Abstract
Purpose
No-till (NT) and fertilization are common land management practices in agricultural production systems to increase soil quality and crop yield. No-till can be reversed to tillage (termed tillage reversal, TR, in this paper) due to changes in management objectives.
Materials and methods
The impact of NT, TR, and TR plus nitrogen (N) fertilization (TRN) treatments on the composition and structure of bacterial communities in a Gray Luvisol was studied in west-central Alberta, Canada.
Results and discussion
The structure of bacterial communities was not affected by the TR treatment (compared with NT). The TRN treatment increased the relative abundance of some bacterial taxa groups, e.g., Gemmatimonadetes, Thermoleophilia and Solibacteres, that have chemolithotrophic nitrifying functions as compared with the TR treatment. The decreased relative abundance of some bacterial taxa groups, such as Alphaproteobacteria, Deltaproteobacteria, Spartobacteria, and Planctomycetia that have denitrifying functions, would change the soil’s denitrification function in the TRN as compared to the TR treatment. There were more dominant bacterial taxa groups, and the bacterial community had greater inter-annual variations in the TRN than in the NT and TR treatments. Moreover, the function of bacterial communities was affected by the TRN as compared to the NT and TR treatments, based on the predicted metagenomes.
Conclusions
We conclude that when TR was applied to the soil with long-term N fertilization, which eliminates N limitation, altered soil bacterial community structure and function over TR applied to the studied Gray Luvisol without long-term N fertilization. Findings from our study have important implications for improving land management practices through tillage and N fertilization to enhance the soil’s function and quality in agroecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Microbes play critical roles in driving the biogeochemical cycling of carbon (C) and other nutrients, affecting plant growth and greenhouse gas emissions in various ecosystems (Ran et al. 2018). In agroecosystems, management practices such as tillage can markedly change soil physicochemical properties (Bogunovic et al. 2018) and, therefore, affect microbial community diversity and function (Bissett et al. 2013; Navarro-Noya et al. 2013; Praeg et al. 2020). The tillage regime may alter the structure, composition, and diversity of soil microbes due to its influence on the availability and pool size of organic C and nutrients in the soil (Nemergut et al. 2008). The increasing need for sustainable agriculture led to the widespread adoption of management practices that have a low frequency and level of disturbance, such as no-till (NT), which can improve soil quality and crop yield; increase the storage of nutrients, moisture, and organic C in soils; and reduce greenhouse gas emissions (Sengupta and Dick 2015; Banerjee et al. 2019). No-till or reduced tillage has commonly been shown to increase the biomass, size, and diversity of microbial populations (Schmidt et al. 2018; Wang et al. 2020). In a Lexington silt loam soil in west Tennessee, NT has also been shown to increase the abundance of Gram-positive bacterial, actinomycetic, and mycorrhizal fungal fatty acid methyl ester (FAME) biomarkers and decrease saprophytic fungal FAME (Mbuthia et al. 2015). No-till was found to significantly affect the relative abundance of several phyla of both Gram-positive and Gram-negative bacteria through 16S rRNA sequencing (Miura et al. 2013; Loaiza et al. 2019).
Long-term NT management, however, leads to the accumulation of crop residues and pesticides, reduction of water infiltration rate, and stratification of nutrients on the soil surface (Mbuthia et al. 2015). Intermittently reversing of NT to conventional tillage (in this paper this practice is termed tillage reversal, TR) provides a means to resolve some of the problems, which at the same time disrupts soil aggregation and exposes labile organic C to microbial decomposition (Xun et al. 2016). A one-time tillage event in the early or late growing season has been shown to affect bacterial biomass (Schmidt et al. 2018). However, the one-time tillage of NT plots had no long-term effect on soil microbial biomass (Vries et al. 2015). Moreover, occasional tillage did not affect microbial enzyme activity, the soil’s capacity to utilize different C sources, or genetic fingerprinting (Rincon-Florez et al. 2016). Since the effect of TR on long-term NT plots has been little studied, how does TR affect soil microbial communities is poorly understood. In addition, the community profiling results using fatty acid methyl ester analysis, or FAMEs (Wortmann et al. 2010), and terminal restriction fragment analysis (T-RFLP; Rincon-Florez et al. 2016) do not provide details of the composition of microbial communities, which can be investigated using high-throughput sequencing.
As a key management practice in agricultural ecosystems, nitrogen (N) fertilization usually affects microbial communities are structured and how they function (Xun et al. 2016; Li et al. 2018). Nitrogen fertilization has been shown to increase plant productivity, with corresponding changes in organic matter input into soils and in microbial community composition (Wang et al. 2016). Microbial taxa, such as nitrifiers, denitrifiers, and N-fixers that are involved in N biogeochemical cycles, often respond to N fertilization (Dai et al. 2018). Nevertheless, N fertilization has been shown to have a positive, a negative, or no effect on both the structure and function of microbial communities that are affected by factors such as the fertilizer type applied, the soil type studied, or the crop species planted (Xun et al. 2016).
Luvisolic soils, covering about 809,000 km2, which is about 8.8% of terrestrial land area in Canada, are one of the three main forest soil Orders in Canada (Lavkulich and Arocena 2011). To increase agricultural production, some of the Luvisolic soils have been cultivated, and NT has been implemented as a long-term management practice (Lavkulich and Arocena 2011). Our previous research has studied how TR and N fertilization affect soil organic C and N storage, water-extractable organic C and N content and their quality (Sun et al. 2015), and formation of soil aggregates and C contained in aggregates in a Gray Luvisol (Sun et al. 2020). The objective of this study was to assess the responses of soil bacterial communities to TR, and TR plus N fertilization (TRN) after long-term NT management on a Gray Luvisolic soil at the Breton Plots, managed by the University of Alberta. In this study, 16S rRNA genes were sequenced to characterize the bacterial community and to predict metagenomes with a bioinformatic approach. In this study, we tested the following two hypotheses: (1) the TR treatment would reduce the abundance and alter the structure of the bacteria community as compared to the NT treatment, as the TR treatment reduces soil water-extractable organic C (WEOC), which provides a measure of organic C availability to microbes, in the studied plots (Sun et al. 2015); and (2) the TRN treatment would change the abundance of some specific bacterial groups and the soil bacterial community in terms of their structure and function as compared to the TR and NT treatments, because the increased N and C availability would influence soil bacterial community (Sun et al. 2020). While many studies have researched the effect of tillage (less so on TR) and N fertilization on the structure and function of bacterial communities in agroecosystems using various microbiological techniques (Bissett et al. 2013; Dai et al. 2018; Mbuthia et al. 2015), but few have studied the effect of TR plus N fertilization.
2 Materials and methods
2.1 Site description and soil characterization
The Breton Plots that we sampled were located near the township of Breton in west-central Alberta (53.07° N, 114.28° W; 830 masl). A detailed description of the long-term weather condition can be found in Izaurralde et al. (2001). The soil at the study site was an Orthic Gray Luvisol (Soi1 Classification Working Group 1998) or a Typic Cryobralf (Soil Survey Staff 1999).
The experiment was set up in 1979 (Nyborg et al. 1995). Barley (Hordeum vulgare L.) was monoculturally cropped from 1983 to 1996. Thereafter, spring wheat (Triticum aestivum L.), canola (Brassica napus L.), triticale (x Triticosecale Wittmack), or pea (Pisum sativum L.) were used as part of the crop rotation. Those long-term plots included NT without N fertilization and NT with N fertilization (at 100 kg N ha−1 year−1 as urea). On June 4, 2010, the TR treatment was tilled each spring prior to seeding using a rotary tiller to a depth of about 8 cm, resulting in the following treatments: NT without N fertilization (NT), NT without N fertilization converted to tillage without N (TR), and NT with N fertilization converted to tillage (TRN). In NT and TRN plots, urea was mid-row banded. Each long-term plot had a size of 6.85 × 1.37 m. Each treatment had four replications. At seeding time, phosphorus was applied at 20 kg P ha−1 (Sun et al. 2015; 2020).
We collected soil samples from the NT, TR, and TRN treatments in June 2012 and June 2013 when barley was planted. We used a soil corer with a 3 cm diameter to collect soil samples. On each date of soil sampling, seven cores were collected from the 0 to 10 cm depth to form a composite sample per plot (Sun et al. 2015; 2020). After removing large roots and crop residue, the soils were stored at − 20 °C in preparation for DNA extraction. The soil had pH, total organic C, total N, C:N ratio, and clay content of 6.60, 13.8 g kg−1, 1.2 g kg−1, 11.5, and 22.0%, respectively; other basic soil properties of different treatment (including soil pH, total organic C, total N, mean weight diameter of aggregates, sand-free water-stable aggregates, and aggregate-associated carbon concentrations in soil) can be found in our previous publications (Sun et al. 2015; 2020).
2.2 DNA analysis
We used the FastDNA SPIN kit for soil manufactured by MP Biomedicals (Solon, OH, USA) for metagenomic DNA extraction. We used NanoDrop and Qubit to check and confirm the quality and purity of the isolated metagenomic DNA (Michele et al. 2013).
We used a 2-PCR method to construct the library (Ma et al. 2018a). A region of the 16 s rRNA gene was amplified by PCR1 (locus-specific amplification) using tagged primers Glenn-F515 and trp1-R806 and the PCR2 (Barcode and Ion Torrent specific adaptor attachment) (Ma et al. 2018a). An Ion PGM400 Sequencing Kit and an Ion316 Chip on an Ion Torrent Personal Genome Machine (PGM) System (Life Technologies) were used to sequence the DNA samples.
After sequencing, the individual sequence reads were filtered to remove low quality and polyclonal sequences. Sequences matching the PGM 3′adaptor were also automatically trimmed. All PGM quality-filtered data were exported as fastq files and subsequently analyzed using open-reference operational taxonomic units (OTUs) pickup strategy in the QIIME pipeline (Ma et al. 2018a). Sequence counts were adjusted based on 16S copy numbers in rrnDB (Stoddard et al. 2015) and were normalized with the negative nominal model (McMurdie and Holmes 2014), which minimized bias associated with sequencing coverage and allowed for comparison of results for all samples. The sequences were deposited in SRA within the study with accession number PRJNA325652.
2.3 Bipartite network
To examine the relationship between dominant microbial OTUs and TR, TRN, or the inter-annual variation of microbial populations, a bipartite network analysis was conducted using the maximal information coefficient in the minerva package in R. The bipartite network analysis was conducted to visualize the associations between OTUs and the different treatments (Hartmann et al. 2015). Dominant OTUs were identified as OTUs with relative abundances greater than 0.1%. The network was generated in the igraph package (version 1.0.1) and visualized in Gephi (version 0.8.2) (Ma et al. 2018b).
2.4 Metagenome prediction
This study employed PICRUSt2 to infer the metagenome of a sample from its phylogenetic composition (Langille et al. 2013; Douglas et al. 2020). The OTUs table mapped to the Greengenes 13_8 reference phylogeny was used as the input file for metagenome imputation of individual soil sample. Predicted gene family abundances were analyzed at KEGG Orthology (KO) group levels 3. The mean nearest sequenced taxon index was lower (0.15 ± 0.03) than that reported for soil microbial communities (0.17 ± 0.02) (Langille et al. 2013), indicating the high quality of our predicted metagenomes. PICRUSt cannot replace whole metagenome profiling and could be problematic when analyzing microbial communities with a large proportion of poorly characterized members. However, it is useful to supplement 16S rRNA analyses in metagenome studies, especially for broad surveys in microbial ecology applications.
2.5 Statistical analyses
In this study, we focused on the influence of TRN and TR (as compared to the NT treatment) on the compositional structure and function of bacterial communities. To measure the difference in composition profiles between treatments, we calculated the odds ratio values by dividing the mean relative abundance values of taxa groups at class level in soils with different treatments (Ma et al. 2018a). We calculated the Shannon index and phylogenetic diversity (PD) index with the R packages vegan and Picante, respectively. We used non-metric multidimensional scaling (NMDS) for ordinations to describe the bacterial community structure (beta-diversity). The NMDS analysis was based on both the Bray–Curtis and UniFrac phylogenetic distance matrix. The analyses were conducted using the R package Phyloseq (McMurdie and Holmes 2014). We used PERANOVA to test differences between bacterial groups. We used R Version 3.2.4 (http://www.r-project.org) for statistical analysis and graphing.
3 Results
3.1 Bacterial community compositions
A total of 138,272 reads were generated with multiplex sequencing to profile bacterial 16S rRNA genes. We achieved 97% sequence identification, and 10,885 OTUs were identified. The bacterial community was dominated by Actinomycetales (22.7%), followed by Gemmatimonadetes (9.9%), Deltaproteobacteria (9.2%), Thermoleophilia (8.7%), Gammaproteobacteria (7.5%), Alphaproteobacteria (6.5%), and Spartobacteria (5.5%). The relative abundances for other classes were all less than 5%.
The profiles of odds ratios were similar in 2 years, but most of the values of odds ratio in 2013 were greater than those in 2012 (Fig. 1). The difference in the odds ratio between NT and TR treatments was small, except for Spartobacteria in 2012 and for Gemmatimonadetes in 2013 (Fig. 1). Compared with the TR and NT treatment, the relative abundance of Gemmatimonadetes, Thermoleophilia, Gammaproteobacteria, and Solibacteria was increased by the TRN treatment, and the odds ratios of TRN/NT and TRN/TR were greater than 0.05. The relative abundance of Deltaproteobacteria, Alphaproteobacteria, Spartobacteria, Saprospirae, and Planctomycetia decreased more than 1.1 times in the TRN treatment, compared with the TR and NT treatments.
3.2 Bacterial community diversity and structure
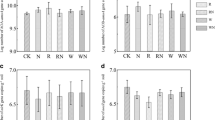
Based on the Shannon index and the PD values, the treatments did not affect bacterial community alpha-diversity (Fig. 2a). The bacterial community structure was different between the TRN and the NT or TR treatments (ANOSIM. R2 = 0.112, P = 0.001; Fig. 2b), indicating that the TRN influenced the soil bacterial community over the TR and NT treatments. For the bacterial community, the NT treatment did not affect its structure as compared to the TR treatment (ANOSIM. R2 = 0.023, P = 0.34). All the changes of dominant bacterial orders had a similar tendency among different treatments, except for Spartobacteria and Planctomycetia (Fig. S1).
Alpha- and beta-diversity of bacterial communities in soils in the no-tillage (NT), tillage reversal (TR), and tillage reversal plus N fertilization (TRN) treatments: a The Shannon index and phylogenetic diversity (PD) in different treatments; and b the non-metric multidimensional scaling (NMDS) ordination based on the Bray–Curtis and UniFrac distance matrices
3.3 Treatment effects on dominant bacterial composition
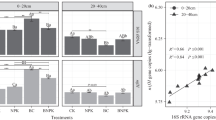
The bipartite network showed that the TRN treatment was associated with more bacterial OTUs with greater relative abundances (Fig. 3a). The number of dominant OTUs associated with TRN (65) was greater than that associated with TR (52) and the inter-annual variation, or year (59; Fig. 3b). The number of dominant OTUs associated with both TRN and TR (17) was greater than the number of dominant OTUs associated with both the inter-annual variation and TRN (7) or TR (6; Fig. 3b). Only four dominant OTUs were associated with all three treatments (Fig. 3b), and three of them were Actinobacteria. The dominant OTUs associated with TRN, TR, and inter-annual variation belong to seven, eight, and nine phyla, respectively (Fig. 3c). The TRN treatment did not influence OTUs belonging to Cyanobacteria (Fig. 3c). Tillage reversal did not influence OTUs belonging to Firmicutes, Cyanobacteria, and Chloroflexi (Fig. 3c). The dominant OTUs belonging to Nitrospirae and Firmicutes had little inter-annual variation (Fig. 3c).
The bipartite network between dominate OTUs and treatment variables, including tillage reversal (TR), tillage reversal plus N fertilization (TRN), and inter-annual variation (Year): a Bipartite network between dominant OTUs and treatment factors; b the shared associating OTUs among treatment factors; and c the association pattern among phyla for different treatment factors
3.4 Predicted metagenomes
The predicted metagenomes were different between the TRN and the other two treatments (Fig. 4, Fig. S2). Compared with NT and TR, the TRN treatment increased the relative abundance of some special genes, which were in the pathways for transcription, replication and repair, cell motility, and glycan biosynthesis. The relative abundance of some special genes for nitrogen and carbon cycling, such as hydroxylamine reductase, glutamate dehydrogenase, pentose phosphate pathway, and amino sugar and nucleotide, were increased in the TRN. Conversely, the TRN decreased the relative abundance of genes for membrane transport, biodegradation and metabolism of xenobiotics, metabolism of terpenoids and polyketides, and lipids. The relative abundance of genes related to nitrogen and carbon cycling, such as nitrilase, narG, narH, narG, narZ, nasB, butanoate metabolism, pyruvate metabolism, were also decreased in the TRN treatment. Within the same year, the predicted metagenomes were similar between the NT and TR treatments (Fig. 4).
4 Discussion
The bacterial community composition and structure was not affected by the TR (as compared with NT) treatment, rejecting our first hypothesis. This result is inconsistent with the decreased soil WEOC and WEON in the TR relative to the NT treatment in the studied plots (Sun et al. 2015; 2020). The NT practice has been shown to be effective for increasing microbial C and N contents and microbial biomass across different soils, experimental durations, and climatic conditions on a global level (Li et al. 2018). However, the tillage reversal was applied for less than 3 years (since 2010) in our study, and a longer period of treatment might be needed for the effect of TR to develop.
Our results support our second hypothesis, and we can conclude that the TRN treatment changed the structure and function of the soil bacterial community as compared with the TR and NT treatments in the studied Luvisolic soil in west-central Alberta. The increased relative abundance of Gemmatimonadetes in soils with TRN in comparison to TR is consistent with Nemergut et al. (2008), suggesting that the long-term N amendment increased the relative abundance of Gemmatimonadetes. The greater abundance of Gammaproteobacteria and Solibacteres and the class Thermoleophilia belonging to the phyla Actinobacteria in soils with TRN was also in agreement with studies on long-term inorganic N fertilization (Zhou et al. 2015). Nitrogen fertilization has been shown to favor the growth of Actinobacteria and Proteobacteria and reduce the abundance of Acidobacteria (Dai et al. 2018). One explanation for the greater abundance of bacterial taxa groups in the TRN treatment might be the increase of nitrification rates in soils with urea application, as a high proportion of chemolithotrophic nitrifying bacteria occurs within the Gammaproteobacteria class (Praeg et al. 2020). Gemmatimonadetes, Thermoleophilia, and Solibacteres are important to decompose soil C (Pitombo et al. 2015). Another explanation might, therefore, be the increase of WEOC and decrease of pH in soils under long-term N fertilization (Sun et al. 2016). Conversely, the TRN treatment decreased the abundance of Alphaproteobacteria, Deltaproteobacteria, Spartobacteria, and Planctomycetia in this study; those are groups that are important for denitrification in the studied soil (Xun et al. 2016). The interannual variation of the bacterial communities was low in the TRN treatment in this study. These suggest that the long-term N fertilization stimulation effect on soil C and N biogeochemical processes was greater than that of changes in the weather condition between years.
The greater effect of the TRN treatment than the TR treatment on the bacterial community explains the larger number of OTUs associated with TRN than those associated with TR or the inter-annual variation. Furthermore, the large number of OTUs associated with TRN and TR at the same time indicates that the trend of effects of TRN and TR were similar. Only a few OTUs were associated with all three treatments, indicating that those three treatments affected different bacterial stains. Nevertheless, in the network analysis, only dominating OTUs with a relative abundance > 0.1% were included, and the responses of rare groups were not evaluated.
The TRN treatment resulted in a soil bacterial community structure different from other treatments, consistent with Nivelle et al. (2016), who found that soil microbial community structure was mainly impacted by tillage and N fertilization even though they did not study a combined tillage and N fertilization treatment. Our previous study found that soil organic matter content and nutrient availability was increased, while soil pH and temperature were decreased by the TRN treatment (Sun et al. 2015; 2020), which can inevitably change microbial community structure (Xun et al. 2016; Wang et al. 2020). Long-term N fertilization increases C and N availability, which could affect bacterial diversity and community composition (Dai et al. 2018). The dominant influence of TRN on bacterial community function is reflected in the changes in the predicted metagenomic profiles. The changes of predicted metagenomic profiles were consistent with the bacterial community response to TRN, suggesting that the TRN treatment had a comparable effect on bacterial community composition and function (Fierer et al. 2012; Loaiza et al. 2019). The higher relative abundance of genes for hydroxylamine reductase, glutamate dehydrogenase, and amino sugar and nucleotide indicated the higher ability of biological nitrogen fixation and microbial carbon use efficiency in the TRN treatment (Domeignoz et al. 2020). The bacterial taxa groups having chemolithotrophic nitrifying functions that had higher relative abundance in the TRN treatment belonged to copiotrophic bacteria (Vries et al. 2015), which have more genes in pathways for transcription, replication and repair, cell motility, and glycan biosynthesis and metabolism (Fierer et al. 2012). In contrast, TRN decreased the relative abundance of oligotrophic bacteria, which have more genes in pathways for membrane transport, biodegradation and metabolism of xenobiotics, and metabolism of terpenoids and polyketides, energy, cofactors, vitamins, and lipids (Ma et al. 2018b). Overall, our results suggest that the TRN treatment changed the soil microbial community function compared to the TR and NT treatments by increasing the abundance of copiotrophic bacteria in the studied Gray Luvisol.
5 Conclusions
We demonstrate that tillage reversal plus N fertilization together rather than tillage reversal alone affected the bacterial community composition, structure, and function that altered nutrient cycling processes in long-term NT management in a Gray Luvisol. We conclude that N fertilization and TR applied together, but not TR alone, altered bacterial community composition and diversity and nutrient cycling processes that are mediated by microbial populations over the NT treatment in the studied soil. In agricultural production systems, proper tillage practices and N fertilization regimes should be used to enhance soil function and quality. Our research design does not allow the test of the interaction between TR and N fertilization, and future studies should focus on interactive effects between TR and N fertilization on the bacterial community and function in different agroecosystems.
References
Banerjee S, Walder F, Büchi L, Meyer M, Held AY, Gattinger A, Keller T, Charles R, Heijden GA (2019) Agricultural intensification reduces microbial network complexity and the abundance of keystone taxa in roots. ISME J 13:1722–1736
Bissett A, Richardson AE, Baker G, Kirkegaard J, Thrall PH (2013) Bacterial community response to tillage and nutrient additions in a long-term wheat cropping experiment. Soil Biol Biochem 58:281–292
Bogunovic I, Pereira P, Kisic I, Sajko K, Sraka M (2018) Tillage management impacts on soil compaction, erosion and crop yield in stagnosols (croatia). CATENA 160:376–384
Dai Z, Su W, Chen H, Barberán A, Zhao H, Yu M, Brookes PC, Schadt CW, Chang SX, Xu J (2018) Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agro-ecosystems across the globe. Glob Change Biol 24(8):3452–3461
Domeignoz-Horta LA, Pold G, Liu X, Frey SD, Melillo JM, Deangelis KM (2020) Microbial diversity drives carbon use efficiency in a model soil. Nat Commun 11:3684
Douglas GM, Maffei VJ, Zaneveld JR (2020) PICRUSt2 for prediction of metagenome functions. Nat Biotechnol 38:685–688
Fierer N, Lauber CL, Ramirez KS, Zaneveld J, Bradford MA, Knight R (2012) Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J 6:1007–1017
Hartmann M, Frey B, Mayer J, Mäder P, Widmer F (2015) Distinct soil microbial diversity under long-term organic and conventional farming. ISME J 9:1177–1194
Izaurralde RC, McGill WB, Robertson JA, Juma NG, Thurston JJ (2001) Carbon balance of the Breton classical plots over half a century. Soil Sci Soc Am J 65:431–441
Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31:814–821
Lavkulich LM, Arocena JM (2011) Luvisolic soils of Canada: genesis, distribution, and classification. Can J Soil Sci 91:781–806
Loaiza Puerta V, Pujol Pereira E, Huang P, Wittwer R, Six J (2019) Soil microhabitats mediate microbial response in organic reduced tillage cropping. Appl Soil Ecol 137:39–48
Li Y, Chang SX, Tian L, Zhang Q (2018) Conservation agriculture practices increase soil microbial biomass carbon and nitrogen in agricultural soils: a global meta-analysis. Soil Biol Biochem 121:50–58
Ma B, Lv X, Cai Y, Chang SX, Dyck MF (2018a) Liming does not counteract the influence of long-term fertilization on soil bacterial community structure and its co-occurrence pattern. Soil Biol Biochem 123:45–53
Ma B, Zhao K, Lv X, Su W, Dai Z, Gilbert JA, Brookes PC, Faust K, Xu J (2018b) Genetic correlation network prediction of forest soil microbial functional organization. ISME J 12:2492–2505
Mbuthia LW, Acosta-Martínez V, DeBruyn J, Schaeffer S, Tyler D, Odoi E, Mpheshea M, Walker F, Eash N (2015) Long term tillage, cover crop, and fertilization effects on microbial community structure, activity: implications for soil quality. Soil Biol Biochem 89:24–34
McMurdie PJ, Holmes S (2014) Waste not, want not: why rarefying microbiome data is inadmissible. PLoS Comput Biol 10:e1003531
Michele S, Marisa G, Vincenzo C, Matteo F, Andrea M, Giorgio M (2013) DNA qualification workflow for next generation sequencing of histopathological samples. PLoS One 8(6):e62692
Miura T, Niswati A, Swibawa IG, Haryani S, Gunito H, Kaneko N (2013) No tillage and bagasse mulching alter fungal biomass and community structure during decomposition of sugarcane leaf litter in Lampung Province, Sumatra, Indonesia. Soil Biol Biochem 58:27–35
Navarro-Noya YE, Gómez-Acata S, Montoya-Ciriaco N, Rojas-Valdez A, Suárez-Arriaga MC, Valenzuela-Encinas C, Jiménez-Bueno N, Verhulst N, Govaerts B, Dendooven L (2013) Relative impacts of tillage, residue management and crop-rotation on soil bacterial communities in a semi-arid agroecosystem. Soil Biol Biochem 65:86–95
Nemergut DR, Townsend AR, Sattin SR, Freeman KR, Fierer N, Neff JC, Bowman WD, Schadt CW, Weintraub MN, Schmidt SK (2008) The effects of chronic nitrogen fertilization on alpine tundra soil microbial communities: implications for carbon and nitrogen cycling. Environ Microbiol 10:3093–3105
Nivelle E, Verzeaux J, Habbib H, Kuzyakov Y, Decocq G, Roger D, Lacoux J, Duclercq J, Spicher F, Nava-Saucedo J-E, Catterou M, Dubois F, Tetu T (2016) Functional response of soil microbial communities to tillage, cover crops and nitrogen fertilization. Appl Soil Ecol 108:147–155
Nyborg M, Solberg ED, Malhi SS, Izaurralde RC (1995) Fertilizer N, crop residue, and tillage alter soil C and N content in a decade. R. Lal, J. Kimble, E. Levine, B.A. Stewart (Eds.), Soil management and greenhouse effect, Lewis Publishers, CRC Press, Boca Raton, FL, pp. 93–99
Praeg N, Seeber J, Leitinger G, Tasser E, Illmer P (2020) The role of land management and elevation in shaping soil microbial communities: insights from the Central European Alps. Soil Biol Biochem 150, 107951
Pitombo LM, do Carmo JB, de Hollander M, Rossetto R, López MV, Cantarella H, Kuramae EE (2015) Exploring soil microbial 16S rRNA sequence data to increase carbon yield and nitrogen efficiency of a bioenergy crop. GCB Bioenergy 8:867–879
Ran JK, Birthe V (2018) Biogeochemical cycling of metals impacting by microbial mobilization and immobilization. J Environ Sci 66:146–154
Rincon-Florez VA, Dang YP, Crawford MH, Schenk PM, Carvalhais LC (2016) Occasional tillage has no effect on soil microbial biomass, activity and composition in Vertisols under long-term no-till. Biol Fertil Soils 52:191–202
Schmidt R, Mitchell J, Scow K (2018) Cover cropping and no-till increase diversity and symbiotroph:saprotroph ratios of soil fungal communities. Soil Biol Biochem 129:99–109
Sengupta A, Dick WA (2015) Bacterial community diversity in soil under two tillage practices as determined by pyrosequencing. Microb Ecol 70:853–859
Soi1 Classification Working Group (1998) The Canadian System of Soil Classification. Agriculture and Agri-Food Canada Publication 1646 (3rd ed.). Ottawa, ON: NRC Research Press. 1998. p. 187 pp. ISBN 0–660–17404–9
Soil Survey Staff (1999) Soil taxonomy: a basic system of soil classification for making and interpreting soil surveys. 2nd edition. Natural Resources Conservation Service. U.S. Department of Agriculture Handbook 436
Stoddard SF, Smith BJ, Hein R, Roller BRK, Schmidt TM (2015) rrnDB: improved tools for interpreting rRNA gene abundance in bacteria and archaea and a new foundation for future development. Nucleic Acids Res 43:593–598
Sun B, Jia S, Zhang S, McLaughlin NB, Zhang X, Liang A, Chen X, Wei S, Liu S (2016) Tillage, seasonal and depths effects on soil microbial properties in black soil of northeast China. Soil and Tillage Research 155:421–428
Sun L, Chang SX, Feng YS, Dyck MF, Puurveen D (2015) Nitrogen fertilization and tillage reversal affected water-extractable organic carbon and nitrogen differentially in a black chernozem and a gray luvisol. Soil and Tillage Research 146:253–260
Sun L, Feng YS, Dyck MF, Puurveen D, Chang SX (2020) Tillage reversal did not reverse N fertilization enhanced C storage in a Black Chernozem and a Gray Luvisol. Geoderma 370:114355
Vries M de, Schöler A, Ertl J, Xu Z, Schloter M (2015) Metagenomic analyses reveal no differences in genes involved in cellulose degradation under different tillage treatments. FEMS Microbiol Ecol 91, fiv069
Wang H, Guo Q, Li X, Li X, Zhang C (2020) Effects of long-term no-tillage with different straw mulching frequencies on soil microbial community and the abundances of two soil-borne pathogens. Appl Soil Ecol 148:103488
Wang Z, Liu L, Chen Q, Wen X, Liao Y (2016) Conservation tillage increases soil bacterial diversity in the dryland of northern China. Agron Sustain Dev 36:1–9
Wortmann CS, Drijber RA, Franti TG (2010) One-time tillage of no-till crop land five years post-tillage. Agron J 102:1302
Xun W, Zhao J, Xue C, Zhang G, Ran W, Wang B, Shen Q, Zhang R (2016) Significant alteration of soil bacterial communities and organic carbon decomposition by different long-term fertilization management conditions of extremely low-productivity arable soil in south China. Environ Microbiol 18:1907–1917
Zhou J, Guan D, Zhou B, Zhao B, Ma M, Qin J, Jiang X, Chen S, Cao F, Shen D, Li J (2015) Influence of 34-years of fertilization on bacterial communities in an intensively cultivated black soil in northeast China. Soil Biol Biochem 90:42–51
Acknowledgements
We thank D. Puurveen and M. Dyck for maintaining the field experiment, and P. Auer, Y.P. Gao, Shujie Ren, Yang Liu, and others for helping with soil sample collection.
Funding
This work was supported by the Natural Science and Engineering Research Council of Canada (NSERC, grant # 249664–2013), the National Natural Science Foundation of China (NSFC, grant #42107419), and the Zhejiang Provincial Natural Science Foundation of China (LD19D060001 and LQ20C030006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Yuan Ge
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lv, X., Ma, B., Sun, L. et al. Long-term nitrogen fertilization, but not short-term tillage reversal, affects bacterial community structure and function in a no-till soil. J Soils Sediments 22, 630–639 (2022). https://doi.org/10.1007/s11368-021-03100-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-021-03100-z