Abstract
Purpose
Molecular organic markers are an effective tool to detect the adverse effects of anthropogenic inputs in the aquatic environment. Linear alkylbenzenes (LABs) are sewage molecular organic markers that are released into the environment as a consequence of the incomplete sulphonation step of industrial detergents. In the current work, the characterisation, sources and degradation of LABs in the surface sediments of Muar River and Johor Bahru Coast, Malaysia, were identified.
Materials and methods
The surface sediment samples were obtained from the Muar River and Johor Bahru Coast area using an Ekman dredge sampler. Both locations were selected to represent the current anthropogenic pollution situation. The sediment samples were subjected to Soxhlet extraction, first purification step, second fractionation step column chromatography and then gas chromatography-mass spectrometry (GC-MS) analysis.
Results and discussion
The results revealed that the LAB concentrations in the samples of the Muar River and Johor Bahru Coast ranged from 87.4 to 188.1 ng g−1 dry weight (dw) and from 87.6 to 188.7 ng g−1 dw, respectively. The ratios of internal isomer (the benzene ring is closed to the centre of the linear alkyl chain) to external isomer (the benzene ring nears the end of the linear alkyl chain) (I/E ratio) of LABs were between 1.7 and 2.2 in Muar River and between 1.8 and 2.7 in Johor Bahru Coast samples.
Conclusions
These findings indicate that the concentrations of LABs in the sediments ranged from low to moderate contamination and the aquatic environment received both primary and secondary sewage effluents. This study emphasises that continuous monitoring of sewage pollution to minimise the environmental pollution in coastal areas is highly recommended.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Coastal zones are important habitats for aquatic organisms. However, these environments are sensitive to the pollutants caused by various anthropogenic activities such as urban and industrial discharges, agricultural activities, organic matter combustion, direct petroleum input, marine, fishing and tourism activities (Wei et al. 2014; Keshavarzifard and Zakaria 2015; Keshavarzifard et al. 2016, 2017a). The molecular markers are used as indicator to trace sources of anthropogenic inputs in the environment (Takada and Eganhouse 1998; Martins et al. 2014). One of these molecular markers is the linear alkylbenzenes (LABs) which have been commonly used due to their high hydrophobicity (log Kow ranging from ~ 7 to ~ 9), which demonstrates the strong partitioning behaviour of LABs to sewage particles. These particles settle down on surface sediments and in anaerobic condition with no biodegradation for long periods (Murray et al. 1987; Raymundo and Preston 1992; Takada et al. 1994; Isobe et al. 2004; Wei et al. 2014; Magam et al. 2015; Alkhadher et al. 2016). LABs, alongside the linear alkyl benzene sulfonate (LASs)–type detergents, are discharged into the environment through applications of household laundry detergents and dishwashing applications. The external isomers of LABs are biodegraded further more easily than the internal ones due to the benzene ring of the external isomer near the terminal of the alkyl chain side (Eganhouse et al. 1983; Takada and Ishiwatari 1987; Valls et al. 1989; Takada and Ishiwatari 1990). LABs consist of a suite of 26 phenyl alkanes with chain lengths ranging from 10 to 14 carbon atoms in a typical commercial formula, which are used to produce linear alkylbenzene sulfonates (LASs) as the major anionic surfactants widely used in the manufacturing of detergents since the 1960s (Eganhouse et al. 1983; Sherblom et al. 1992). Therefore, the LABs represent 13% of the incomplete sulphonation that resides in the final products, and thus its discharge with the surfactant wastewater into the aquatic systems (Eganhouse et al. 1983). Hence, the current work focused on detecting the current input of LABs released from municipal wastewater to the aquatic environment.
The final synesthetic detergents (LASs-type detergents) contain low amounts of LABs. However, the widespread consumption of detergents with high quantities leads to accumulate LABs in the aquatic environments (Eganhouse et al. 1983; Takada and Ishiwatari 1987; Alkhadher et al. 2015; Magam et al. 2015).
The I/E ratio of total LABs (6-C12+5-C12)/(4-C12+3-C12+2-C12) is developed to be used as indicator for LAB biodegradation in marine environments. Moreover, the differences in the biodegradation capacities of the internal and external isomers of the LABs and I/E ratio are scientifically referenced as LAB indicators (Takada and Ishiwatari 1990; Gustafsson et al. 2001; Tsutsumi et al. 2002; Isobe et al. 2004; Luo et al. 2008; Zhang et al. 2012; Martins et al. 2014; Wei et al. 2014; Dauner et al. 2015). LABs have been utilised as molecular markers for sewage contamination in various locations around the world (Takada and Ishiwatari 1987; Díez et al. 2006; Luo et al. 2008; Ni et al. 2008; Venkatesan et al. 2010; Rinawati et al. 2012; Alkhadher et al. 2015; Dauner et al. 2015; Magam et al. 2015; Masood et al. 2015). The high concentrations of LABs have been recorded in different environmental compartments, for instance, riverine water column particles (Takada and Ishiwatari 1987; Ni et al. 2008; Dauner et al. 2015), sediments (Eganhouse et al. 1983; Ishiwatari et al. 1983; Wang et al. 2012; Martins et al. 2014), biota such as fish and mussels (Peterman and Defino 1990; Tsutsumi et al. 2002), municipal wastes (Eganhouse et al. 1983; Takada and Ishiwatari 1987) and industrial wastes (Zhang et al. 2012).
The southern part of Peninsular Malaysian, with high population and urbanisation activities, is associated with high disposal of the industrial and municipal effluents into the natural water systems. Therefore, these natural water systems are potentially influenced by different anthropogenic inputs (Shahbazi et al. 2010; Keshavarzifard et al. 2016; Keshavarzifard et al. 2017a, b). Muar River passes through three states in Malaysia including Negeri Sembilan, Pahang and finally flows to Malacca straits at Kuala Muar (Abu Samah et al. 2011). Kuala Muar city is located at the mouth of the Muar River with population of 49,000 people (Abu Samah et al. 2011). The Muar River is one of the main rivers in Peninsular Malaysia and is famous for its fresh prawn and some species of fish. Johor Bahru oversees the South coast and is the second largest state in Peninsular Malaysia with an area of approximately 19,000 km2 and a population of 3.5 million people (DOSM 2016). The coastal zone of Johor Bahru is characterised by rapid development such as tourism and fishing activities (Hadibarata et al. 2012). LABs are strongly related to anthropogenic activities. Therefore, they can indicate the extent of anthropogenic impacts on the aquatic environments of Malaysia. The criteria of choosing a marker depend on the availability and persistence of the marker in the environment as well as the specific source influence of the marker (Takada et al. 1997). For instance, coprostanol is used as an indicator for faecal steroid contamination due to its persistent and positive correlation with faecal bacteria (Isobe et al. 2002). In contrast, LABs are used as an indication of sewage treatment efficiencies and the modernity of sewage contamination in the environment based on the different biodegradation capacities of the internal and external isomers of LABs (Tsutsumi et al. 2002). The studies in the literature have investigated the composition of LABs in some parts of Peninsular Malaysia such as Penang Estuary, Port Klang, Negeri Sembilan, Malacca and Sarawak Rivers (Isobe et al. 2004; Magam et al. 2012). However, there is no data available from other locations in Peninsular Malaysia. The main aim of the present study is to assess the anthropogenic inputs of LABs in the aquatic environments of the Muar River and Johor Bahru Coast. Therefore, the concentrations of LABs were determined and the possible sources and degradation level of LABs in the studied areas were evaluated.
2 Methods
2.1 Sampling
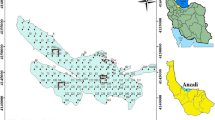
The research areas investigated in this study are situated in Johor state, Malaysia. The sampling areas and description of the sampling sites of the study areas are illustrated in Fig. 1. The studied area was selected based on presence of high and less anthropogenic activity across south and west Peninsular Malaysia.
In order to assess the anthropogenic input of LABs in the study areas, the collection of surface sediment samples was done from eight sampling locations in these areas (Table 1). The top 4 cm of each sediment sample was taken from the eight areas representing current introductions of anthropogenic pollution along the Muar river, including upstream (nearer to the source of the river), midstream and downstream (moving in the direction in which the river flows) of the river (Fig. 1), and from Johor Bahru Coast using an Ekman dredge sampler. The sediments were then put into pre-solvent rinsed stainless steel containers, transferred to the laboratory with dry ice in a cooler box and kept in a freezer at − 20 °C. Moisture was removed from the sediments using a freeze-dryer prior to further chemical analysis.
2.2 Chemical analysis
The analysis methods of LABs in the sediment extraction were carried out using two columns with purification (remove polar compounds) and fractionation of the resulting organic extracts as described by authors in the literature (Hartman et al. 2000; Zakaria et al. 2002; Magam et al. 2015; Masood et al. 2015).
A fixed weight of the dried sediments (10 g) was put in a cellulose thimble and then extracted with 250 mL of dichloromethane (DCM) in a Soxhlet apparatus for 8–10 h according to Masood et al. (2015) and Vaezzadeh et al. (2014). Fixed volumes of 50 μL of “1-Cn” LABs as surrogate standards (SS) of LABs were added to the samples prior to extraction for the recovery correction of target LABs. In “1-Cn” LAB, 1- represents the first isomer of each LAB homologue, and n represents the carbon number (8–14). The extract was further subjected to activated copper to eliminate sulphur to avoid any interference with the final GC-chromatograms.
The extract volume was reduced by utilising a rotary evaporator and moved to the top of 5% H2O deactivated silica gel (60–200 mesh size, Sigma Chemical Company, USA) in a chromatography column (0.9 cm i.d, 9 cm height). Hydrocarbons were eluted in this step using 20 mL of a purity hexane/DCM mixture (3:1, v/v). The elution volume was reduced to 1–2 mL and then, a fully activated silica gel column (0.47 cm i.d, 18 cm height) was used at this step in order to obtain LAB fractions using 4 mL of hexane. Next, the fractions of LABs were moved to a 2-mL amber vial and decreased to dryness using a gentle stream of nitrogen. Internal standards (IS = biphenyl-d10, m/z = 164) were put into the fraction before GC-MS analysis.
A GC-MS of 7890A series, gas chromatograph, Agilent Technologies, interfaced with a C5975 MSD split/splitless injector, was used to analyse the LABs. In brief, a 30-m fused silica capillary column of 0.25-mm internal diameter (i.d.) and a DB-5MS capillary column of 0.25-μm film thickness were used. The carrier gas was helium at a constant pressure of 60 kg cm−2. Mass spectral data were done using the selective ion monitoring (SIM) mode and the LABs were detected at m/z = 91, 92 and 105. The GC-MS operating condition was set at 70 eV for the ionisation process, with the source at 200 °C and electron multiplier voltage at ~ 1250 eV. The injection port was kept at 300 °C when the sample was injected via splitless mode, followed by a 1-min purge. The column temperature was kept at 70 °C for 2 min, maintained at 30 °C min−1 to 150 °C and finally, increased from 4 °C min−1 to 310 °C for 15 min.
2.3 Quality control and assessment
The surrogate standards (1-Cn LABs) were recovered with a reasonably efficient proportion within the accepted range (between 60 and 120%), showing only a minimal loss of the target compound in the analysis procedures. The range of recoveries for LAB surrogates was within 87–98% for the sediment samples analysed in this study. Each batch of samples (four samples) was processed with a blank that contained in an empty glassware all the chemicals and the standards (SS, IS and native standards) present in the normal samples to avoid any potential cross contamination from different sources during the analytical procedures. Standards such as SS, IS and native standards were freshly prepared daily and spiked with known concentration into the sediment samples. The GC-MS was set in selected ion monitoring mode at m/z = 91, 92 and 105 to analyse the target LAB congener. Five concentrations of a LAB standard mixture (SIS, IIS and native standards) within the range of 0.25–5.0 ppm were injected together with samples and a 5-point calibration curve was used to quantify the LAB target compounds. The limits of quantification (LOQ) and limits of detection (LOD) were calculated by the lowest concentration level of each calibration curve divided by the mean sample weight (Takada and Eganhouse 1998). They ranged within 0.1–2 ng g−1 and 0.02–0.1 ng g−1, respectively.
2.4 Total organic carbon analysis
The total organic carbon (TOC) was measured in sediments utilising a LECO CR-412 analyser. Sediment samples were kept in an oven at 60 °C overnight to be dried; then, a mortar and pestle were used to ground the dried samples. 1–2 g of each dried sediment sample was weighed and then, 1–2 mL of 1 M HCl was added to the sample until it was completely moist by HCl to remove inorganic carbon (carbonates). Afterward, the HCl was removed from the sediment sample through drying at 100 °C for 10 h. Finally, a LECO CR-412 Carbon Analyser (with furnace temperature of 1350 °C and O2 boost time of 1 min) was used to determine the TOC% (Nelson and Sommers 1996). The determined TOC percentage is shown in Table 2.
3 Results and discussion
3.1 Composition and distribution of ΣLABs
Structural congeners of the LABs are shown in Fig. 2, with their sum expressed as ΣLABs. The structure of the individual congener is indicated as “n-Cm” LAB, where n shows the position of the benzene ring on the straight alkyl chain and m represents the number of alkyl carbons.
Gas chromatograms of LABs in the surface sediments of (a) the Muar River and (b) Johor Bahru Coast. IIS (internal injection standard-biphenyl, d10); surrogates 1-Cn-LABs (n, 8–14) from left to right indicated by asterisks. Subscripts indicate the alkyl chain length. Numbers on the peaks indicate the phenyl substituted position on the alkyl chain
The LABs C10–C14 were detected in all investigated sediments. As shown in Table 2, ∑LABs C10–C14 ranged from 87.4 to 188.1 and 87.6 to 188.7 ng g−1 dw in the surface sediments of the Muar River and Johor Bahru Coast, respectively. These concentrations are higher than those recorded in the sediment samples at Southern California Bight sediments (1.7–92.9 ng g−1), while are lower than those reported in northern Tokyo Bay, Japan (1000–3270 ng g−1) (Table 3) (Takada et al. 1992; Hartmann et al. 2000; Macıas-Zamora and Ramırez-Alvarez 2004).
The highest concentration of total LABs in the current research was detected at the station SJB3 in the Johor Bahru Coast (188.7 ng g−1 dw), whereas the lowest concentration was found at SMu3 of the Muar River (87.4 ng g−1 dw) (Table 2). The maximum concentration of LABs in the Muar River was detected in the upstream (SMu1, 188.1 ng g−1 dw). The concentrations are higher than ∑LABs previously observed in the Muar River sediments and in the Kim Kim River (32 and 122 ng g−1 dw, respectively) but lower than the LAB concentrations found in the Port Klang and Penang Estuaries, Malaysia (8590 and 3000 ng g−1 dw, respectively) (Isobe et al. 2004; Table 3). The explanations for these differences might be related to the rapid industrialisation and urbanisation in the upstream portion of the Muar River which are associated with the spatial distributions of LABs in this river (Alkhadher et al. 2016).
The decrease in concentrations of ∑LABs in the Muar River from upstream to downstream is depicted in Fig. 3. The results are comparable to previous findings (68–242 ng g−1 dw) reported by Magam et al. (2015). The Muar River flows through a highly urbanised area with high anthropogenic activities (Abu Samah et al. 2011). The highest concentration of ∑LABs at the upstream and midstream locations could be explained by the fact that the sampling sites are close to LAB input sources, where both untreated and treated effluents are being released into the river.
The lower concentrations of ∑LABs in SMu3 might be due to the location of the station, which is in the river mouth and close to Malacca Sea where the salinity concentration is expected to be high and the salinity effect contributes to reduce their isomeric species. Another explanation for the lower concentrations of ∑LABs in SMu3 could be the heavy rainfall which washed out the high mass of eroded soil to the Muar River mouth (SMu3), hence diluting the concentrations of ∑LABs. The ∑LAB distribution in the riverine ecosystem is predominated by the lateral movement of sewage effluents combined with the intensity of sewage input (Zeng et al. 1997; Isobe et al. 2004).
The total LAB concentrations detected at SJB1, SJB2, SJB3, SJB4 and SJB5 were 116.8, 87.6, 188.7, 115.8 and 130.7 ng g−1 dw, respectively. The concentration of ∑LABs in the Johor Bahru Coast sediments varied in distribution with the trend of SJB3 > SJB5 > SJB1 > SJB4 > SJB2, showing that the distance of the sites from the input sources of ∑LABs is probably the reason for the variability of LAB concentrations in these stations. The ∑LAB sources of the sediments in Johor Bahru Coast might be resulted from higher industrialisation and urbanisation along Johor Bahru Coast and the transportation of these contaminants to the coastal locations are likely responsible for these spatial distributions. Intensive tourism activities in the area and sewage inputs that are coming directly from the fishing boats could also be amongst the important sources of LABs in Johor Bahru Coast. The high utilisation of synthetic detergents in bordering urbanised sites in the sampling stations can contribute to the concentrations of LABs (Ni et al. 2008). Marine effects that can be affected by organic input, pollutant input and pathogenic microorganisms are well reported (Wang et al. 2012). Therefore, it is satisfactory to define an environmental policy for this area in order to avoid further anthropogenic impact. The hydrodynamic process combined with lateral movements could be the major input pathway of LABs in the sediments. It has been reported in the literature that wastewater release, in addition to horizontal flow, was responsible for the existence of ∑LABs in aquatic environment sediments (Sherblom et al. 1992; Luo et al. 2008; Ni et al. 2008; Montone et al. 2010).
The LABs and PAHs are persistent organic pollutants (POPs) and have similar physiochemical properties. In this study, both compounds exhibited similar spatial distribution trend with previous detection by Vaezzadeh et al. (2014). These findings are in agreement with Luo et al. (2008) who reported moderate to high correlation coefficients (from 0.59 to 0.77, p < 0.05) between PAHs and LABs revealed in sediments from South China Sea (SCS), China. The study suggested that both PAHs and LABs were associated with sewage-derived particulates and have similar sources and/or input routes in these regions.
Due to recreational activities such as fishing in the Muar River (Samah et al. 2011), large amounts of waste and detergents have been directly discharged from boats and fishing vessels into the Muar River and Johor Bahru Coast. As a result, it can be indicated that the sources of LABs in the present study areas may result from the release of domestic sewage into aquatic environments together with direct waste release from boats and fishing vessels. It was inferred from the outcomes of LAB concentrations that the sewage treatment plants had a direct impact on the concentrations of LABs, since a lower concentration of ∑LABs was observed in sampling locations with sewage treatment plants. The isomeric compositions of ∑LABs in the sediments are illustrated in Fig. 4.
A relative abundance of 13-, 12- and 14-LAB homologs was detected in the sediments, while 10- and 11-LAB homologs showed less abundance. These outcomes are in perfect agreement with the results of Phillips et al. (2001) and Martins et al. (2010), who revealed significant amounts of 13- and 14-LAB homologs in sediments from the near-outfall sites of Southern California, while 10- and 11-LAB homologs showed lower proportions. One possible reason behind this composition of LABs in the sediments could be the selective loss of C10 and C11 homologs while sewage particles are settling down. Furthermore, the isomeric compositions of LABs in the sediments shown in Fig. 4 indicated a difference between the distribution of ∑LAB chain length with lower amounts of C10 and C11 homologs similar to ∑LABs in detergents and sludge (Luo et al. 2008). The enrichment of 12-LAB homologs has been detected in the first station of the Muar River and explained by the fact that this station is the nearest to sewage outfalls and this is consistent with those of 12-LAB homologs in sediments from Southern California sites (Phillips et al. 2001). An abundance of the C13 homologs found in sediments in this research was also reported elsewhere (Luo et al., 2008). A major reason can be more biodegradation of short homologs (C10, C11 and C12) in the sediments, along with higher Kow of long-chain LABs, and therefore, their higher tendency to be adsorbed to particulate matter and settle down as sediments can be another explanation.
The LABs are used to evaluate the types and efficiency of STPs. Takada and Eganhouse (1998) proposed that the I/E ratios in primary effluents are generally low, ranging from 0.5 to 0.9, and the secondary effluents show a much higher I/E ratio ranging from 2 to 7. The high I/E ratios in the secondary effluents belong to the bacterial degradation of LABs. Therefore, the I/E ratios in the industrial and domestic sewage depend on the bacterial load, which plays an important role in the secondary treatment process.
3.2 Evaluation of ∑LAB biodegradation and sources using ∑LAB ratio
Molecular ratios are suitable tools for the identification of LAB sources in aquatic environments. The researchers in the literature have applied LAB ratios as indicators of sources in sediments (Takada and Ishiwatari 1990; Magam et al. 2015; Masood et al. 2015). One of these ratios is an internal (I) isomer to the external (E) one (I/E ratio). The internal isomer of LABs is more difficult to degrade than the external one (Ishiwatari et al. 1983; Isobe et al. 2004). The I/E ratios increase during ∑LAB biodegradation under aerobic conditions (Takada and Ishiwatari 1990). Therefore, LAB ratios have been proposed for application (Takada and Ishiwatari 1990; Alkhadher et al. 2015) as indicators of the extent of ∑LAB degradation. The ratio of I/E ranged between 1.7 and 2.2 in the Muar River and between 1.8 and 2.7 in Johor Bahru Coast sediments, showing increased biodegradation of LABs (Fig. 5).
The I/E ratio in the Muar and Johor Bahru Coast sediment samples. The horizontal lines are drawn as stated in Takada and Eganhouse (1998)
The I/E ratios indicate that primary and secondary effluents are being released to the aquatic environments of the study area. The I/E ratios of the Muar River and Johor Bahru Coast sediments are higher than the ∑LABs of the Pearl River Estuary, China, ranging from 0.6 to 1.5 (Luo et al. 2008), suggesting a high release of treated effluents in the Muar and Johor Bahru Coast.
A high I/E ratio was detected upstream of the Muar River (SMu1) as compared with the other sites of the Muar River and Johor Bahru Coast. Therefore, it seems that the sediment of SMu1 has gone through greater degradation, which might be due to the higher bacterial activity in this station. The I/E ratios in the Muar River are different from the expectations from the experiments of incubation degradation (Takada and Ishiwatari 1990). This result supports that LAB isomers are converted via a process that causes the phenyl group to migrate after a H-abstraction of the alkyl chain, followed by reacquisition of hydrogen from attaching organic matter (Gustafsson et al. 2001).
The I/E ratios in the Johor Bahru Coast sediments showed constant values ranging between 1.8 and 2.7, indicating aerobic degradation. The high I/E ratio in the Johor Coast stations (1.8–2.7) was attributed into the effective coverage of sewage treatment plants (STPs) in recent years where 77% of Johor Bahru was equipped with STPs (Malaysian Water Industry Guide 2015).
It is proved that the sewage released from medium- and small-sized industries with poor sewage treatment facilities is the main pollution source into the west and south of Malaysian coastal (Rosnani 2001).
The accuracy of I/E ratio as an indicator of LAB biodegradation in offshore areas has been reported in the literature. For example, a decreasing trend was detected in the values of I/E ratios in Boston Harbour with increasing in the distance from the sources to offshore (Gustafsson et al. 2001), which differ from incubation degradation experiments. A similar trend of I/E ratios was observed by Luo et al. (2008) where the ratios ranged from 0.2 to 0.9 in offshore sediments of the South China Sea, while the range of ratios was between 0.7 and 1.9 in terrestrial rivers. Therefore, the L/S ratio, defined as (5-C13+5-C12)/(5-C11+5-C10), was also suggested as a LAB biodegradation indicator in riverine areas (Gustafsson et al. 2001). The L/S ratios of the sediments in this study are illustrated in Table 2. A similar trend with I/E ratios was found in the L/S ratios of the Muar River sediments (SMu1 > SMu2 > SMu3). Alternatively, the ratio of L/S in Johor Bahru Coast was comparable (2.4 to 2.5) with its I/E ratios (1.8–2.7), all of which are in line with the expectations that the ∑LABs of this area should have undergone a higher degree of degradation compared with I/E ratio range. These variables suggest that the L/S ratio can be a useful indicator for the extent of ∑LAB biodegradation in ecosystems.
A relative decrease in C12 homologs was reported by Luo et al. (2008) in the sediment of the Pearl River Estuary and North South China Sea. Therefore, ratio of C13/C12 (6-, 5-, 4-, 3- and 2-C13 LABs)/(6-, 5-, 4-, 3- and 2-C12 LABs) was proposed to evaluate the distribution of carbon chain lengths (Fig. 6). The ratio of C13/C12 decreases seaward, which can be explained by the higher hydrophobicity of C13 homologs, resulting in higher attachment to suspended particulate matters and settling down in the sediment (Sherblom et al. 1992). Takada and Eganhouse (1998) developed a log linear regression to measure the relationship between the I/E ratios and the degree of LAB degradation with the following equation: ∑LABs deg (%) = 81 × log (I/E ratios) + 15 (r2) = 0:96. The percentage of ∑LAB biodegradation was measured for the sediments in this study based on this formula and the results ranged from 33 to 43% and from 34 to 49%, respectively (Table 2).
The high LAB degradation indices with high abundance of 13-LAB were detected in the Muar River compared with the near shore of the adjacent South China Sea (Luo et al. 2008; Fig. 4). This is a sign of improvement in the wastewater treatment in the studied area and indicates a greater extent of degradation.
According to the ∑LAB ratios, it was difficult to come to a unique conclusion for sediments of the study area. Similarly, the contradiction of I/E, L/S and C13/C12 ratios was reported earlier in the sediment of the Pearl River Delta (Luo et al. 2008), oceanic regions off Boston Harbour (Gustafsson et al. 2001) and Guangdong Province coastal areas (Liu et al. 2013). Moreover, the occurrence of LAB congener inter-conversions involving phenyl group migration or the enrichment of external isomers might have been the reason behind the anticipated increase in the I/E values (Gustafsson et al. 2001; Luo et al. 2008). Therefore, the aerobic conditions combined with high concentration of ∑LABs could have affected the evaluation of biodegradation, and L/S and C13/C12 ratios are reported as sensitive ratios showing biodegradation in sediment from the Johor Bahru Coast area.
The ∑LAB concentrations in the Muar River have a moderate correlation with the amount of organic compound (R2, 0.54) in the surface sediment (Fig. 7(a)). Therefore, the increase of the ∑LAB concentrations in the Muar River is explained as a result for increasing the organic matter input coming from domestic waste and sewage discharge due to increasing population growth in this area (Bakhtiari et al. 2011). This is inconsistent with earlier hypothesis that stated strong correlation between ∑LABs and TOC suggesting sewage release as the main source for organic carbon to the river sediments (Zhang et al. 2012; Magam et al. 2015). On the other hand, Fig. 7 (b) demonstrates that the correlation of TOC and ∑LABs at the Johor Bahru Coast is very weak (R2 = 0.29). These findings indicated that TOC was not a controlling factor for the distribution of ∑LABs. This confirmed the earlier work done on California’s coastal region (Macıas-Zamora et al. 2004), which concluded that there was no linear relationship between ∑LABs and TOC, which suggested that TOC is not a controlling factor in determining the distribution of ∑LABs in the surface sediments of the south end of the Southern California Bight. Therefore, anthropogenic input from demotic waste and sewage discharge of Johor Bahru could be the controlling factors for the ∑LAB distribution in coastal water. The poor correlation of TOC in this area may attribute to the different sources of ∑LABs and TOC.
4 Conclusions
The concentrations of ΣLABs in surface sediments of the Muar River were lower than those of Johor Bahru Coast. High LAB concentrations were recorded in the upstream study sites of the Muar River, while the stations downstream displayed low concentrations, indicating that upstream urban wastewater was the main source of ∑LABs. The intensity of ∑LAB inputs is the main predominant factor in the ∑LAB distribution of the study area. ∑LAB ratios of I/E, L/S and C13/C12 were observed to be high in both study areas, indicating that LAB degradation in the Muar River is lower than in Johor Bahru Coast. The biodegradation ratios indicate that the study areas received primary and secondary treated effluents. The results of this research showed the necessity for incessant investigation of potential pollution in coastal ecosystems in order to avoid possible contamination from sewage pollution in the future. The installation of more efficient sewage treatment plants is required at the surrounding areas of the study locations to minimise sewage pollution in this area.
References
Abu Samah B, Yassin SM, Shaffril HAM, Hassan MS, Othman MS, Abu Samah A, Ramli SA (2011) Relationship to the river: the case of the Muar River community. Am J Environ Prot 7:362–369
Alkhadher SAA, Zakaria MP, Yusoff FM, Kannan N, Suratman S, Keshavarzifard M, Magam SM, Masood N, Vaezzadeh V, Sani MSA (2015) Baseline distribution and sources of linear alkylbenzenes (LABs) in surface sediments from Brunei Bay, Brunei. Mar Pollut Bull 101(1):397–403
Alkhadher SAA, Zakaria MP, Yusoff FM, Kannan N, Suratman S, Magam SM, Masood N, Keshavarzifard M, Vaezzadeh V, Sani MSA (2016) Distribution and sources of linear alkylbenzenes (LABs) in surface sediments from Johor Bahru Coast and the Kim Kim River, Malaysia. Environ Forensic 17(1):36–47
Bakhtiari AR, Zakaria MP, Yaziz MI, Lajis MNH, Bi X (2011) Variations and origins of aliphatic hydrocarbons in sediment cores from Chini Lake in Peninsular Malaysia. Environ Forensic 12(1):79–91
Dauner ALI, Hernández EA, Mac Cormack WP, Martins CC (2015) Molecular characterisation of anthropogenic sources of sedimentary organic matter from Potter Cove, King George Island, Antarctica. Sci Total Environ 502:408–416
Department of Statistics Malaysia (DOSM). Population and demography report no. 1; intercensal mid-year population estimates, June 2016, www.statistics.gov.my (accessed 8 June /2016)
Díez S, Jover E, Albaigés J, Bayona JM (2006) Occurrence and degradation of butyltins and wastewater marker compounds in sediments from Barcelona Harbor, Spain. Environ Int 32:858–865
Eganhouse RP, Blumfield DL, Kaplan IR (1983) Long-chain alkylbenzenes as molecular tracers of domestic wastes in the marine environment. Environ Sci Technol 17(9):523–530
Eganhouse RP, Olaguer DP, Gould BR, Phinney CS (1998) Use of molecular markers for the detection of municipal sewage sludge at sea. Mar Environ Res 25(1):1–22
Gustafsson Ö, Long CM, MacFarlane J, Gschwend PM (2001) Fate of linear alkylbenzenes released to the coastal environment near Boston Harbor. Environ Sci Technol 35(10):2040–2048
Hadibarata T, Abdullah F, Yusoff ARM, Ismail R, Azman S, Adnan N (2012) Correlation study between land use, water quality, and heavy metals (Cd, Pb and Zn) content in water and green lipped mussels Perna viridis (Linnaeus) at the Johor Strait. Water Air Soil Pollut 223:3125–3136
Hartmann PC, Quinn J, King JW, Tsutsumi S, Takada H (2000) Intercalibration of LABs in marine sediment SRM1941a and their application as a molecular marker in Narragansett Bay sediments. Environ Sci Technol 34(5):900–906
Ishiwatari R, Takada H, Yun SJ, Matsumoto E (1983) Alkylbenzene pollution of Tokyo Bay sediments. Nature 301(5901):599–600
Isobe KO, Tarao M, Zakaria MP, Chiem NH, Minh LY, Takada H (2002) Quantitative application of fecal sterols using gas chromatography-mass spectrometry to investigate fecal pollution in Tropical waters: Western Malaysia and Mekong Delta, Vietnam. Environ Sci Technol 36:4497–4507
Isobe KO, Zakaria MP, Chiem NH, Minh LY, Prudente M, Boonyatumanond R, Saha M, Sarkar M, Takada H (2004) Distribution of linear alkylbenzenes (LABs) in riverine and coastal environments in South and Southeast Asia. Water Res 38(9):2449–2459
Keshavarzifard M, Zakaria MP (2015) Polycyclic aromatic hydrocarbons (PAHs) contamination of surface sediments from Port Dickson, Malaysia: distribution, sources and ecological risk assessment. Environ Forensic 16(4):322–332
Keshavarzifard M, Zakaria MP, Keshavarzifard S (2016) Evaluation of polycyclic aromatic hydrocarbons contamination in the sediments of the Johor Strait, Peninsular Malaysia. Polycycl Aromat Compd 39(1):44–59
Keshavarzifard M, Zakaria MP, Hwai TS (2017a) Bioavailability of polycyclic aromatic hydrocarbons (PAHs) to short-neck clam (Paphia undulata) from sediment matrices in mudflat areas of West coast of Peninsular Malaysia. Environ Geochem Health 39:591–610
Keshavarzifard M, Zakaria MP, Sharifi R (2017b) Ecotoxicological and health risk assessment of polycyclic aromatic hydrocarbons (PAHs) in short-neck clam (Paphia undulata) and contaminated sediments in Malacca Strait, Malaysia. Arch Environ Contam Toxicol 73(3):474–487
Liu LY, Wang JZ, Wong CS, Qiu JW, Zeng EY (2013) Application of multiple geochemical markers to investigate organic pollution in a dynamic coastal zone. Environ Toxicol Chem 32(2):312–319
Luo XJ, Chen SJ, Ni HG, Yu M, Mai BX (2008) Tracing sewage pollution in the Pearl River Delta and its adjacent coastal area of South China Sea using linear alkylbenzenes (LABs). Mar Pollut Bull 56(1):158–162
Macıas-Zamora J, Ramırez-Alvarez N (2004) Tracing sewage pollution using linear alkylbenzenes (LABs) in surface sediments at the south end of the Southern California Bight. Environ Pollut 130(2):229–238
Magam SM, Zakaria MP, Halimoon N, Masood N, Alsalahi MA (2012) Aliphatic distribution of linear alkylbenzenes (LABs) in sediments of Sarawak and Sembulan Rivers, Malaysia. Environ Asia 5(1):48–55
Magam SM, Halimoon N, Zakaria MP, Aris AZ, Kannan N, Masood N, Mustafa S, Alkhadher SAA, Keshavarzifard M, Vaezzadeh V, Sani MSA (2015) Evaluation of distribution and sources of sewage molecular marker (LABs) in selected rivers and estuaries of Peninsular Malaysia. Environ Sci Pollut Res Int 23(6):5693–5704
Malaysia Water Industry Guide (MWIG) (2015) http://www.mwaorg.my/publication-guideline.html. (accessed 8 June 2016)
Martins CC, Ferreira JA, Taniguchi S, Mahiques MM, Bícego MC, Montone RC (2008) Spatial distribution of sedimentary linear alkylbenzenes and faecal steroids of Santos Bay and adjoining continental shelf, SW Atlantic, Brazil: origin and fate of sewage contamination in the shallow coastal environment. Mar Pollut Bull 56(7):1359–1363
Martins CC, Bícego MC, Mahiques MM, Figueira RC, Tessler MG, Montone RC (2010) Depositional history of sedimentary linear alkylbenzenes (LABs) in a large South American industrial coastal area (Santos Estuary, Southeastern Brazil). Environ Pollut 158(11):3355–3364
Martins CC, Aguiar SN, Bícego MC, Montone RC (2012) Sewage organic markers in surface sediments around the Brazilian Antarctic station: results from the 2009/10 austral summer and historical tendencies. Mar Pollut Bull 64(12):2867–2870
Martins CC, Cabral AC, Barbosa-Cintra SCT, Dauner ALL, Souza FM (2014) An integrated evaluation of molecular marker indices and linear alkylbenzenes (LABs) to measure sewage input in a subtropical estuary (Babitonga Bay, Brazil). Environ Pollut 188:71–80
Masood N, Halimoon N, Zakaria MP, Aris AZ, Kannan N, Mustafa S, Ali MM, Keshavarzifard M, Vaezzadeh V, Alkhadher SAA, Al-Odaini NA (2015) Anthropogenic waste indicators (AWI) particularly PAHs and LABs in Malaysian sediments: application of aquatic environment for identifying anthropogenic pollution. Mar Pollut Bull 102(1):160–175
Montone RC, Martins CC, Bícego MC, Taniguchi S, Silva DAM, Campos LS, Weber RR (2010) Distribution of sewage input in marine sediments around a maritime Antarctic research station indicated by molecular geochemical indicators. Sci Total Environ 408(2010):4665–4671
Murray A, Gibbs C, Kavanagh P (1987) Linear alkyl benzenes (LABs) in sediments of Port Phillip Bay (Australia). Mar Environ Res 23(1):65–76
Nelson D, Sommers L (1996) Total carbon, organic carbon and organic matter. In Sparks DL (ed) Methods of soil analysis. Part 3: Chemical methods. Soil Science Society of America, Madison, WI, USA, pp 961–1010
Ni HG, Lu FH, Wang JZ, Guan YF, Luo XL, Zeng EY (2008) Linear alkylbenzenes in riverine runoff of the Pearl River Delta (China) and their application as anthropogenic molecular markers in coastal environments. Environ Pollut 154(2):348–355
Peterman PH, Delfino JJ (1990) Identification of isopropylbiphenyl, alkyl diphenylmethanes, diisopropylnaphthalene, linear alkyl benzenes and other polychlorinated biphenyl replacement compounds in effluents, sediments and fish in the Fox River system. Wisconsin Biol Mass Spectrom 19(12):755–770
Phillips CR, Venkatesan MI, Lin T (2001) Linear alkylbenzenes in muscle tissues of white croaker near a large ocean outfall in southern California, USA. Environ Toxicol Chem 20(2):231–238
Raymundo C, Preston M (1992) The distribution of linear alkylbenzenes in coastal and estuarine sediments of the Western North Sea. Mar Pollut Bull 24(3):138–146
Rinawati KT, Koike H, Kurumisawa R, Ito M, Sakurai S, Togo A, Saha M, Arifinc Z, Takada H (2012) Distribution, source identification, and historical trends of organic micro pollutants in coastal sediment in Jakarta Bay, Indonesia. J Hazard Mater 217–218:208–216
Rosnani I (2001) River water quality status in Malaysia. In Proceedings of national conference on sustainable river basin management in Malaysia, Kuala Lumpur, Malaysia, November. 13–14, 2000
Samah BA, Yassin SM, Shaffril HA, Hassan MS, Othman MS (2011) Relationship to the river: the case of the Muar River community. Am J Environ Sci 7(4):362–369
Shahbazi A, Zakaria MP, Yap CK, Tan SG, Surif S (2010) Use of different tissues of Perna viridis as biomonitors of polycyclic aromatic hydrocarbons (PAHs) in the coastal waters of Peninsular Malaysia. Environ Forensic 11(3):248–263
Sherblom PM, Gschwend PM, Eganhouse RP (1992) Aqueous solubilities, vapor pressures, and 1-octanol-water partition coefficients for C9-C14 linear alkylbenzenes. J Chem Eng Data 37(4):394–399
Takada H, Ishiwatari R (1987) Linear alkylbenzenes in urban riverine environments in Tokyo: distribution, source, and behavior. Environ Sci Technol 21(9):875–883
Takada H, Ishiwatari R (1990) Biodegradation experiments of linear alkylbenzenes (LABs): isomeric composition of C12 LABs as an indicator of the degree of LAB degradation in the aquatic environment. Environ Sci Technol 24(1):86–91
Takada H, Ishiwatari R, Ogura N (1992) Distribution of linear alkylbenzenes (LABs) and linear alkylbenzene sulphonates (LAS) in Tokyo Bay sediments. Estuar Coast Shelf Sci 35(2):141–156
Takada H, Farrington JW, Bothner MH, Johnson CG, Tripp BW (1994) Transport of sludge-derived organic pollutants to deep-sea sediments at Deep Water Dump Site 106. Environ Sci Technol 28(6):1062–1072
Takada H, Satoh F, Bothner MH, Tripp BW, Johnson CG, Farrington JW (1997) Anthropogenic molecular markers: tools to identify the sources and transport pathways of pollutants. ACS Symposium Series, Vol 671. Chapter 12:178–195
Takada H, Eganhouse RP (1998) Molecular markers of anthropogenic waste: their use in determining sources, transport pathways and fate of wastes in the environment. In Encyclopedia of environmental analysis and remediation 5:2883–2940
Tsutsumi S, Yamaguchi Y, Nishida I, Akiyama K, Zakaria MP, Takada H (2002) Alkylbenzenes in mussels from South and South East Asian coasts as a molecular tool to assess sewage impact. Mar Pollut Bull 45(1):325–331
Vaezzadeh V, Zakaria MP, Mustafa S, Ibrahim ZZ, Shau-Hwai AT, Keshavarzifard M (2014) Distribution of polycyclic aromatic hydrocarbons (PAHs) in sediment from Muar River and Pulau Merambong, Peninsular Malaysia. From Sources to Solution, Kuala Lumpur, Malaysia. Springer, Singapore, pp 451–455
Valls M, Bayona JM, Albaiges J (1989) Use of trialkylamines as an indicator of urban sewage in sludges, coastal waters and sediments. Nature 337(6209):722–724
Venkatesan MI, Merino O, Baek J, Northrup T, Sheng Y, Shisko J (2010) Traceorganic contaminants and their sources in surface sediments of Santa Monica Bay, California, USA. Mar Environ Res 69(5):350–362
Wang JZ, Zhang K, Liang B (2012) Tracing urban sewage pollution in Chaohu Lake (China) using linear alkylbenzenes (LABs) as a molecular marker. Sci Total Environ 414:356–363
Wei GL, Liu LY, Bao LJ, Zeng EY (2014) Tracking anthropogenic influences on the continental shelf of China with sedimentary linear alkylbenzenes (LABs). Mar Pollut Bull 80:80–87
Zakaria MP, Takada H, Tsutsumi S, Ohno K, Yamada J, Kouno E, Kumata H (2002) Distribution of polycyclic aromatic hydrocarbons (PAHs) in rivers and estuaries in Malaysia: a widespread input of petrogenic PAHs. Environ Sci Technol 36(9):1907–1918
Zeng EY, Khan AR, Tran K (1997) Organic pollutants in the coastal environment off San Diego, California. 3. Using linear alkylbenzenes to trace sewage-derived organic materials. Environ Toxicol Chem 16(2):196–201
Zhang K, Wang JZ, Liang B, Shen RL, Zeng EY (2012) Assessment of aquatic wastewater pollution in a highly industrialized zone with sediment linear alkylbenzenes. Environ Toxicol Chem 31(4):724–730
Acknowledgements
This research is funded by University Tun Hussein Onn Malaysia (UTHM) under the postdoctoral scheme grant and Inisiatif Putra Berkumpulan Grant, UPM (9412401).
Funding
The research was funded by Inisiatif Putra Berkumpulan (IPB) Grant through Universiti Putra Malaysia (Grant No. 9412401).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This paper does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the paper.
Additional information
Responsible editor: Jan Schwarzbauer
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alkhadher, S.A.A., Kadir, A.A., Zakaria, M.P. et al. Monitoring of sewage pollution in the surface sediments of coastal ecosystems using linear alkylbenzenes (LABs) as molecular markers. J Soils Sediments 20, 3230–3242 (2020). https://doi.org/10.1007/s11368-020-02671-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-020-02671-7