Abstract
Purpose
Mining areas are low-quality habitats for macro- and microorganisms’ development, mainly due to the degradation of the soil quality by metal pollution. The present work aimed to analyze the influence of metal contamination and of plant species on the rhizospheric microbial communities of four indigenous metallophytes (Ononis natrix, Haloxylon scoparium, Peganum harmala, and Aizoon canariense) growing along a metal contamination gradient in Kettara mine near Marrakech, Morocco.
Materials and methods
In pyrrhotite mining areas (Kettara mine, Morocco), rhizosphere soil samples were collected from four predominant indigenous metallophytes (O. natrix, H. scoparium, P. harmala, and A. canariense) growing along a metal contamination gradient (ZC, control zone; Z1, high metal contamination; Z2, moderate metal contamination; Z3, low metal contamination). Microbial communities were analyzed by using microbial counts and by denaturing gradient gel electrophoresis (DGGE). The physicochemical properties (pH, conductivity, total organic carbon, nitrogen, P Olsen, and metal concentrations) of soils were also determined.
Results and discussion
The physicochemical analysis revealed that rhizospheric soils from Z1, Z2, and Z3 were relatively poor in nutrients as they presented low levels of total organic carbon and nitrogen, organic matter and available P. Moreover, these rhizospheric soils showed high concentrations of metals, especially Cu and Pb, which significantly reduced the abundance of the different groups of soil microorganisms (bacteria, fungi, and actinomycetes) and the activity of soil dehydrogenase. The analysis of bacterial communities by DGGE revealed that bacterial diversity was not negatively affected by metal contamination being higher in the most contaminated area (Z1).
Conclusions
Overall, the microbial abundance, the composition, and the diversity of rhizospheric bacterial communities were more influenced by the environmental factors in sampling zones than by plant cover. Microbial counts and enzymatic activity were both systematically affected throughout the metal gradient, evidencing as good indicators of the harmful effects of anthropogenic disturbances in soils. H. scorparium and P. harmala proved to be good candidates for the development of phytotechnological programs aiming the revegetation of mining degraded areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Soil contamination by anthropogenic activities has widely increased in last decades. Among soil pollutants, there is a particular concern with metals, as most of them cannot be transformed into less toxic species, persisting indefinitely in the environment (Ali et al. 2013; Ayangbenro and Babalola 2017). In addition to their harmful effects on plants, animals, and soil biodiversity, metals can also compromise human health, being a cross-cutting problem to several countries (Science Communication Unit 2013).
Mining industry is one of the most polluting activities in the world constituting a permanent threat for ecosystems and public health (Li et al. 2014a). Mining operations (e.g., milling, disposal of tailings) generate large amounts of toxic materials, including metals, which can be easily diffused into the surrounding environment (El Khalil et al. 2008; Mokhtari et al. 2018), even after the end of mining works (Acosta et al. 2011). The high concentrations of metals and the harsh soil conditions (e.g., low pH and organic matter content, erosion) registered in the vicinity of mining areas significantly impair plant survival and development, resulting in a scarce vegetation cover in these areas, which increase the risk of wind and hydraulic dispersion of contaminated particles (Monterroso et al. 2014).
Metallophytes are plants able to grow in highly metal-contaminated soils as they have developed mechanisms to tackle the harmful effects of metals in their tissues (Alford et al. 2010). Several studies have shown the potential of native metallophytes present in many metalliferous sites to be used in phytotechnological approaches for the remediation and revegetation of environmental degraded areas (Whiting et al. 2004; Nadgórska-Socha et al. 2015). In Kettara mine, despite the relatively high levels of metals in the soils, most metallophyte species growing in the area present low levels of metals in their shoots (Boularbah et al. 2006a). As such, according to the criteria of Baker and Brooks (1989), the indigenous species of Kettara mine should be classified as hypertolerant but not as hyperaccumulators, being good candidates for phytostabilization strategies.
The microorganisms present in the rhizosphere of these metallophytes have a crucial role on the speciation and bioavailability of metals in soil (Li and Wong 2011), while they can improve plant growth and their establishment in metal-enriched soils (Moreira et al. 2016a, 2016b). Metals are known to adversely affect the abundance, activity, and diversity of soil microbial communities (Yuangen et al. 2006; Khan et al. 2010). As such, these biological parameters have been used as indicators of soil quality and health, since they are known to be very sensitive to anthropogenic soil disturbances (Alkorta et al. 2003).
The composition of the rhizospheric microbial communities may be shaped by several factors, including soil physicochemical properties, environmental stresses, and type of vegetation cover (Berg and Smalla 2009; Li et al. 2014b). However, several authors also consider the plant species the key factor influencing the composition and abundance of rhizospheric microbial communities (Wieland et al. 2001; Steinauer et al. 2016), being the amount and type of root exudates released by the different plant species the driving force behind these changes (Berg and Smalla 2009). Increasing the knowledge on the response of microorganisms inhabiting the rhizosphere of metallophytes to metal pollution is an important stepping stone for the development of microbial-assisted phytoremediation solutions.
The present work aimed (i) to evaluate the effect of metal contamination and of the plant species colonizing the Kettara mining area on the microbial abundance and on the rhizospheric bacterial community composition and diversity; (ii) to assess the potential use of soil microbial parameters as soil quality indicators, and (iii) to assess the potential of native metallophytes to be used in phytoremediation strategies in mining affected areas.
2 Materials and methods
2.1 Site description and sampling
The present study was conducted in an abandoned pyrrhotite mine located in Kettara village, approximately 32 km northwest of Marrakech (31° 52’ 15” N 8° 10’ 31” W). As a result of more than 30 years of mining and milling operations in Kettara mine, more than 3 million tons of mine waste have been deposited over an area of approximately 16 ha (Hakkou et al. 2008).
Following a gradient of metal contamination previously defined (Boularbah et al. 2006a; Benidire et al. 2016), three sampling sites located at different distances from tailings ponds were chosen: Zone 1 (Z1), high metal contamination; zone 2 (Z2), moderate metal contamination; and zone 3 (Z3), low metal contamination (Fig. 1). A site located upstream of the mining area, 10 km away from the source of pollution, was used as control (ZC). Mine tailings were also sampled for physicochemical and microbiological analysis.
Four metallophyte species were commonly found in the selected zones—Ononis natrix, Haloxylon scoparium, Peganum harmala, and Aizoon canariense (Boularbah et al. 2006b). All plant species were found in ZC and Z2, while A. canariense and O. natrix were absent in Z1 and P. harmala and A. canariense were absent in Z3. A total absence of vegetation was noted in mine tailings.
Three entire plants were collected in spring, from the three sampling zones as well as from the control site. Plants were placed in sterile plastic bags and kept in a cooler until the laboratory. The rhizospheric soil was obtained by shaking the roots and a composite sample was made for each plant of each zone. Subsequently, the obtained soils were sieved (< 2 mm) and separated into different portions. The first portion was air-dried at room temperature for soil physicochemical analyzes, while the second one was stored in sterile plastic bags at 4 °C and assayed within 48 h for microbiological and enzymatic analyzes. The remaining soil was stored at − 20 °C for denaturing gradient gel electrophoresis (DGGE) analysis.
2.2 Soil and mine tailing physicochemical characterization
Soil pH and electrical conductivity (EC) were measured in H2O using 1:2.5 and 1:5 soil:solution ratio, respectively. Total organic carbon (TOC) was determined using dichromate oxidation method (Blakemore et al. 1972), while soil total nitrogen (TN) and available phosphorus (P Olsen) content were determined using Kjeldahl and molybdenum blue method (Olsen and Sommers 1982), respectively. The extractable fraction of metals was determined using 0.01 M CaCl2 as extractant. Total metal concentrations were determined after digestion of the soil with H2SO4 and HCl according to standard methods (NF EN ISO 11 466, 1995). The concentrations of metals were determined by flame atomic absorption spectrometry (Thermo Scientific iCE 3000) in the Laboratory of Hydrobiology, Ecotoxicology, Sanitation and Global Changes (FSSM, Marrakech, Morocco). All measurements were carried out in triplicate.
2.3 Germination test
In order to evaluate the phytotoxicity of the rhizospheric soils collected from each metallophyte, a germination test was carried out according to the AFNOR X 31-201 protocol (AFNOR 1986). The germination test was performed with seeds of common wheat var. Achtar (Sonacos) and lettuce var. Soleilan (Syngenta) due to their high sensitivity to chemical compounds, as recommended in different guidelines (EPA 1996; OECD 2006). Forty seeds of each plant species were placed in Petri dishes containing 40 g of soil and incubated for 7 days in darkness at 28 °C and 50% relative humidity in environmental chambers. A control was prepared using Petri dishes with Whatman paper moistened with distilled water instead of soil. The germination rate (G) was calculated according to the following formula:
Where A is the mean seed germination in the control test, and B is the mean seed germination in the tested soil.
2.4 Soil microbiological parameters
2.4.1 Dehydrogenase activity
Dehydrogenase activity (DHA) activity was performed according to Thalmann (1968). Briefly, 5 g of fresh soil was incubated with 5 ml of 2,3,5-triphenyltetrazoliumchloride (TTC) in 1 M Tris–HCl buffer (pH 7.5) for 24 h at 30 °C. The triphenyl formazan (TPF) produced was extracted with 40 ml of acetone. The suspension was filtered and the optical density of the supernatant was measured at 546 nm (UV-visible spectrometer, UV-3100PC Model).
2.4.2 Enumeration of soil culturable microorganisms
The number of culturable heterotrophic bacteria, fungi, and actinomycetes in the rhizosphere of each plant species and in mine tailings was determined by plating serial dilutions. Tryptone soy agar (TSA; Fluka) and potato dextrose agar (PDA; Difco) media were used for neutrophilic heterotrophic bacteria and fungi enumeration, respectively, since they are non-selective media and widely used for microbial enumeration in environmental samples (Aboudrar et al. 2007; Pereira et al. 2015; Naylo et al. 2019). The International Streptomyces Project 2 (ISP2) media was used for actinomycetes as it is the recommended media for the isolation and cultivation of actinobacteria (Shirling and Gottlieb 1966). Plates were incubated at 25–30 °C for 5 days. Data from triplicate counts were expressed as colony-forming units (CFU) g-1 dry soil.
2.4.3 Denaturing gradient gel electrophoresis
DNA was extracted from each rhizospheric soil composite sample using the Power Soil DNA Isolation Kit (MOBIO Laboratory, Inc., USA) according to the manufacturer’s protocol. DNA was stored at − 20 °C.
In order to increase sensitivity and to facilitate the denaturing gradient gel electrophoresis (DGGE) analyses, a nested PCR technique was applied. The first round was performed with the universal primers 27F (5′-GAGTTGATCCTGGCTCAG-3′) and 1492R (5′-ACCTTGTTACGACTT-3′) (NZYTech) to amplify the bacterial 16S rDNA gene (Lane 1991), and the second PCR was carried out using the primers 338F-GC (50-GACTCCTACGGGAGGCAGCAG-30) and 518R (50-ATTACCGCGGCTGCTGG-30) (NYZTech) to amplify the V3 variable region of the 16S rRNA gene (Muyzer et al. 1993). The reactions mixtures and the PCR conditions were performed according to the protocol described in Pereira and Castro (2014). A slight modification was made in the first amplification, where it was used 1.25 μl of DMSO instead of 0.4 mg ml-1 of bovine serum albumin. All PCR products were verified in a 1.5% agarose gel in 1x TAE buffer.
DGGE was carried out in a Bio-Rad DcodeTM Universal Mutation Detection System (Bio-Rad, USA). The nest-PCR products containing ca. 900 ng of DNA were loaded onto 8% (w/v) polyacrylamide gels (37.5:1, acrylamide/bisacrylamide) using a denaturing gradient from 35% to 60% of urea and formamide. After 16 h of migration at 70 V in 1x TAE buffer at a constant temperature of 60 °C, the gels were stained with a 5x Gel-Green Nucleic Acid Stain solution (Biotium, Inc.) for 10 min. DGGE images were obtained using a Safe ImagerTM blue light transilluminator (InvitrogenTM) and a microDOC gel documentation system (Cleaver Scientific Ltd.).
2.5 Metal concentrations in shoots of metallophytes
Metal concentrations in shoots of the metallophytes were determined as described by El Hamiani et al. (2010). Briefly, shoots were thoroughly cleaned with pressurized tap water followed by ultrapure water and air-dried at room temperature. The aboveground tissues were ground and stored in polythene bags at room temperature until analysis. Samples (0.5 g) were mixed with 8 ml of HNO3 and 2 ml of H2O2 and placed in a hot plate at 100 °C until the total evaporation of the extractant under a fume hood. The mineralized material was recovered by the addition of ultrapure water and filtered with an ash-less filter. The volume was adjusted to 25 ml with ultrapure water and acidified with 1% HNO3. The concentrations of metals in shoots were determined by flame atomic absorption spectrometry (Thermo Scientific iCE 3000).
Bioconcentration (BCF) factor was calculated as follows:
where C tissue is the concentration of the target metal in the plant harvested tissue and C soil is the concentration of the same metal in soil (Ali et al. 2013).
2.6 Data analysis
Statistical analysis was carried out with the program SPSS (IBM, Armonk, NY, USA, version 25.0.) Results were analyzed by ANOVA, considering treatments as the independent variable. Significant statistical differences were established by Student-Newman-Keuls’ test (P < 0.05). A correlation matrix between all physicochemical and microbiological parameters was calculated. The significance level reported (P < 0.01 and P < 0.05) is based on Pearson’s coefficients.
DGGE profiles were analyzed with the Bionumerics software (version 6.6, Applied Maths, Saint-Martens, Belgium). Based on the presence or absence of individual bands, a binary matrix was constructed. A dendrogram was generated using the unweight pair group mean average (UPGMA) cluster analysis.
Species richness (S) was calculated based on the total number of distinct bands in a lane. The Shannon index (H) (Shannon and Weaver 1949) was determined by using the intensity of the DGGE bands given by peak heights in the densitometric curve, as follows:
where ni is the height of the peak and N is the sum of all peak heights of the densitometric curve for all bands in a given sample (used as estimates of species abundance). Pearson correlation analysis was also used to evaluate the relationships among α-diversity and soil microbiological and physicochemical properties by using SPSS (version 25.0).
The influence of sampling zones, plants, or their interactions on the composition of bacterial communities was evaluated through permutational multivariate analysis of variance (PERMANOVA) with 999 permutations using the adonis function from vegan package in R version 3.5.1 (R Core Team 2018). PERMANOVA was performed in Jaccard distances matrixes constructed from the DGGE dataset. Information in the Jaccard dissimilarity matrix was then graphically assessed with non-metric multidimensional scaling (NMDS) using metaMDS function (from vegan package in R) to create the NMDS ordination. The environmental variables were normalized using the function scale and their corresponding vectors were found and fitted in the NMDS ordination using the function envfit (from vegan package in R).
3 Results
3.1 Physicochemical characterization of rhizospheric soils and mine tailings
The physicochemical properties of mine tailings and of rhizospheric soils of the metallophytes species collected at Kettara mine are summarized in Tables 1 and 2. Mine tailings were extremely acid (2.8 ± 0.02) and showed high conductivity’s values (2.45 ± 0.04). Soil rhizospheric pH varied between sampling zones and plant species ranging between 4.49 ± 0.03 in the rhizosphere of H. scoparium in Z1 and 8.17 ± 0.03 in the rhizosphere of P. harmala in ZC. In ZC, the rhizosphere of metallophytes was slight alkaline, while in Z1 and Z2 were considered very acidic and acidic, respectively. In Z3, the rhizospheric pH varied between 6.01 ± 0.15 in O. natrix and 7.20 ± 0.01 in H. scoparium. Concerning conductivity, in general no remarkable differences were observed between plant species sampled from ZC and Z2, except in rhizospheric soil of A. canariense from Z2, which presented high values of conductivity compared to other species (0.58 ± 0.04 mS cm-1). In Z3, conductivity was slightly higher if compared to ZC and Z2, with values varying between 0.29 ± 0.01 and 0.31 ± 0.02 mS cm-1. However, the highest conductivity values were observed in rhizospheric soils from Z1, ranging from 1.24 ± 0.03 to 1.31 ± 0.08 mS cm-1, reflecting a high salinity in this location. The levels of TOC, OM, and extractable P in rhizospheric soils decreased with increasing metal contamination, being in average 6.6, 6, and 3.7 times lower, respectively, in the three contaminated zones than in ZC. TOC and OM contents were significantly higher in ZC followed by Z3, while in Z1 and Z2, no significative differences were observed. These parameters were quite similar between the different plant species sampled from the same location, with exception of P. harmala rhizospheric soil in Z2, where TOC (1.21 ± 0.151%) and OM (2.08 ± 0.10b%) values were significantly higher. A similar trend was observed for TN, and the maximum values were determined in the rhizospheric soils sampled from ZC (0.09–0.40%), followed by Z3 (0.13–0.19%), Z2 (0.05–0.18%), and Z1 (0.04–0.12%). Extractable P was significantly higher in the rhizospheric soils of ZC (0.12 ± 0.005–0.61 ± 0.060 mg kg1) followed by the Z3 (0.13 ± 0.015–0.20 ± 0.007 mg kg1).
Total metal concentrations varied between plant species and zones. Cu was the main contaminant present in the rhizosphere of all plant species and mine tailings, followed by Zn and Pb. Overall, metal concentrations were higher in mine tailings than in rhizospheric soils. As expected, the rhizospheric soils from Z1 showed the highest levels of metals, followed by Z2, while soils from Z3 showed the lower contamination. In fact, the concentration of total Cu was in average 19, 16, and 6 times higher in soils from Z1, Z2, and Z3, respectively, than in soils collected from ZC. A similar trend was observed for Pb; however, for Zn and Cr, the tendency was different with rhizospheric soils from ZC showing higher levels of both metals. Some differences were also observed between plant species in each sampling zone. In general, the rhizosphere of P. harmala presented higher levels of metals, especially Cu, when compared to the other plant species in all sampling zones where this plant was found. The highest Cu- and Zn-extractable concentrations were recorded in the rhizosphere of metallophytes from Z1, while in the other sampling zones, the levels were very similar.
3.2 Germination test
The germination rates of common wheat and lettuce seeds growing in mine tailings and in the different rhizospheric soils are presented in Fig. 2. In comparison to wheat, lettuce seeds were more sensitive to the phytotoxic effects of metals and to the acid pH. Complete inhibition of seed germination of both species was observed in mine tailings, contrary to what happened in ZC, where seeds germinated perfectly. Seed germination rates were lower in rhizospheric soils from Z1 than in soils collected from moderate (Z2) and low (Z3) metal-contaminated zones. This tendency was particularly evident for lettuce seeds. Germination rates of lettuce seeds were similar in both Z1 rhizospheric soils, while higher germination rates were observed in the rhizospheric soils of P. harmala and H. scoparium from Z2 compared to O. natrix and A. canariense. Moreover, the germination rates in the rhizospheric soils of O. natrix and H. scoparium from Z3 were higher if compared to the soils of the same plant species in Z2.
Effect of mine tailings (MT) and different rhizospheric soils on the germination of wheat and lettuce seeds. ZC, control; Z1, high metal contamination; Z2, moderate metal contamination; Z3, low metal contamination; MT, mine tailing). Results are expressed as mean ± SD (n = 3). Bars with different letters (lowercase, wheat; uppercase, lettuce) differ significantly from each other (P < 0.05)
3.3 Soil microbiology parameters
3.3.1 Dehydrogenase activity
Figure 3 shows the results of the DHA activity in mine tailings and in rhizospheric soils. The activity of DHA was completely undetectable in tailings. Soils in ZC showed high DHA activity, ranging from 23 to 47 μg TPF g-1 of dry soil 24 h-1, with the highest values being recorded in H. scoparium and P. harmala rhizospheres. In general, DHA activity decreased with increasing metal contamination with the lowest activity being observed in the rhizosphere of H. scoparium and P. harmala in Z1 and of O. natrix and A. canariense in Z2, which did not exceed 1.9 μg TPF g-1 of dry soil 24 h-1. In the low metal-contaminated zone (Z3), the DHA activity recorded in the rhizosphere of H. scoparium was similar to the values found in control soils.
Dehydrogenase activity (DHA) in mine tailing (MT) and in the rhizosphere of the different metallophytes (O. natrix, H. scoparium, P. harmala, A. canariense) collected in each sampling zone (ZC, Z1, Z2, Z3). Results are expressed as mean ± SD (n = 3). Bars with different letters differ significantly from each other (P < 0.05)
3.3.2 Quantification of culturable soil microorganisms
The microbial abundance in mine tailings and in rhizospheric soils is shown in Fig. 4. As expected, no neutrophilic culturable microorganisms were found in mine tailings. Overall, the numbers of culturable microorganisms were much higher in soils from ZC, particularly in the rhizospheres of H. scoparium and P. harmala, than in metal contaminated soils. In control soils, the numbers of bacteria varied from 2.30 × 106 to 8.13 × 106 CFU g-1 dry soil, while actinomycetes and fungi ranged from 1.22 × 104 to 9.92 × 104 and from 1.73 × 103 to 6.20 × 103 CFU g-1 dry soil, respectively.
Number of culturable heterotrophic bacteria (A), actinomycetes (B), and fungi (C) in mine tailing (MT) and in the rhizosphere of the different metallophytes (O. natrix, H. scoparium, P. harmala, A. canariense) collected in each sampling zone (ZC, Z1, Z2, Z3). Results are expressed as mean ± SD (n = 3). Bars with different letters differ significantly from each other (P < 0.05)
Overall, no significant differences were observed between rhizospheric soils from metal-contaminated areas with exception of the rhizosphere of H. scoparium in the low-contaminated zone (Z3) that showed higher microbial density when compared to the size of microbial populations found in the rhizospheric soils from moderate (Z2) and high (Z1) metal-contaminated areas.
3.3.3 Bacterial community structure and diversity in the rhizosphere of metallophytes
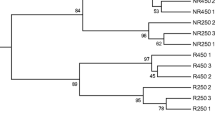
The DGGE band profiles obtained from the rhizosphere of metallophytes growing along the gradient of metal contamination, as well as the species richness (S) and the Shannon-Weaver (H) index, are shown in Fig. 5. The total number of band positions detected in the gel was 37 (data not shown). The species richness was generally different in each sample, varying between 7 (Z3_H) and 29 (Z1_H). In general, samples from Z1 (most contaminated area) showed in average higher species richness (25) when compared to ZC (16), Z2 (15), and Z3 (13) samples. A similar trend was observed for H’ which also varied according to the metal contamination in sampling zones. The rhizosphere of H. scoparium collected in Z3 showed the lowest bacterial diversity (H’ = 0.842), while the highest diversity was found in the rhizosphere of the same plant species collected in Z1 (H’ = 1.462). Indeed, when comparing the bacterial diversity in rhizospheric soils of metallophytes species common to the different zones, it was observed that diversity increased with increasing metal contamination in the rhizosphere of H. scoparium and P. harmala plants. The diversity of bacterial communities of H. scoparium (present in the four sampling zones) was greater in the most contaminated zones (Z1 and Z2) than in less contaminated ones (ZC and Z3). Similarly, for P. harmala, the diversity index was higher in Z1 (H’ = 1.301) and Z2 (H’ = 1.176) than in ZC (1.145). Conversely, in the rhizosphere of A. canarensis, it was observed a decrease in bacterial diversity from ZC (H’ = 1.342) to Z2 (H’ = 1.175), while in the rhizospheric soils of O. natrix, no remarkable differences were recorded.
The cluster analysis also indicated differences in the composition of rhizospheric bacterial communities among metallophytes (Fig. 5), which seems to be mainly related to sampling zones. Overall, bacterial communities clustered into two main groups with a similarity of around 40% (Fig. 5). One of the groups enclosed only 2 samples corresponding to the rhizospheres of O. natrix and H. scoparium from ZC and Z3, respectively, while the other included the remining samples. The latter presented 2 sub-clusters, the first one clustered essentially rhizospheric soils collected in the most contaminated areas (Z1 and Z2), whereas the second comprised mostly soils retrieved from ZC. The highest percentage of similarity (74%) was found between the rhizospheres of H. scoparium and P. harmala both collected in Z2. Statistical analysis (PERMANOVA) of the DGGE data confirmed that the composition of rhizospheric bacterial communities was significantly (P < 0.05) influenced by sampling zone (Table S1 – Electronic Supplementary Material). The environmental variables that influenced the distribution of rhizospheric samples were represented as vectors in NMDS (Fig. 6). The total concentrations of Zn, Cr, and Pb, as well as TOC and OM contents in soils, were the variables that most impacted the composition of bacterial communities, as proved by its high correlation coefficients (> 0.5). The composition of rhizospheric bacterial communities in the same metallophyte sampled from different zones was quite different and no particular trend was observed. In accordance, PERMANOVA analysis showed that plant species and the interaction between both factors (Zone:Plant) did not significantly (P < 0.05) influence communities’ composition.
Non-metric multi-dimensional scaling (NMDS) based on DGGE data showing samples colored by zone (Z). Vectors represent the correlation (Pearson’s) between the environmental variables (EC, pH, TOC, TN, OM, P Olsen, Zn, Cu, Cr, Pb, ext Cu, ext Zn) and the ordination coordinates. EC, electrical conductivity; TOC, total organic carbon; TN, total nitrogen; OM, organic matter; P Olsen, P assimilable; ext, extractable metal content. O, O. natrix; H, H. scoparium; P, P. harmala; A, A. canariense
3.4 Plant analysis
The concentrations of Cu and Zn in the shoots of metallophytes collected from the metal-contaminated zones (Z1, Z2, and Z3) were generally higher than those measured in the plants collected from ZC (Table 3). In all contaminated zones, the highest concentrations of Cu were detected in shoots of A. canariense and O. natrix, while Zn was mainly found in shoots of O. natrix and P. harmala.
The BCFs for Cu and Zn were lower than 1 in all sampling zones and plants (Table 3). For Cu, BCFs were slightly higher in control soils (ZC) than in metal-contaminated ones, while an opposite trend was observed for Zn. The higher Zn BCFs were observed for O. natrix plants in Z2 and Z3.
3.5 Pearson’s correlations
Pearson correlation coefficients between the different physicochemical properties and microbiological parameters of rhizospheric soils collected from Kettara mine region are shown in Table 4. The microbial abundance was positively influenced by pH, TOC, and TN contents, while the total concentrations of Cu and Pb negatively affected the numbers of all groups of microorganisms. As expected, a similar trend was observed for DHA activity, with a positive correlation of this parameter to the same physicochemical properties that influenced microbial abundance and a negative correlation to total concentrations of Cu and Pb and to extractable forms of Cu and Zn. The correlations between α-diversity and soil microbiological and physicochemical properties are shown in Table 5. Species richness was positively correlated to EC (r = 0.662, P < 0.05) and extractables fractions of Zn and Cu (r = 0.781, P < 0.01 and r = 0.691, P < 0.05, respectively), whereas H index was significantly correlated to EC (r = 0.581*), total Zn (r = 0.578, P < 0.05), and Cu extractable concentrations in soils (r = 0.691*).
4 Discussion
The indigenous metallophytes growing in the vicinity of Kettara mine can withstand high concentrations of metals and are well adapted to the semiarid conditions of this region. Most of them were either annual or perennial species (Midhat et al. 2016). The dominance of this kind of vegetation is due to the typical climate of arid and semi-arid zones, which is characterized by low annual precipitation. In the present work, among the four studied metallophyte species, only two (H. scoparium and P. harmala) were able to grow in the highly metal-contaminated zone (Z1, 1079.93–1234.43 mg Cu kg-1, 80.50–141.5 mg Pb kg-1, and 137.62–309.25 mg Zn kg-1). In fact, some of these plants showed visible signs of toxicity in the field, including a slow growth when compared to the same plant species in ZC, suggesting that the bioavailable metal concentrations were high enough to induce toxicity. These findings are in agreement with the results of germination tests which clearly showed that high levels of total and extractable metals in rhizospheric soils collected from Z1 and Z2 impaired the germination of lettuce and wheat seeds.
The metallic concentrations found in the shoots of metallophytes of Kettara mine were in the same range of concentrations observed by other authors in native plants of mining sites (Bennisse et al. 2004; Boularbah et al. 2006b; Yoon et al. 2006; Ha et al. 2011). The concentrations of Cu in shoots of O. natrix and of A. canariense exceeded the phytotoxic concentration thresholds of 20 mg kg-1 proposed by Kabata- Pendias and Pendias (2001), corroborating the results previously obtained by Boularbah et al. (2006b). However, the accumulation of this metal in the aboveground tissues was very low when compared to the accumulation capacity of other metallophytes, like Elsholtzia splendens growing in Cu-contaminated soils (Wang et al. 2008), making their use unfeasible for phytoextraction purposes. According to Baker and Brooks (1989), the four plants sampled in the different zones of Kettara mine are not hyperaccumulators, as their BCF values were lower than 1 (Table 3), being classified as hypertolerant metallophytes (Boularbah et al. 2006b). As a result, all studied plants seem to be good candidates for improving metal stabilization and vegetation cover of mining sites, reducing the risk of off-site contamination.
The pH of soils sampled in Kettara mine was not a limiting factor for plant growth, except in mine tailings where no vegetation was found, probably as a consequent of the extremely acidic pH (2.8). A total inhibition of seed germination was also observed in mine tailing samples, which is in line with what was observed in the field, confirming the crucial role of pH and metal pollution on plant development. Indeed, highly acidic pH is one of the main factors involved in the inhibition of the natural colonization of plants in mining wastes (Anawar et al. 2013; Courtney and Pietrzykowski 2018). Consequently, in the course of time, the open cast tailings become more vulnerable to runoff and wind erosion leading to a huge metal contamination of the surrounding areas (Mian and Yanful 2003; Boularbah et al. 2006a; El Khalil et al. 2008; El Hamiani et al. 2010). In this work, the pH varied from slight acidic to slight alkaline in the different sampling zones, except in Z1, where rhizospheric soils are very acidic. This may be explained by the proximity of Z1 to the mining site and consequently to a higher exposure to sulfur-rich dust. These kinds of materials suffer biological and chemical oxidation leading to soil acidification and consequent inhibition of the development of dense vegetation, which may explain the absence of some plant species, including O. natrix and A. canariense, in this site.
According to the Canadian Soil Quality Guidelines, total Cu (87.25 to 1234.43 mg Cu kg-1) concentrations in rhizospheric soils of contaminated soils were in most cases above the limit recommended for agricultural, parkland, commercial, and industrial land (63–91 mg Cu kg-1), use, while total Pb concentrations (40.53 to 141.25 mg Pb kg-1) were above the limit recommended for agricultural and parkland (70–140 mg Pb kg-1) use. In addition, according to the Dutch Standards, the levels of Cu, Pb and Zn in soils were, in general, above the target values (36 mg Cu kg-1, 85 mg Pb kg-1, and 140 mg Zn kg-1).
Previous studies have shown that soil microbial abundance and diversity are highly dependent on environmental conditions, including soil properties (e.g., pH, EC, metals) (Yuangen et al. 2006; Khan et al. 2010), vegetation cover (Berg and Smalla 2009; Hu et al. 2010), and seasonal changes (Bamborough and Cummings 2009; Yao et al. 2011; Zhang et al. 2018). In the present work, total concentrations of Cu and Pb had a significative and negative effect on microbiological parameters as shown by the correlation factors obtained between metal concentrations and: DHA activity (− 0.89, P < 0.01 and − 0.68, P < 0.01), bacterial (r = − 0.75, P < 0.01 and − 0.70, P < 0.05), fungi (r = − 0.65, P < 0.01 and − 0.61, P < 0.05), and actinomycetes (r = − 0.69, P < 0.05 and − 0.67, P < 0.05) counts. DHA activity decreased with increasing metal concentrations confirming the negative effects of these pollutants on soil microbial activity. Similar results were obtained by Xie et al. (2009) that reported a decrease in DHA activity in soils contaminated with 100 mg Cu kg-1. DHA activity is usually more affected by metal pollution than other soil enzymes. In fact, several studies reported dehydrogenases as very sensitive enzymes to metal pollution being frequently used as biological indicators in metal-affected soils (Fernández-Calviño et al. 2010; Hu et al. 2014; Xiao et al. 2017).
The low bacterial counts registered in most contaminated soils could be influenced by the choice of growth medium, since TSA favors the growth of heterotrophic in detriment of oligotrophic bacteria. Nonetheless, DHA was strongly correlated to microbial counts (r > 0.8), confirming that soil with low pH and high concentrations of metals have probably a low number of total microorganisms in their soils.
As described previously, the microbial abundance in rhizospheric soils was affected by metal pollution. However, it has been reported that pH, OM content, and soil nutrient status were also important factors influencing microorganisms in metal-contaminated sites, since they can often help to offset the harmful effects of metallic pollutants (Stefanowicz et al. 2012). In the current work, Pearson’s correlations showed that TOC and TN contents were beneficial for the development of microorganisms in the rhizospheric soils. Beside to its contribution to improve soil nutrient status (e.g., C and N), the increase of OM contents will contribute to increase soil porosity, improving air circulation and water retention capacity, stimulating the growth of microorganisms (Lal 2006; Park et al. 2011). In addition, high levels of OM can promote the immobilization of trace elements in stable organic complexes with humic substances (Spark et al. 1997; Farrell et al. 2010), reducing its bioavailability (Giller et al. 2009), and creating a more suitable environment for the establishment of microorganisms and vegetation.
In our study, very low densities of actinomycetes and fungi were detected in the different rhizospheric soils, suggesting that these soils are preferred niches for bacterial populations. This could be related to the less favorable conditions in soils, including the levels of metals that cannot be tolerated by these groups of microorganisms (Deneux-Mustin et al. 2003). Khan and Scullion (2000) have also noted higher bacterial rates than fungi in metal-contaminated soils, suggesting that fungi are more affected by metallic compounds. This could be explained by the fact that fungi generally colonize large pores and soil macro-aggregates, which makes them more exposed and vulnerable to the metal toxicity than bacteria, which are often associated to smaller pores and soil micro-aggregates (Carrasco et al. 2010).
Several studies have shown that metal contamination negatively affects the abundance, composition, and structure of bacterial communities, which generally results in a decreased diversity (Khan et al. 2007; Wang et al. 2008). The areas in the vicinity of the old Cu mine have been subject to heavy metal pollution over a long period of time, which may explain the reduction of the microbial abundance and the depletion of the DHA activity in contaminated zones (Z1, Z2, and Z3). A different trend was observed by Pereira et al. (2015) that reported higher bacterial numbers in the rhizosphere of Juncus effuses and Phragmites australis growing in a heavily metal-polluted soil than in the less contaminated one. Nevertheless, the analysis of DGGE profiles did not show a negative effect of metal pollution on bacterial diversity; quite in the contrary, in comparison to ZC, the highest bacterial diversity was recorded in the rhizospheric soils retrieved from the most metal-contaminated area (Z1), suggesting that long-term metal exposure led to the development of well adapted and diversified bacterial populations. These findings were corroborated by the positive and significant correlations obtained between both diversity indexes and total Zn concentrations and Zn and Cu extractable fractions in soils. In accordance, Martínez-Iñigo et al. (2009) also reported new bands in DGGE profiles of the rhizospheric communities of Silene vulgaris in response to metal exposure. Xiao et al. (2017) also found that the diversity of microbial communities increased after the application of high levels of vanadium in the soil, suggesting that new species of metal tolerant bacteria have replicated under these conditions.
The composition and diversity of bacterial communities may also be strongly influenced by the quantity and quality of root exudates (Marschner et al. 2004), which in turn is dependent on the type and concentration of metals (Qin et al. 2007; Luo et al. 2017). This could explain, in part, the differences observed in the bacterial diversity of rhizospheric soils of H. scoparium among sampling sites, which varied from very low in Z3 to very high in Z1. However, it is important to note that the lower diversity registered in Z3 was accompanied by a large increase of bacterial numbers.
In the present work, the composition and diversity of rhizobacterial communities were more influenced by sampling zones and the associated environmental factors than by plant species, which is in agreement with the results obtained by Lopez et al. (2017) that showed that bacterial diversity in the rhizosphere of Alyssum mural was more conditioned by the physicochemical factors than by plant species. Conversely, Pacwa-Płociniczak et al. (2018) showed that plants have a higher impact on soil microbial community than metallic pollutants.
5 Conclusions
The indigenous metallophytes growing in the vicinity of Kettara mine are not metal hyperaccumulators, making them good candidates for phytostabilization purposes, in particular H. scoparium and P. harmala. Both species provided an improvement of microbial abundance and bacterial diversity in their rhizospheres if compared to other metallophytes, especially under metal contamination, evidencing their ability to be used in the revegetation of mining areas, reducing the risk of dispersion of metals through erosion and leaching.
Microbial abundance and DHA activity are good tools to evaluate the effect of anthropogenic disturbances on soil microbial functions, since both parameters were systematically impacted by increasing metal concentrations in soils, while the influence of plant species was poorly noticed.
References
Aboudrar W, Schwartz C, Benizri E, Morel JL, Boularbah A (2007) Soil microbial diversity as affected by the rhizosphere of the hyperaccumulator Thlaspi Caerulescens under natural conditions. Inter J Phytorem 9:41–52
Acosta JA, Faz A, Martínez-Martínez S, Zornoza R, Carmona DM, Kabas S (2011) Multivariate statistical and GIS-based approach to evaluate heavy metals behavior in mine sites for future reclamation. J Geochem Explor 109:8–17
AFNOR, Norme expérimentale X31-201 (1986) Qualité des sols-Essai d'inhibition de germination de semences par une substance
Alford ER, Pilon-Smits EAH, Paschke MW (2010) Metallophytes—a view from the rhizosphere. Plant Soil 337:33–50
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals—concepts and applications. Chemosphere 91:869–881
Alkorta I, Aizpurua A, Riga P, Albizu I, Amézaga I, Garbisu C (2003) Soil enzyme activities as biological indicators of soil health. Rev Environ Health 18:65–73
Anawar HM, Canha N, Santa-Regina I, Freitas MC (2013) Adaptation, tolerance, and evolution of plant species in a pyrite mine in response to contamination level and properties of mine tailings: sustainable rehabilitation. J Soils Sediments 13:730–741
Ayangbenro AS, Babalola OO (2017) A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int J Environ Res Public Health 14(1). https://doi.org/10.3390/ijerph14010094
Baker AJM, Brooks RR (1989) Terrestrial higher plants which hyperaccumulate metallic elements—review of their distribution, ecology, and phytochemistry. Biorecovery 1:81–126
Bamborough L, Cummings SP (2009) The impact of zinc and lead concentrations and seasonal variation on bacterial and actinobacterial community structure in a metallophytic grassland soil. Folia Microbiol 54:327–334
Benidire L, Pereira SIA, Castro PML, Boularbah A (2016) Assessment of plant growth promoting bacterial populations in the rhizosphere of metallophytes from the Kettara mine, Marrakech. Environ Sci Pollut Res 23:21751–21765
Bennisse R, Labat M, ElAsli A, Brhada F, Chandad F, Lorquin J, Liegbott PP, Hibti M, Qatibi AI (2004) Rhizosphere bacterial populations of metallophyte plants in heavy metal-contaminated soils from mining areas in semiarid climate. World J Microbiol Biotechnol 20:759–766
Berg G, Smalla K (2009) Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol Ecol 68:1–13
Blakemore LC, Searle PL, Daly BK (1972) Methods for chemical analysis of soils New Zealand soil bureau report 10A. Government printer, Wellington. https://doi.org/10.7931/DL1-SBSR-10A
Boularbah A, Schwartz C, Bitton G, Morel JL (2006a) Heavy metal contamination from mining sites in South Morocco: 1. Use of a biotest to assess metal toxicity of tailings and soils. Chemosphere 63:802–810
Boularbah A, Schwartz C, Bitton G, Aboudrar W, Ouhammou A, Morel JL (2006b) Heavy metal contamination from mining sites in South Morocco: 2. Assessment of metal accumulation and toxicity in plants. Chemosphere 63:811–817
Carrasco L, Gattinger A, Fließbach A, Roldán A, Schloter M, Caravaca F (2010) Estimation by PLFA of microbial community structure associated with the rhizosphere of Lygeum spartum and Piptatherum miliaceum growing in semiarid mine tailings. Microb Ecol 60:265–271
Courtney R, Pietrzykowski M (2018) Soil quality indices for evaluation of acid mine spoil. In: Prasad MN, Favas PJ, Maiti SK (eds) Bio-Geotechnologies for Mine Site Rehabilitation. E-Publishing Inc, Chicago, pp 33–48. https://doi.org/10.1016/B978-0-12-812986-9.00002-6
Deneux-Mustin S, Roussel-Debet S, Mustin C, Henner P, Munier-Lamy C, Colle C, Berthelin J, Garnier-Laplace J, Leyval C (2003) Mobilité et transfert racinaire des éléments en traces (influence des micro-organismes du sol). Tec & Doc Lavoisier, Paris, p 282
El Hamiani O, El Khalil H, Lounate K, Sirguey C, Hafidi M, Bitton G, Schwartz C, Boularbah A (2010) Toxicity assessment of garden soils in the vicinity of mining areas in Southern Morocco. J Hazard Mater 177:755–761
El Khalil HE, Hamiani OE, Bitton G, Ouazzani N, Boularbah A (2008) Heavy metal contamination from mining sites in South Morocco: monitoring metal content and toxicity of soil runoff and groundwater. Environ. Monit Assess 36:147–160
EPA (1996) Ecological effects test guidelines. Seed germination/root elongation toxicity test. Office of Prevention, Pesticides and Toxic Substances, United States Environmental Protection Agency
Farrell M, Perkins WT, Hobbs PJ, Griffith GW, Jones DL (2010) Migration of heavy metals in soil as influenced by compost amendments. Environ Pollut 158:55–64
Fernández-Calviño D, Soler-Rovira P, Polo A, DíazRaviña M, Arias-Estévez M, Plaza C (2010) Enzyme activities in vineyard soils long-term treated with copper-based fungicides. Soil Biol Biochem 42:2119–2127
Giller KE, Witter E, McGrath SP (2009) Heavy metals and soil microbes. Soil Biol Biochem 41:2031–2037
Ha NTH, Sakakibara M, Sano S, Nhuan MT (2011) Uptake of metals and metalloids by plants growing in a lead–zinc mine area, Northern Vietnam. J Hazard Mater 186:1384–1391
Hakkou R, Benzaazoua M, Bussière B (2008) Acid mine drainage at the Abandoned Kettara Mine (Morocco): 1. Environmental Characterization. Mine Water Environ 27:145–159
Hu C, Fu B, Liu G, Jin T, Guo L (2010) Vegetation patterns influence on soil microbial biomass and functional diversity in a hilly area of the Loess Plateau, China. J Soils Sediments 10:1082–1091
Hu X-F, Jiang Y, Shu Y, Hua X, Liu L, Luo F (2014) Effects of mining wastewater discharges on heavy metal pollution and soil enzyme activity of the paddy fields. J Geochem Explor 147:139–150
Kabata- Pendias A, Pendias H (eds) (2001) Trace elements in soils and plants, 3rd edn. CRC Press Inc, Boca Raton
Khan M, Scullion J (2000) Effect of soil on microbial responses to metal contamination. Environ Pollut 110:115–125
Khan S, Qing CAO, Hesham AEL, Yue X, He JZ (2007) Soil enzymatic activities and microbial community structure with different application rates of Cd and Pb. J Environ Sci 19:834–840
Khan S, Hesham AEL, Qiao M, Rehman S, He JZ (2010) Effects of Cd and Pb on soil microbial community structure and activities. Environ Sci Pollut Res 17:288–296
Lal R (2006) Enhancing crop yields in the developing countries through restoration of the soil organic carbon pool in agricultural lands. Land Degrad Dev 17:197–209
Lane DJ (1991) 16S/23S sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. John Wiley, Chichester, pp 171–204
Li WC, Wong MH (2011) Effects of bacteria on metal bioavailability, speciation, and mobility in different metal mine soils: a column study. J Soils Sediments 10:313–325
Li Z, Ma Z, Van der Kuijp TJ, Zengwei Yuan Z, Huang L (2014a) A review of soil heavy metal pollution from mines in China: pollution and health risk assessment. Sci Total Environ 468–469:843–853
Li X, Rui J, Maoa Y, Yannarell A, Mackie R (2014b) Dynamics of the bacterial community structure in the rhizosphere of a maize cultivar. Soil Biol Biochem 68:392–401
Lopez S, Piutti S, Vallance J, Morel JL, Echevarria G, Benizri E (2017) Nickel drives bacterial community diversity in the rhizosphere of the hyperaccumulator Alyssum murale. Soil Biol Biochem 114:121–130
Luo Q, Wang S, Sun LN, Wang H (2017) Metabolic profiling of root exudates from two ecotypes of Sedum alfredii treated with Pb based on GC-MS. Sc Rep 7:39878. https://doi.org/10.1038/srep39878
Marschner P, Crowley D, Yang CH (2004) Development of specific rhizosphere bacterial communities in relation to plant species, nutrition and soil type. Plant Soil 261:199–208
Martínez-Iñigo MJ, Pérez-Sanz A, Ortiz I, Alonso J, Alarcón R, García P, Lobo MC (2009) Bulk soil and rhizosphere bacterial community PCR–DGGE profiles and β-galactosidase activity as indicators of biological quality in soils contaminated by heavy metals and cultivated with Silene vulgaris (Moench) Garcke. Chemosphere 75:1376–1381
Mian MH, Yanful EK (2003) Tailings erosion and resuspension in two mine tailings ponds due to wind waves. Adv Env Res 7:745–765
Midhat L, Ouazzani N, Esshaimi M, Ouhammou A, Mandi L (2016) Assessment of heavy metals accumulation by spontaneous vegetation: screening for new accumulator plant species grown in Kettara mine-Marrakech, Southern Morocco. Int J Phytorem 19:191–198
Mokhtari A, Feiznia S, Jafari M, Tavili A, Ghaneei-Bafghi MJ, Rahmany F, Kerry R (2018) Investigating the role of wind in the dispersion of heavy metals around mines in arid regions (a case study from kushk Pb–Zn mine, Bafgh, Iran). Bull Environ Contam Toxicol 101:124–130
Monterroso C, Rodríguez F, Chaves R, Diez J, Becerra-Castro C, Kidd PS, Macías F (2014) Heavy metal distribution in mine-soils and plants growing in a Pb/Zn-mining area in NW Spain. Appl Geochem 44:3–11
Moreira H, Pereira SIA, Marques APGC, Rangel AOSS, Castro PML (2016a) Mine land valorization through energy maize production enhanced by the application of plant growth-promoting rhizobacteria and arbuscular mycorrhizal fungi. Environ Sci Pollut Res 23:6940–6950
Moreira H, Pereira SIA, Marques APGC, Rangel AOSS, Castro PML (2016b) Selection of metal resistant plant growth promoting rhizobacteria for the growth and metal accumulation of energy maize in a mine soil—effect of the inoculum size. Geoderma 278:1–16
Muyzer G, De Waal EC, Uiterlinden AG (1993) Profiling of complex microbial populations by denaturating gradient gel electrophoresis analysis of polymerase chain reaction amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Nadgórska-Socha A, Kandziora-Ciupa M, Ciepał R (2015) Element accumulation, distribution, and phytoremediation potential in selected metallophytes growing in a contaminated area. Environ Monit Assess 187:1–15
Naylo A, Pereira SIA, Benidire L, El Khalil H, Castro PM, Ouvrard S, Schwartz C, Boularbah A (2019) Trace and major element contents, microbial communities, and enzymatic activities of urban soils of Marrakech city along an anthropization gradient. J Soils Sediments 19:2153–2165
NF ISO 11466 (1995) Qualité du sol-Extraction des éléments en traces solubles dans l’eau régale. In: AFNOR. 1999. Qualité des sols. Volume 1. AFNOR Paris, p 566
OECD (2006) OECD Guidelines for the Testing of Chemicals, Section 2. Effects on Biotic Systems. Test No. 208: Terrestrial Plant Test: Seedling Emergence and Seedling Growth Test. ISSN: 20745761 (online). https://doi.org/10.1787/20745761
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis part 2, 2nd edn. Agronomy Society of America, Madison, pp 403–430
Pacwa-Płociniczak M, Płociniczak T, Yu D, Kurola JM, Sinkkonen A, Piotrowska-Seget Z, Romantschuk M (2018) Effect of Silene vulgaris and heavy metal pollution on soil microbial diversity in long-term contaminated soil. Water Air Soil Pollut 229:1–13
Park JH, Lamb D, Paneerselvam P, Choppala G, Bolan N, Chung JW (2011) Role of organic amendments on enhanced bioremediation of heavy metal (loid) contaminated soils. J Hazard Mater 185:549–574
Pereira SIA, Castro PML (2014) Phosphate-solubilizing rhizobacteria enhance Zea mays growth in agricultural P-deficient soils. Ecol Eng 73:526–535
Pereira SIA, Pires C, Henriques I, Correia A, Magan N, Castro PML (2015) Assessment of rhizospheric culturable bacteria of Phragmites australis and Juncus effuses from polluted sites. J Basic Microbiol 55:1–12
Qin R, Hirano Y, Brunner I (2007) Exudation of organic acid anions from poplar roots after exposure to Al, Cu and Zn. Tree Physio 27:313–320
R Core Team (2018) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna
Science Communication Unit (2013) Science for environment policy in-depth report: soil contamination: impacts on human health. Report Produced for the European Commission DG Environment. University of the West of England, Bristol http://ec.europa.eu/science-environment-policy
Shannon CE, Weaver W (1949) The mathematical theory of communication. The University of Illinois Press, Urbana, p 117
Shirling EB, Gottlieb D (1966) Methods for characterization of Streptomyces species. Int J Syst Evol Microbiol 16:313–340
Spark KM, Wells JD, Johnson BB (1997) The interaction of a humic acid with heavy metals. Soil Res 35:89–102
Stefanowicz AM, Kapusta P, Szarek-Łukaszewska G, Grodzińska K, Niklińska M, Vogt RD (2012) Soil fertility and plant diversity enhance microbial performance in metal-polluted soils. Sci Total Environ 439:211–219
Steinauer K, Chatzinotas A, Eisenhauer N (2016) Root exudate cocktails: the link between plant diversity and soil microorganisms? Ecol Evol 6:7387–7396
Thalmann A (1968) Zur Methodik der bestimmung der dehydrogenaseaktivität im boden mittels triphenyltetrazoliumchlorid (TTC). In: Alef K, Nannipieri P (eds) (1995) Methods in Applied Soil Microbiology and Biochemistry. Academic Press, London, pp 228–230. https://doi.org/10.1016/B978-0-12-513840-6.X5014-9
Wang Y, Li Q, Shi J, Lin Q, Chen X, Wu W, Chen Y (2008) Assessment of microbial activity and bacterial community composition in the rhizosphere of a copper accumulator and a non-accumulator. Soil Biol Biochem 40:1167–1177
Whiting SN, Reeves RD, Richards D, Johnson MS, Cooke JA, Malaisse F, Paton A, Smith JAC, Angle JS, Chaney RL, Ginocchio R, Tanguy J, Johns R, McIntyre T, Purvis OW, Salt DE, Schat H, Zhao FJ, Baker AJM (2004) Research priorities for conservation of metallophyte biodiversity and their potential for restoration and site remediation. Restoration Ecol 12:106–116
Wieland G, Neumann R, Backhaus H (2001) Variation of microbial communities in soil, rhizosphere, and rhizoplane in response to crop species, soil type, and crop development. Appl Environ Microbiol 67:5849–5854
Xiao XY, Wang MW, Zhu HW, Guo ZH, Han XQ, Zeng P (2017) Response of soil microbial activities and microbial community structure to vanadium stress. Ecotox Environ Safe 142:200–206
Xie W, Zhou J, Wang H, Chen X, Lu Z, Yu J, Chen X (2009) Short-term effects of copper, cadmium and cypermethrin on dehydrogenase activity and microbial functional diversity in soils after long-term mineral or organic fertilization. Agric Ecosyst Environ 129:450–456
Yao H, Bowman D, Shi W (2011) Seasonal variations of soil microbial biomass and activity in warm-and cool-season turfgrass systems. Soil Biol Biochem 43:1536–1543
Yoon J, Cao X, Zhou Q, Ma LQ (2006) Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ 368:456–464
Yuangen Y, Campbell CD, Clark L, Cameron CM, Paterson E (2006) Microbial indicators of heavy metal contamination in urban and rural soils. Chemosphere 63:1942–1952
Zhang J, Li M, Jia K, Zheng G, Long XE (2018) Seasonal variation rather than stand age determines bacterial diversity in the rhizosphere of wolfberry (Lycium barbarum L.) associated with soil degradation. J Soils Sediments 18:1518–1529
Acknowledgments
The authors would like to thank the scientific collaboration of Fundação Ciência e Tecnologia (FCT) project UID/Multi/50016/2019. We are also grateful to Dr. A. El Gharmali for his constant help in the analysis of heavy metals in different samples and to Dr. Marta Alves for her help in DGGE data analysis.
Funding
This study was financially supported by the “Convention de coopération CNRST-Morocco/FCT-Portugal,” Centre National de Recherche Scientifique et Techniques [grant no. PPR 22/2015] and by the project PhytoSudoe (SOE1/P5/E0189)—Demostração de melhorias na biodiversidade do solo, funcionalidade e serviços ambientais de locais contaminados e/ou degradados sob intervenção de fitotecnologias dentro da região Interreg Sudoe, funded by FEDER—Fundo Europeu de Desenvolvimento Regional under Programa INTERREG.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Yanfen Wang
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Benidire, L., Pereira, S.I.A., Naylo, A. et al. Do metal contamination and plant species affect microbial abundance and bacterial diversity in the rhizosphere of metallophytes growing in mining areas in a semiarid climate?. J Soils Sediments 20, 1003–1017 (2020). https://doi.org/10.1007/s11368-019-02475-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-019-02475-4