011) Soft X-ray induced photoreduction of organic Cu(II) compounds probed by X-ray absorption near-edge (XANES) spectroscopy. Anal Chem 83(20):7856–7862. https://doi.org/10.1021/ac201622g
Yi KX, Fan W, Chen JY, Jiang SH, Huang SJ, Peng L, Zeng QR, Luo S (2018) Annual input and output fluxes of heavy metals to paddy fields in four types of contaminated areas in Hunan Province, China. Sci Total Environ 634:67–76. https://doi.org/10.1016/j.scitotenv.2018.03.294
Yu HY, Liu CP, Zhu JS, Li FB, Deng DM, Wang Q, Liu CS (2016) Cadmium availability in rice paddy fields from a mining area: the effects of soil properties highlighting iron fractions and pH value. Environ Pollut 209:38–45. https://doi.org/10.1016/j.envpol.2015.11.021
Yu HY, Chang CY, Li FB, Wang Q, Chen M, Zhang J (2018) Thallium in flowering cabbage and lettuce: Potential health risks for local residents of the Pearl River Delta, South China. Environ Pollut 241:626-635. https://doi.org/10.1016/j.envpol.2018.05.090
Zeng FR, Ali S, Zhang HT, Ouyang YB, Qiu BY, Wu FB, Zhang GP (2011) The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ Pollut 159:84–91. https://doi.org/10.1016/j.envpol.2010.09.019
Zeng XY, Xiao ZH, Zhang GL, Wang AD, Li ZH, Liu YH, Wang H, Zeng QR, Liang YS, Zou DS (2018) Speciation and bioavailability of heavy metals in pyrolytic biochar of swine and goat manures. J Anal Appl Pyrol 132:82–93. https://doi.org/10.1016/j.jaap.2018.03.012
Zhang JR, Li HZ, Zhou YZ, Dou L, Cai LM, Mo LP, You J (2018) Bioavailability and soil-to-crop transfer of heavy metals in farmland soils: a case study in the Pearl River Delta, South China. Environ Pollut 235:710–719. https://doi.org/10.1016/j.envpol.2017.12.106
Zhao KL, Liu XM, Zhang WW, Xu JM, Wang F (2011) Spatial dependence and bioavailability of metal fractions in paddy fields on metal concentrations in rice grain at a regional scale. J Soil Sediment 11(7):1165. https://doi.org/10.1007/s11368-011-0408-6
Zheng SN, Wang Q, Yu HY, Huang XZ, Li FB (2020) Interactive effects of multiple heavy metal(loid)s on their bioavailability in cocontaminated paddy soils in a large region. Sci Total Environ 708:135126. https://doi.org/10.1016/j.scitotenv.2019.135126
Acknowledgements
The authors greatly thank all the people for giving help to this research.
Funding
This study was financially supported by the National Key Research and Development Program of China (2016YFD0800306) and Science and Technology Program of Jiangsu (BE2018679).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by HW, FS, HD, TS, HL, JW, and XH. The first draft of the manuscript was written by HW. The final manuscript was critically revised by LL, MD, SJ, CD, and GP. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
This manuscript is new and not being considered elsewhere. All authors have approved the submission of this manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible editor: Kitae Baek
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, H., Sui, F., Duan, H. et al. Comparison of heavy metal speciation, transfer and their key influential factors in vegetable soils contaminated from industrial operation and organic fertilization. J Soils Sediments 22, 1735–1745 (2022). https://doi.org/10.1007/s11368-022-03187-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-022-03187-y