Abstract
Purpose
The effects of soil properties on biochar-induced soil phosphate sorption and availability are not well investigated. An alkaline biochar-induced soil phosphate sorption, phosphorus (P) availability, and related mechanisms (including the effects of calcium/magnesium (Ca/Mg) minerals in biochar and the liming effect) in different soils were studied in the laboratory.
Materials and methods
Two soils (sandy soil and clay soil) with contrasting P-retention capacities were amended with four rates of alkaline peanut biochar. Soil phosphate sorption–desorption indices were characterized by a multiple-point isotherm batch experiment. Soil P availability was determined by sequential fractionation method.
Results and discussion
The alkaline biochar addition increased the phosphate sorption of sandy soil but decreased the phosphate sorption of the clay soil. The interaction between the biochar and external phosphate on the biochar surface was mainly controlled by adsorption process, rather than precipitation. The liming effect of the biochar decreased the Langmuir phosphate sorption maxima (Smax) of clay soil. However, the biochar increased Smax of clay soil when the liming effect was eliminated, because Ca/Mg minerals in biochar can provide phosphate sorption sites. The dominant P fraction of the biochar was Ca-bounded P, and therefore the biochar addition increased HCl-extractable P of both sandy and clay soils. Generally, the biochar addition increased P availability of clay soil because it increased NaHCO3-extractable P content and decreased phosphate sorption. P mobility of sandy soil was also increased by biochar addition, because water-extractable P and EPC0 were increased.
Conclusions
Our results suggest that both the biochar (particularly the contents of Ca/Mg minerals within) and soil properties (particularly the initial phosphate sorption capacity) determine phosphate sorption and availability following the biochar application. The alkaline biochar application to sandy soils with low P buffering capacity should consider the amount of P input from biochar and the corresponding environmental risk, although biochar addition can enhance phosphate sorption in these soils in the short term.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biochar is a stable, carbon-rich material, which is produced through thermochemical reaction under conditions of limited or no oxygen (Lehmann 2007a). Biochar has received a considerable research attention because it is an easily accessible input for agriculture (Biederman and Harpole 2013) and a promising amendment for achieving environmental objectives such as soil and water remediation (Ahmad et al. 2014).
Biochar amendment can supply nutrients to plants in nutrient-deficient, low-fertility, and degraded soils (Lehmann 2007b; Novak et al. 2009). A better understanding of the effects of biochar on soil nutrient availability and the related mechanisms can advance biochar application to agricultural soils. Biochar is commonly alkaline which contributes to its liming effect and influences soil nutrients availability, although biochars with lower pH also exist (e.g., neutral and acidic biochars) (Ippolito et al. 2016; Qi et al. 2017; Zhang et al. 2019). Previous studies have shown inconsistent results as to whether alkaline biochar application enhances phosphorus (P) fixation or availability (Atkinson et al. 2010; Yao et al. 2012; Morales et al. 2013; Bornø et al. 2018). Zhai et al. (2015) have reported that the application of maize straw biochar increased the content of soil available P such as Olsen P. In addition, Xu et al. (2014) showed that biochar application increased pH in acid soil because of its high alkalinity and consequently reduced P fixation by iron (Fe) and aluminum (Al) minerals. However, other studies reported that alkaline biochar application decreased P availability mostly in alkaline soils, which was probably due to substantial release of cations including calcium (Ca) and magnesium (Mg) cations from biochar, which complex with P at alkaline conditions (DeLuca et al. 2015).
P availability in soil depends heavily on the extent of sorption and precipitation reactions. In acid soils, P is mainly fixed by Fe and Al oxide and hydroxide minerals via precipitation and ligand exchange reactions, whereas P fixation in alkaline and calcareous soils mainly occurs through the formation of Ca-phosphate compounds and by sorption and precipitation with CaCO3 (Havlin et al. 2005). Biochar may directly or indirectly influence soil P sorption. Zhang et al. (2016) reported that the enhancement of P retention in biochar-amended soil can be attributed to P sorption by Ca minerals in biochar. In contrast, other studies suggested that biochar addition decreased phosphate sorption of acid soils due to increases of pH, which can increase the net negative charge repulsing phosphate ions from sorption to soil minerals (DeLuca et al. 2015); and enhancing release of dissolved organic carbon, which can compete for phosphate sorption sites in soil (Schneider and Haderlein 2016; Liu et al. 2018). Soinne et al. (2014) reported that a woody alkaline biochar had a very low phosphate affinity and the biochar addition did not increase phosphate sorption in soil, but the biochar addition can reduce particulate P loss by enhancing soil aggregate stability. Investigation of the direct effect of Ca/Mg minerals in alkaline biochar and the indirect effect (e.g., changing positive or negative charge density) of liming of alkaline biochar on phosphate sorption characteristics can advance our knowledge on phosphate sorption in response to the biochar application.
Soil phosphate adsorption isotherms provide a useful means for studying soil phosphate sorption–desorption dynamics (Graetz and Nair 2000; Huang et al. 2014). In this study, we measured phosphate sorption–desorption isotherms in two types of soils (i.e., sandy soil and clay soil) amended with alkaline biochar at varying rates. Our objectives were (1) to determine the effects of Ca/Mg minerals in biochar (direct effect) and the liming effect (indirect effect) on soil phosphate sorption–desorption characteristics and P availability, and (2) to assess the effects of soil properties on biochar-induced phosphate sorption and availability.
2 Materials and methods
2.1 Soil and biochar
Two types of soils (sandy soil and clay soil) with contrasting P sorption capacities were selected for this experiment. The sandy soil, which had the lower P sorption capacity, was collected from Nanchang, China (28° 44′ 46.2″ N, 115° 55′ 19.2″ E). It is classified as an Inceptisol (Soil Survey Staff 2010) formed on alluvium of Gan River, and is mainly used for vegetable cultivation (Brassica chinensis L., Lactuca sativa Linn.). The clay soil, which had the higher P sorption capacity, was collected from Jinxian, China (28° 41′ 45.2″ N, 116° 12′ 29.7″ E). This soil is classified as an Ultisol developed from Quaternary red clay, and is mainly used for tangerine (Citrus tangerina) cultivation. The sandy soil can represent the soils with low P retention capacity but under intensive fertilizer input. With continuous excessive application of P fertilizer and/or manure for vegetable farming, the sandy soil showed significant P enrichment and higher soil organic carbon content (Table 1). The clay soil can represent the low fertility soils with high P retention capacity while receiving little fertilizer application. The soil (~ 20 cm layer) was collected using a spade, thoroughly mixed, air-dried, and ground to pass a 2-mm sieve prior to use.
A commercial biochar was used for the experiment (Sanli New Energy Plant, China). It was made from peanut husk slowly pyrolyzed at 350–500 °C. The biochar had pH of 9.16, organic carbon of 323.76 g kg−1, total nitrogen of 10.52 g kg−1, total P of 6.11 g kg−1, total potassium of 15.51 g kg−1, total Ca of 36.65 g kg−1, total Mg of 6.95 g kg−1, total iron of 4.34 g kg−1, total aluminum of 3.79 g kg−1, and total silicon 17.90 g kg−1. The biochar was crushed, homogenized, and passed through a 2-mm sieve.
The biochar was mixed with 100-mg L−1 phosphate solution (KH2PO4, 1:10, solid:liquid) and incubated at 25 °C for 14 days to determine whether new phosphate-containing minerals can be formed after biochar and phosphate interaction. After incubation, the biochar was washed with deionized water and oven-dried at 80 °C. The incubated biochar and original biochar (i.e., without incubation with phosphate solution) samples were then ground in an agate mortar and pestle for X-ray diffraction (XRD) analysis (D8 Advance, Bruker AXS, Germany) and morphology analysis by field emission scanning electron microscopy (SEM, Hitachi, S-4800, Japan). Meanwhile, surface element analysis was also conducted simultaneously with the SEM at the same surface locations using energy dispersive X-ray spectroscopy (EDS, Oxford Instruments Link ISIS).
2.2 Phosphate adsorption–desorption isotherm determination
The biochar was added to samples from each of the two soils at rates of 0, 2, 4, and 8% (w/w), designed to test a broad range of biochar addition rates on soil phosphate sorption–desorption behavior. The soil mixtures were incubated at 25 °C for 14 days. A constant moisture content (70% water-holding capacity) was maintained throughout the whole incubation period. The experiment was replicated three times, and thus twelve samples for each type of soil were obtained. At the end of the incubation, soil and biochar mixtures were air-dried and sieved through a 2-mm sieve before the batch experiment.
The phosphate adsorption isotherms were determined according to the method of Graetz and Nair (2000). One gram of soil and biochar mixture sample was equilibrated in a 50-mL centrifuge tube with 25 ml of 0.01 M CaCl2 solution containing phosphate (KH2PO4) concentrations of 0, 0.1, 1, 2.5, 5, 10, 25, 50, and 100 mg P L−1. Three drops of chloroform were added to each tube to inhibit microbial activity. The tubes were then shaken at 25 ± 1 °C for 24 h on an end-over-end shaker at a rate of 150 rpm, and the supernatants were filtered through 0.45-μm membrane filters. The phosphate in solution was then measured following the method of Murphy and Riley (1962).
The phosphate desorption experiment was performed on soil residues immediately after the sorption experiment. After removing the supernatant solution from the sorption experiment, each soil was resuspended with 25 ml of 0.01 M phosphate-free CaCl2 solution and again shaken for 24 h at 25 °C. Thereafter, desorbed phosphate was measured in filtrates as described in adsorption experiments after filtering through 0.45-μm membrane filters.
A modified Langmuir equation was used to describe the sorption curves for sorption experiments (Siemens et al. 2004):
where S is the sorbed phosphate (mg kg−1), c is the phosphate concentration in the equilibrium solution (mg L−1), Smax is the maximum sorption capacity of soil phosphate (mg kg−1), k is a constant related to the bonding strength (L mg−1), and S0 is a fitting parameter for an estimation of initial soil sorbed phosphate (mg kg−1) (Siemens et al. 2004).
The equilibrium phosphate concentration (EPC0, mg L−1), defined as the solution phosphate concentration supported by a soil sample at which no net sorption or desorption occurs, can be calculated by setting S = 0 in Eq. (1):
To study the liming effect on phosphate sorption due to biochar addition, another set of soils was treated with NaOH solution to obtain approximately the same pH value as biochar addition treatments, and was incubated for 14 days as described above. Only clay soil was chosen for this experiment because biochar addition had a profound effect on pH of the clay soil (increased from 4.48 to 6.19) and had limited effect on that of sandy soil (increased from 7.24 to 7.75). This incubation experiment was conducted in duplicate.
2.3 P fractionation
A modified Hedley sequential fractionation was employed to examine the effect of biochar on soil P mobility and availability (Liu et al. 2018; Xu et al. 2018). The peanut biochar and biochar-amended soil were extracted at 25 ± 1 °C for 16 h sequentially by deionized water, 0.5 M NaHCO3, 0.1 M NaOH, and 1 M HCl. After each successive extraction, the suspension was centrifuged and supernatants were filtered through a 0.45-μm membrane filter. The NaHCO3 and NaOH extracts were divided into two aliquots in order to measure the total P and inorganic P. Organic P was calculated as a difference between total P and inorganic P. Total P of NaHCO3 and NaOH extracts were determined by persulfate digestion. The P concentration in solution was determined using the ascorbic acid molybdenum blue method (Murphy and Riley 1962).
2.4 Statistical analyses
All analyses were performed in the statistical program R (version 3.1.2, R Core Team 2014). The nonlinear fitting of the Langmuir adsorption equation was conducted using the nls function in R base packages. Differences in soil phosphate sorption–desorption parameters and P fractions among the four biochar addition rates in each type of soil were analyzed statistically using one-way analysis of variance (significance p < 0.05). Data were checked for normality and homogeneity, and were transformed using log10 when test assumptions were not met. Multiple comparisons of means were performed by Tukey’s honest significant difference test using the agricolae package for R software (de Mendiburu 2014). The relationship between soil P sorption parameters and water-extractable P was investigated by linear regression analysis using the lm() function in R base packages.
3 Results
3.1 Soil and biochar P-retentive capacity evaluation
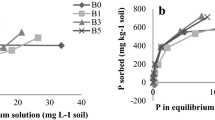
In general, clay soil had greater phosphate sorption capacity than sandy soil (Fig. 1). Based on the Langmuir equation, the clay soil had higher Smax (692 vs. 253 mg kg−1), k (3.29 vs. 0.06 L mg−1), and S0 (34.84 vs. − 0.90 mg kg−1) values but lower EPC0 (0.015 vs. − 0.095 mg L−1) compared with the sandy soil. In comparison with the clay soil, the sandy soil also had higher Olsen P (34.04 vs. 3.62 mg kg−1) and Bray P (41.78 vs. 1.65 mg kg−1) concentrations (Table 1). Biochar had affinity to phosphate, as phosphate sorption by biochar tended to increase linearly with initial phosphate concentrations, and a linear regression was able to describe this sorption curve (S = 32.35 × c – 9.93, r2 = 0.99, p < 0.0001). As a result, biochar had higher EPC0 value (0.307 mg L−1) than the two soils. The pH of sandy soil was 7.25 and that of clay soil was 4.48. Both soils had lower pH compared with the biochar (9.16).
Phosphate (P) sorption isotherm of sandy soil, clay soil, and biochar. Data points are the arithmetic mean of triplicate determinations (n = 3). The P sorption curves of sandy soil and clay soil were well fitted by the Langmuir equation, whereas P sorption by biochar was better fitted by a linear regression
The XRD analysis revealed that biochar contained quartz, Ca/Mg phosphate, and Ca silicate minerals (Fig. 2). The original biochar and phosphate-loaded biochar (i.e., incubated with 100 mg L−1 phosphate solution) had similar XRD spectrum. In addition, SEM-EDS analysis showed that element composition on biochar surface was not apparently changed after incubation with phosphate-containing solution (Fig. 3).
3.2 Phosphate sorption behavior
Biochar addition consistently increased phosphate sorption of sandy soil but appeared to decrease the phosphate sorption of clay soil (Fig. 4). The Smax values increased from 253 to 373 mg kg−1 in sandy soil after biochar addition (0 to 8% biochar addition, p = 0.028; Table 2). In addition, biochar addition tended to increase S0 (p = 0.16) and EPC0 (p = 0.048) values of sandy soil. The highest values of S0 (10.61 mg kg−1) and EPC0 (0.564 mg L−1) were found in the 8% biochar-amended sandy soil, while the lowest were found in the sandy soil without biochar addition (S0 = − 0.90 mg kg−1, EPC0 = − 0.095 mg L−1). In contrast, in clay soil, Smax values were decreased by about 23–32% in biochar addition treatments compared with the treatment without biochar addition (p = 0.024). There were no significant differences in k, S0, and EPC0 among different biochar addition treatments in clay soil.
Biochar addition also increased soil pH, a critical factor regulating phosphate sorption, of the two types of soils (p < 0.001), and the increment in clay soil was the greatest (Table 2). We further examined the liming effect of biochar addition on phosphate sorption of clay soil by comparing Smax values of NaOH-adjusted clay soil, biochar-addition treatments, and original clay soil. The Smax of clay soil tended to decrease as pH increased. Moreover, Smax of NaOH-adjusted clay soil was lower than those for biochar-addition treatments (i.e., 4 and 8% biochar addition, p < 0.0001), although these treatments had approximately the same soil pH (Fig. 5).
Comparison of the Langmuir phosphate sorption maxima (Smax) for clay soil amended with biochar or NaOH, which had approximately the same pH. Both biochar and NaOH decreased Smax of the clay soil (blank clay soil had Smax of 692 mg kg−1 as indicated by the solid black line in the figure), but the decrement from biochar addition was smaller. Values are means ± 1 standard error. Different letters indicate significant difference at p < 0.05
3.3 Phosphate desorption behavior
Phosphate adsorbed by the two types of soils can be partially desorbed, and both soils exhibited varying degrees of hysteresis (Fig. 4). Phosphate desorbability was used to describe the amount of phosphate desorbed from biochar-amended soils (Fig. 6). In general, the desorbabilities of biochar-amended sandy soils were higher than those of biochar-amended clay soils. In sandy soil, biochar additions tended to decrease phosphate desorbability when high concentrations of initial phosphate were added, particularly at 50 mg L−1 of initial phosphate addition (p = 0.014). In clay soil, the fraction of desorbed phosphate to newly sorbed phosphate increased with the amount of phosphate added in the sorption experiment, but no more than 5% of newly sorbed phosphate by soil and biochar mixtures could be desorbed after 24 h of desorption, even when 100 mg L−1 of phosphate was added. In contrast to sandy soil, phosphate desorbability of clay soil was increased by biochar addition when 50 mg L−1 of initial phosphate was added (p = 0.007).
Effect of initial phosphate (P) concentration (25, 50, and 100 mg P L−1) on P desorption in (a) sandy and (b) clay soils amended with different rates of biochar (0, 2, 4, and 8%). Values are means ± 1 standard error. Different letters for each initial P concentration indicate significant differences among different biochar addition rates. NS, not significant
3.4 P fractions
The contents of different P fractions of biochar-amended soil were determined to examine the effect of biochar on soil P mobility and availability. The dominant P fraction of biochar was HCl-extractable P (mean value 4.87 g kg−1, 82% of total P), which is mainly Ca-bounded P. As a result, biochar addition increased HCl-extractable P contents of both sandy and clay soils (p < 0.001, Fig. 7). In fact, the increment of HCl-extractable P was the greatest among all the P fractions, which were 74–309% for sandy soil and 133–1559% for clay soil compared with each soil without biochar addition. In general, biochar addition had no significant effect on organic P fraction (e.g., NaHCO3- and NaOH-extractable organic P) for both sandy and clay soils. However, biochar addition increased NaHCO3- and NaOH-extractable inorganic P of clay soil. P fraction extracted in water, which is most labile, was increased by biochar addition in sandy soil (p = 0.003, Fig. 7), and was linearly correlated with S0 (r = 0.38, p = 0.03) and EPC0 (r = 0.36, p = 0.04) calculated from the Langmuir equation (Fig. 8). For clay soil, however, the water-extractable P content was very low, ranging between 1.46 and 2.55 mg kg−1, and biochar addition had no significant effect on water-extractable P.
4 Discussion
Our results showed contrasting effects of biochar addition on phosphate sorption for sandy soil (neutral, low P-retentive) and clay soil (acid, high P-retentive). Biochar addition increased the Smax value of sandy soil (Table 2), suggesting that biochar can provide more sorption sites for phosphate after its application in sandy soil. The XRD showed that the biochar in our study contained Ca/Mg minerals (e.g., Ca silicates, Ca/Mg phosphates), and these minerals can contribute to phosphate sorption (Fig. 2). In contrast, biochar addition tended to decrease the Smax value of clay soil. Similarly, some previous studies also reported that the addition of biochar derived from lignocellulosic feedstock (e.g., corn stover, rice husk, wood residue) could reduce phosphate sorption in acid soil, but could increase P retention in neutral or alkaline soils in the short term (Chintala et al. 2014; Eduah et al. 2019). The decrease of Smax for biochar-amended clay soil was probably due to the liming effect of biochar, as the pH value of clay soil increased from 4.48 to 6.19 after biochar addition. As the soil pH increases, the positive charges on the Fe/Al oxide and hydroxide minerals surfaces also decrease although both positive and negative sites exist, resulting in decreases of phosphate sorption (Smyth and Sanchez 1980).
NaOH-addition experiment suggested that biochar addition may also contribute to phosphate sorption for clay soil, because Smax of biochar-amended clay soil (i.e., 4 and 8% biochar addition) was higher than that of clay soil with approximately the same pH value as biochar addition treatments (Fig. 5). In this context, the alkaline biochar addition increased phosphate sorption of both sandy and clay soils irrespective of the liming effect. The increased phosphate sorption (as indicated by Smax) due to biochar addition was mainly because Ca/Mg minerals in biochar can provide considerable phosphate sorption sites (Hollister et al. 2013). In general, phosphate adsorbs on the surface of Ca/Mg minerals under low phosphate concentration whereas phosphate can precipitate on the Ca/Mg minerals surface under high phosphate concentration (Zhou and Li 2001). When the biochar was incubated with 100 mg L−1 of phosphate solution, we hypothesized that some crystalline Ca/Mg-phosphate minerals may be formed. However, we did not observe any new crystalline Ca/Mg-phosphate minerals (i.e., stable P forms) formed on the surface of biochar after interaction with phosphate (Figs. 2, 3). Thus, phosphate adsorption controlled the interaction between biochar and phosphate, rather than precipitation, in the phosphate concentration range (i.e., 0–100 mg L−1) of our experiment. Some studies, contradictorily, reported that biochar derived from spruce and pine chips (Soinne et al. 2014, Ca content of 4.8 g kg−1) and wheat straw (Xu et al. 2014, Ca content of 10.2 g kg−1) had limited phosphate sorption capacity. The contrast results are partly because these biochar had lower contents of Ca and Mg than the biochar used in our study (Ca content of 36.7 g kg−1). We did not investigate the specific effect of biochar from which Ca/Mg minerals had been removed on soil phosphate sorption. Previous studies have suggested that removing Ca/Mg minerals as well as other metals from biochar by using acid or water extraction could drastically reduce the phosphate sorption of biochar (Hollister et al. 2013; Takaya et al. 2016). They showed that the metal-removed biochar had little or no sorption of phosphate, because of a limited anion-exchange capacity (Hollister et al. 2013; Takaya et al. 2016).
Consistent with other studies (e.g., Huang et al. 2014; Eduah et al. 2019), we found that phosphate desorption increased with the concentration of initial added P. However, more than 95% (biochar-amended clay soil) and 75% (biochar-amended sandy soil) of the newly adsorbed phosphate remained in the adsorbed phase after 24 h of desorption, even when 100-mg L−1 phosphate was added (the highest concentration of initial phosphate). This indicates a short-term (i.e., 24 h) irreversible sorption of phosphate by biochar-amended soils, especially for biochar-amended clay soil. In our study, biochar addition tended to increase phosphate desorbability of clay soil while decreasing that of sandy soil when a higher concentration of phosphate was added (e.g., 50 mg L−1). Phosphate desorption has been suggested to be controlled by bonding energy between phosphate and soil minerals (Havlin et al. 2005). In general, the sorbed phosphate is held with five times more bonding energy in acid soil than in neutral or calcareous soils (Havlin et al. 2005). Thus, it is expected that biochar addition to the acid clay soil at a certain rate will result in increased phosphate desorption, as Ca/Mg input through biochar addition can weaken the bonding energy of acid soil to phosphate.
P mobility and availability were also controlled by both soil and biochar properties. The alkaline biochar addition increased the P mobility of low P-retentive sandy soil because it increased water-extractable P content, although the biochar tended to increase the soil phosphate sorption capacity. If biochar contains labile P, its application would directly increase the soil labile P content (Wang et al. 2015; Zhai et al. 2015). In our study, increases in P availability of sandy soil was also evidenced by the fact that sandy soil S0, an estimate of initial sorbed phosphate calculated from Langmuir equation, was linearly increased by biochar addition (Table 2). In addition, the EPC0 of sandy soil was also increased by biochar addition, and this may further result in higher P mobility (Dari et al. 2016). For the highly P-retentive clay soil, biochar addition generally increased P availability because it increased NaHCO3-extractable P and decreased phosphate sorption. Overall, the biochar effect on soil phosphate sorption and mobility tended to be increased by a lower P buffering capacity of soil (e.g., the sandy soil in our study) and by higher biochar application rates. Application of biochar with considerable P content (e.g., biochar produced from manure or biosolids) to neutral sandy soil with a low P buffering capacity should be undertaken with care, as this may result in a high leaching potential of P, inducing eutrophication in groundwater, which is similar to amendment with manure or commercial fertilizer (Pizzeghello et al. 2016; Yan et al. 2015). Additional studies investigating phosphate sorption characteristics from soils amended with biochar at the field scale will be helpful. Some studies conducted field trials with biochar application but they did not pay much attention to the changes of phosphate sorption in response to biochar application (e.g., Cao et al. 2020; Gao et al. 2016; Jin et al. 2019).
5 Conclusions
Our results demonstrate that both the alkaline biochar and soil properties determine phosphate sorption following the biochar application. The decreases in Smax in acid clay soil were mainly due to the biochar liming effect, but biochar contributed to phosphate sorption of the clay soil when the liming effect was eliminated. Abundant Ca/Mg minerals in the biochar can provide phosphate sorption sites and thus increase Smax.
Since the dominant P fraction of the alkaline biochar was Ca/Mg-bounded P, the biochar addition increased HCl-extractable P of both sandy and clay soils. The biochar addition increased P availability of clay soil because it increased NaHCO3-extractable P content. In addition, the biochar addition increased P mobility of sandy soil because water-extractable P and EPC0 were increased by biochar, which suggests the biochar application in low P-retentive soil may have environmental concern. The alkaline biochar-induced phosphate sorption via Ca/Mg minerals was relatively weak because no new stable Ca/Mg-P minerals were formed. Thus, application of P-containing biochar to low P buffering soils should be careful. Further studies of P sorption and mobility in response to biochar addition at field scale are necessary.
References
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS (2014) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99:19–33
Atkinson CJ, Fitzgerald JD, Hipps NA (2010) Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 337:1–18
Biederman LA, Harpole WS (2013) Biochar and its effects on plant productivity and nutrient cycling: a meta–analysis. GCB Bioenergy 5:202–214
Bornø ML, Müller-Stöver DS, Liu F (2018) Contrasting effects of biochar on phosphorus dynamics and bioavailability in different soil types. Sci Total Environ 627:963–974
Cao D, Lan Y, Liu Z, Yang X, Liu S, He T, Wang D, Meng J, Chen W (2020) Responses of organic and inorganic phosphorus fractions in brown earth to successive maize stover and biochar application: a 5-year field experiment in Northeast China. J Soils Sediments 20:2367–2376
Chintala R, Schumacher TE, McDonald LM, Clay DE, Malo DD, Papiernik SK, Clay SA, Julson JL (2014) Phosphorus sorption and availability from biochars and soil/biochar mixtures. Clean Soil Air Water 42:626–634
Dari B, Nair VD, Harris WG, Nair P, Sollenberger L, Mylavarapu R (2016) Relative influence of soil- vs. biochar properties on soil phosphorus retention. Geoderma 280:82–87
de Mendiburu F (2014) Agricolae: statistical procedures for agricultural research. R package version 1.1. 4. 2013
DeLuca TH, Gundale MJ, MacKenzie MD, Jones DL (2015) Biochar effects on soil nutrient transformations. In: Lehmann J, Joseph S (eds) Biochar for environmental management: science, Technology and Implementation. Earthscan, London, pp 251–270
Eduah JO, Nartey EK, Abekoe MK, Breuning-Madsen H, Andersen MN (2019) Phosphorus retention and availability in three contrasting soils amended with rice husk and corn cob biochar at varying pyrolysis temperatures. Geoderma 341:10–17
Gao S, Hoffman-Krull K, Bidwell AL, DeLuca TH (2016) Locally produced wood biochar increases nutrient retention and availability in agricultural soils of the San Juan Islands, USA. Agric Ecosyst Environ 233:43–54
Graetz DA, Nair VD (2000) Phosphorus sorption isotherm determination. In: Pierzynski GM (ed) Methods of phosphorus analysis for soils, sediments, residuals, and waters. Southern cooperative series bulletin, vol. 369, vol 369. North Carolina State University, Raleigh, pp 35–38
Havlin J, Beaton JD, Tisdale SL, Nelson WL (2005) Soil fertility and fertilizers: an introduction to nutrient management, 7th edn. Pearson Prentice Hall, Upper Saddle River
Hollister CC, Bisogni JJ, Lehmann J (2013) Ammonium, nitrate, and phosphate sorption to and solute leaching from biochars prepared from corn stover (Zea mays L.) and oak wood (Quercus spp.). J Environ Qual 42:137–144
Huang LM, Thompson A, Zhang GL (2014) Long-term paddy cultivation significantly alters topsoil phosphorus transformation and degrades phosphorus sorption capacity. Soil Tillage Res 142:32–41
Ippolito J, Ducey T, Cantrell K, Novak J, Lentz R (2016) Designer, acidic biochar influences calcareous soil characteristics. Chemosphere 142:184–191
Jin Z, Chen C, Chen X, Jiang F, Hopkins I, Zhang X, Han Z, Billy G, Benavides J (2019) Soil acidity, available phosphorus content, and optimal biochar and nitrogen fertilizer application rates: a five-year field trial in upland red soil, China. Field Crop Res 232:77–87
Lehmann J (2007a) A handful of carbon. Nature 447:143–144
Lehmann J (2007b) Bio–energy in the black. Front Ecol Environ 5:381–387
Liu Y, Zhu ZQ, He XS, Yang C, Du YQ, Huang, YD, Su P, Wang S, Zheng XX, Xue YJ (2018) Mechanisms of rice straw biochar effects on phosphorus sorption characteristics of acid upland red soils. Chemosphere 207:267–277
Morales MM, Comerford N, Guerrini IA, Falcão NPS, Reeves JB (2013) Sorption and desorption of phosphate on biochar and biochar–soil mixtures. Soil Use Manag 29:306–314
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Novak JM, Busscher WJ, Laird DL, Ahmedna M, Watts DW, Niandou MA (2009) Impact of biochar amendment on fertility of a southeastern coastal plain soil. Soil Sci 174:105–112
Pizzeghello D, Berti A, Nardi S, Morari F (2016) Relationship between soil test phosphorus and phosphorus release to solution in three soils after long-term mineral and manure application. Agric Ecosyst Environ 233:214–223
Qi F, Dong Z, Lamb D, Naidu R, Bolan NS, Ok YS, Liu C, Khan N, Johir M, Semple KT (2017) Effects of acidic and neutral biochars on properties and cadmium retention of soils. Chemosphere 180:564–573
R Core Team (2014) R: A language and environment for statistical computing. R foundation for statistical computing, Vienna
Schneider F, Haderlein SB (2016) Potential effects of biochar on the availability of phosphorus–mechanistic insights. Geoderma 277:83–90
Siemens J, Ilg K, Lang F, Kaupenjohann M (2004) Adsorption controls mobilization of colloids and leaching of dissolved phosphorus. Eur J Soil Sci 55:253–263
Smyth TJ, Sanchez PA (1980) Effects of lime, silicate, and phosphorus applications to an oxisol on phosphorus sorption and ion retention. Soil Sci Soc Am J 44:500–505
Soil Survey Staff (2010) Keys to soil taxonomy, 11th edn. USDA-natural resource conservation service, Washington, DC
Soinne H, Hovi J, Tammeorg P, Turtola E (2014) Effect of biochar on phosphorus sorption and clay soil aggregate stability. Geoderma 219:162–167
Takaya C, Fletcher L, Singh S, Anyikude K, Ross A (2016) Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere 145:518–527
Wang Y, Lin Y, Chiu PC, Imhoff PT, Guo M (2015) Phosphorus release behaviors of poultry litter biochar as a soil amendment. Sci Total Environ 512:54–463
Xu G, Sun J, Shao H, Chang SX (2014) Biochar had effects on phosphorus sorption and desorption in three soils with differing acidity. Ecol Eng 62:54–60
Xu G, Shao H, Zhang Y, Sun J (2018) Nonadditive effects of biochar amendments on soil phosphorus fractions in two contrasting soils. Land Degrad Dev 29:2720–2727
Yan X, Wei Z, Wang D, Zhang G, Wang J (2015) Phosphorus status and its sorption-associated soil properties in a paddy soil as affected by organic amendments. J Soils Sediments 15:1882–1888
Yao Y, Gao B, Zhang M, Inyang M, Zimmerman AR (2012) Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 89:1467–1471
Zhai L, CaiJi Z, Liu J, Wang H, Ren T, Gai X, Xi B, Liu H (2015) Short-term effects of maize residue biochar on phosphorus availability in two soils with different phosphorus sorption capacities. Biol Fertil Soils 51:113–122
Zhang H, Chen C, Gray EM, Boyd SE, Yang H, Zhang D (2016) Roles of biochar in improving phosphorus availability in soils: a phosphate adsorbent and a source of available phosphorus. Geoderma 276:1–6
Zhang X, Li H, Li M, Wen G, Hu Z (2019) Influence of individual and combined application of biochar, Bacillus megaterium, and phosphatase on phosphorus availability in calcareous soil. J Soils Sediments 19:3688–3698
Zhou M, Li Y (2001) Phosphorus-sorption characteristics of calcareous soils and limestone from the southern Everglades and adjacent farmlands. Soil Sci Soc Am J 65:1404–1412
Acknowledgments
We thank the two anonymous reviewers for their very helpful comments and revisions of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 41867019, 41967014) and the National Key Research and Development Program of China (NO. 2017YFD0301601).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest to this work.
Human and animal rights and informed consent
This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Responsible editor: Yongfu Li
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zheng, X., Wu, J., Yan, X. et al. Biochar-induced soil phosphate sorption and availability depend on soil properties: a microcosm study. J Soils Sediments 20, 3846–3856 (2020). https://doi.org/10.1007/s11368-020-02713-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-020-02713-0