Abstract
Purpose
The purposes of this study were to investigate the activation and transport of atrazine in the presence of dissolved organic matter (DOM) and the surfactant (Triton X-100) and to understand interactions between DOM, Triton X-100, and atrazine.
Materials and methods
Uncontaminated soils collected from Nanjing, China, along with DOM extracted from rice straw and Triton X-100 (TX-100), were used in the study. The sorption and desorption experiments were carried out using the standard batch equilibration analysis. Soil column leaching was conducted with soil samples packed into PVC columns. Soil thin-layer chromatography was performed using soil and water mixture spread on a 0.5–0.7-mm-thick layer over 20 × 10-cm glass plates. Atrazine accumulation in maize was determined by planting maize in plastic pots (1 L) containing 1 kg soil mixed with 1.0 mg kg−1 atrazine. Soils were watered with different solutions, with the relative water content of 60%.
Results and discussion
Using batch experiment and soil thin-layer chromatography, application of DOM and surfactant reduced sorption and increased desorption of atrazine in soil. In column experiment, DOM and surfactant significantly promoted the mobility of atrazine in soil and the total concentration of atrazine in leachate of the soil column. Accumulation of atrazine in both maize roots and shoots increased with the elevated concentration of surfactant, whereas the content of atrazine declined with the increase of the DOM concentration.
Conclusions
Dissolved organic matter and TX-100 affected the partitioning and transport of atrazine in soil–water and soil–plant ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Over the last five decades, substantial pesticides have been applied to farmland for protecting crops against pests and herbs (Shi et al. 2011). Atrazine (2-chloro-4-ethylamino-6-isopropylamino-1,3,5-triazine) is one of the major herbicides predominantly applied to the fields of grain production. It was estimated that 10–15 million kg of atrazine is yearly imputed on the farmland in China, leading to the widespread residues in the environment (Deng et al. 2005). As atrazine is environmentally persistent, with a half-life of about 57 weeks (Scott et al. 2009), the accumulating residues of atrazine in soil are far more than those of natural degradation (Udiković-Kolić et al. 2012). Thus, much attention has been paid to the research on its environmental behaviors such as sorption–desorption, chemical and biological degradation, plant uptake, and surface runoff and leaching, as well as the environmental risks to wildlife and human health (Mandelbaum et al. 1995; Solomon et al. 1996; Arias-Estévez et al. 2005; Scott et al. 2009; Udiković-Kolić et al. 2012; Zhang et al. 2014, 2017).
Dissolved organic matter is the major component playing pivotal roles in distribution, mobilization, and degradation of soil pollutants such as pesticides and other organic toxicants between the soil interface and soil solution (Ma et al. 2001; Huo et al. 2008; Song et al. 2007; Jiang et al. 2008; Chen et al. 2010). On the other hand, the molecular size and abundance of DOM in soil are affected by various environmental factors such as microbial population, the level of oxygen, and even concentration and speciation of some metal ions (Gao and Zepp 1998; Voelker et al. 2000). DOM can be strongly adsorbed onto the soil surfaces, resulting in low DOM levels in the soil solution (Kalbitz et al. 2000). When DOM interacts with organic pollutants and made them fixed in soil (Zsolnay 2003), it may increase desorption of the organic pollutants (Torrents and Jayasundera 1997).
Recently, the focus of the study has been shifted to the presence of surfactants in the soil–water system (Cao et al. 2008; Rodriguez-Escales et al. 2012). The soil surfactants fall into the amphiphilic category of organic compounds. Given that the concentration of surfactant reaches to the critical micelle concentration (CMC), the surfactant monomers will form micelles (Rodriguez-Escales et al. 2012). The soil surfactants basically increase the solubility of organic toxicants and decrease the partition coefficient of organic toxicants with the soil matrix (Abu-Zreig and Rudra 2002; Cao et al. 2008). Both anionic and nonionic surfactants are the most abundant organic chemicals in municipal wastewater. Domestic and industrial wastewater is often applied to soil with or without minimal treatment. Thus, it is required to investigate the effect of surfactants and DOM on mobility of pesticides in soil.
The herbicide atrazine is widely used for maize production. The fate of atrazine through degradation and detoxification pathways in plant or soil alone has been investigated (Raveton et al. 1997; Blume et al. 2004; Singh et al. 2004; Su and Zhu 2006; Zhang et al. 2014, 2017). However, reports on the fate of atrazine in soil–water system and soil–plant system as affected by DOM and surfactants have been limited (Celis et al. 1998; Abu-Zreig and Rudra 2002). In this study, we experimented with sorption and desorption, soil column leaching, soil thin-layer, and atrazine accumulation in maize and investigated the effect of straw-derived DOM and the surfactant (TX-100) on the behavior of atrazine in soil. The aim of the study was to investigate transport of atrazine with DOM and the surfactant (TX-100) and to understand interactions between DOM, the surfactant (TX-100), and atrazine. The outcome of this study would improve our understanding of atrazine transfer in soil–water system and soil–plant system and provide information for strategy to control transport of pesticides to groundwater, soil surface, and plants.
2 Materials and methods
2.1 Pesticide, soil, seed, and chemicals
Atrazine was obtained from the Institute of Pesticide Science, Academy of Agricultural Sciences in Jiangsu, Nanjing, China, with a purity of 98%. Maize seeds (cv. Su Yu13) were obtained from the Institute of Crop Science, Academy of Agricultural Sciences in Jiangsu, Nanjing, China. Uncontaminated soils were collected from Xiamafang Park in Jiangsu, Nanjing, China. Some physico-chemical features of soil are listed in Table S1 of the Electronic Supplementary Material (Jiang et al. 2008). The sampled soils were air-dried, gently crumbled, and sieved through a 2-mm sieve (used for sorption–desorption, atrazine uptake in maize, and column leaching experiments) and a 100-μm sieve (used for soil thin-layer chromatography). Methanol was chromatographic grade (USA, TEDIA Company, INC). Acetone, petroleum ether, NaClO, and CaCl2 used in the experiment were all of analytical grade.
2.2 Dissolved organic matter extraction and surfactant prepared
Dissolved organic matter was extracted from rice straw collected from the experimental station of Nanjing Agricultural University, Nanjing, China. DOM was prepared according to the method of Jiang et al. (2008). The straw was extracted with distilled water using a solid: water ratio of 1:20 (w/v, dry weight basis) in a shaker at 200 rpm−1and 20 °C for 24 h. The suspensions were centrifuged at 10,000g and 4 °C for 10 min and filtered through a 0.45-μm sterilized membrane (GN-6 Mctrice, Gelman Sciences Ann Arbor, MI). The total organic carbon in filtrates was determined (TOC 5000A, Shimadzu, Japan) (Song et al. 2008).
Triton X-100 (TX-100) was provided by Sinopharm Chemical Reagent Co., LTD, China. Its critical micelle concentration is 192 mg L−1. TX-100 was prepared to 0.5 CMC (96 mg L−1), 1.0 CMC (192 mg L−1), and 1.5 CMC (288 mg L−1) solutions with distilled water respectively (Cao et al. 2007).
2.3 Sorption and desorption experiments
Sorption and desorption experiments were carried out using the standard batch equilibration method (OECD 2000). Two grams of soil and 10 mL of 0.01 M calcium chloride solution with atrazine were mixed into 50-mL polypropylene centrifuge tubes. This treatment was labeled as a control. Two grams of soil was mixed with 10 mL of 0.01 M CaCl2 containing atrazine and DOM or TX-100. The initial concentrations of atrazine were set at 1, 2, 4, 6, 8, and 16 mg L−1. The initial concentrations of DOM and TX-100 were set at 80 mg DOC L−1 and 160 mg DOC L−1, at 0.5 CMC, 1.0 CMC, and 1.5 CMC. Each treatment with a concentration was repeated three times. Samples were shaken at 200 rpm−1 and 20 °C for 24 h. After that, the suspensions were centrifuged at 6000g for 10 min. Concentration of atrazine in the supernatant was measured.
Desorption experiments were performed immediately after the sorption experiments. The tube was placed in inverse order for 24 h to remove the supernatants. Ten milliliters of 0.01 M fresh CaCl2 solution was added into the tube. The shaking, centrifuging, and detecting of experiments were conducted as described above.
2.4 Soil thin-layer chromatography
Soil thin-layer chromatography (TLC) experiment was undertaken according to the method of Jiang et al. (2008). Fifteen grams of soil sample and 11 mL water were mixed and spread on a 20 × 10-cm glass plate with the help of a TLC spreading device. The thickness of the soil layer was within 0.50–0.75 mm. After air-drying, the plates were marked with two horizontal lines at a distance of 2 cm (baseline) and 18 cm (foreland line) from the base, respectively. Then, 100 μL of 400 mg L−1 atrazine solution in acetone was spotted onto the baseline of plate with the help of a microsyringe.
The plates were placed in closed individual glass chromatographic chambers. The solutions at 80 mg DOC L−1, 160 mg DOC L−1, 0.5 CMC, 1.0 CMC, and 1.5 CMC and distilled water (control) were used as developing solvents. After the developing solvents migrated to the foreland of plates, the plates were taken out and dried at room temperature. Soil on plates was divided into eight parts in average between the baseline and foreland. The content of atrazine in soil of each segment was measured, respectively. Mobility factor (Rf) of atrazine was calculated by following formulae (Li et al., 2007a):
where ZP is the average moving distance of pesticide from start point, Zw is the moving distance of the developing solvent from start point, i is the number of segments, Zi is the distance of segment i from start point, and Mi is the pesticide content in segment i.
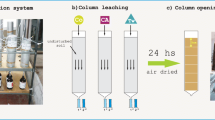
2.5 Column leaching
Soil samples were packed into PVC columns (30 × 4.5 cm i.d.) with a bulk density of about 1.27 g cm−3 and 25-cm height (Jiang et al. 2008). The columns were saturated with 0.01 M calcium chloride solution and stored overnight at room temperature. Atrazine (400 μg) in 1 mL acetone was applied to the column surface and allowed to air-dry for 2 h to evaporate acetone. A layer (1 cm) of acid-washed sand was laid on the top of each column during the experiment. Columns were eluted for 20 h with 1000 mL of different percolating solutions as the following: 0.01 M calcium chloride solution containing 80 mg DOC L−1, 160 mg DOC L−1, 0.5 CMC, 1.0 CMC, and 1.5 CMC and 0.01 M calcium chloride solution as a control. The percolating solution was kept approximately 5 cm on the column surface throughout the leaching. The leachate fractions were collected in 50-mL portions and analyzed for atrazine concentration. Each treatment of column was replicated three times. After leaching, columns were left for drainage for 24 h and dissected into 5-cm sections. Each section of soil was air-dried and analyzed for atrazine content.
2.6 Uptake of atrazine by maize
Maize seeds were sterilized with 5% sodium hypochlorite solution, rinsed several times with distilled water, and placed in culture plate with some water at 30 °C for 2 days. After germination, the seeds were sown on soils in plastic pots (1 L) containing 1000 g soil with atrazine at 1.0 mg kg−1 and watered each day with different solutions (80 mg DOC L−1, 160 mg DOC L−1, 0.5 CMC, 1.0 CMC, 1.5 CMC solutions, and distilled water (control)) to maintain 60% relative water content in soils. Plant was grown at 24/20 °C (day/night) under a light intensity of 300 μmol m−2 s−1. After growing for 10 days, the roots and shoots were separately harvested. The soil on the surface of roots were thoroughly washed by water and blotted with the filter paper to remove any excess water. The roots and shoots were immediately frozen in liquid nitrogen or stored in an − 80 °C freezer for analysis. All treatments were repeated in triplicate.
2.7 Atrazine extraction and analysis
2.7.1 Water samples
LC-C18 cartridges (Supelco Park Bellefonte, PA, USA) were conditioned with 5 mL methanol, followed by 5 mL water. The water sample was transferred onto the column and allowed to percolate at 2–3 mL min−1. The column was dried with a strong stream of air for at least 5 min. The eluates were discarded. The column was extracted with 4 mL methanol. The eluted methanol was collected for analysis. All experiments were independently conducted in triplicate.
2.7.2 Soil samples
Soil sample (20 g) was extracted with 30 mL mixed acetone–water (3:1, v/v) using a mechanical shaker at 200 rpm for 20 min, followed by centrifugation at 5000g for 15 min. The extracting process was repeated for three times. The acetone in extracting supernatant was evaporated by a vacuum rotary evaporator at 40 °C. The remaining water was loaded onto an LC-C18 cartridge. The eluates were discarded. The column was extracted with 4 mL methanol. The eluted methanol was collected for analysis. All experiments were independently performed in triplicate.
2.7.3 Maize samples
Leaves and roots of maize (2–4 g) were extracted with mixed acetone–water (3:1, v/v) for three times (each time 20 mL) using a mechanical shaker at 200 rpm for 20 min, followed by centrifugation at 5000g for 15 min. The extracting solution was evaporated to remove acetone by rotary evaporator at 40 °C. The residual water was transferred into a funnel and extracted with petroleum ether for three times, each time 15 mL. The water phase was discarded. The petroleum ether was evaporated to dryness by rotary evaporator at 40 °C. The residues were re-dissolved by adding 20-mL solution of methanol and distilled water (1:40, v/v) and loaded onto an LC-C18 cartridge. Elutes were discarded. The column was washed with 4 mL methanol. The washing methanol was collected for analysis. To investigate the uptake and distribution of atrazine in maize, we calculated the bioconcentration factor (BCF), a quotient between the organism and medium substance concentration, as well as the translocation factor (TF), the ratio of atrazine concentrations in shoots to roots (Zhang et al. 2014). All experiments were independently conducted in triplicate.
2.7.4 Atrazine analysis
Atrazine in water, soil, and maize samples was quantified by high-performance liquid chromatography (HPLC, Waters 515, Waters Technologies Co. Ltd., USA) with ultraviolet detector (UVD) at a wavelength of 235 nm. The operating conditions were the following: Thermo reversed phase C8 column (250 mm × 4.6 mm i.d. 5 μm); mobile phase, methanol:water (80:20; v/v); flow rate, 0.6 mL min−1; injection volume, 20 μL; room temperature. Under the above conditions, the retention time of atrazine was about 10.5 min.
Atrazine was spiked to pollution-free atrazine water, soil, or shoot and root of maize. The concentration of atrazine in soil was 0.1, 1, and 2 mg/kg. The concentration of atrazine in the shoot or root was 0.04, 0.2, and 1 mg/kg. The concentration of atrazine in water was 0.1, 1, and 5 mg/L. After all spiked samples were laid up for 1 h, extraction and analysis were performed according to the above method. Recoveries of extracting atrazine from water, soil, and maize (shoot and root) were presented in Table S2 of the Electronic Supplementary Material.
3 Results
3.1 Effect of TX-100 and DOM on sorption and desorption of atrazine
The sorption and desorption isotherms of atrazine on soil were examined in the presence of DOM and surfactant TX-100. The Freundlich equation was used to describe the process. The Freundlich model is presented by the following equation:
where Cs is the sorbed concentration (mg kg−1); Ce is the concentration in the solution phase (mg L−1); Kf is the Freundlich sorption coefficient, representing the amount of atrazine sorbed at 1 mg L−1 equilibrium concentration; and 1/n is a linearity factor indicating the sorption intensity. The regression coefficient r ranged from 0.9742 to 0.9932 (Table 1). Kf values appeared to decrease with increasing concentrations of DOM and surfactant. Kf values were 2.09 for 0.01 M CaCl2 (control), 1.83 for 0.5 CMC, 1.27 for 1.0 CMC, 0.60 for 1.5 CMC, 1.12 for 80 DOM, and 0.91 for 160 DOM (Table 1). These results indicated that application of DOM and surfactant led to obvious reduction in atrazine sorption to the soil (Fig. 1a, c). Meanwhile, addition of DOM and surfactant to soil significantly increased atrazine desorption as compared to the control (Fig. 1b, d). The respective Kf values were 6.84 for control, 5.21 for 0.5 CMC, 4.55 for 1.0 CMC, 2.13 for 1.5 CMC, 3.17 for 80 DOM, and 2.03 for 160 DOM (Table 1).
3.2 Effect of TX-100 and DOM on mobility of atrazine in soil microstructures
To investigate the mobility of atrazine within soil microstructures, the distilled water (control), and surfactant (TX-100), DOM solutions were used as developing solvents. As shown in Table 2, the Rf values (within 0.56 and 0.72) were different between the control and various treatments, in the order of Rf control < 0.5 CMC < 1.0 CMC < 80 DOM < 160 DOM < 1.5 CMC. The contents of atrazine in each segment with distilled water, DOM, and surfactant as developing solvents were also found to be different (Fig. 2). Compared with control, the transport of atrazine was enhanced with the DOM and surfactant. The maximal concentration of atrazine for the control was examined in the third segment (control), the fourth segment for 0.5 CMC, the fifth segment for 1.0 CMC, and the sixth segment for 1.5 CMC (Fig. 2a). The maximal concentration of atrazine for 1.5 CMC was 1.67 times the control. The maximal concentration of atrazine for the control was found in the third segment, the fifth segment for 160 DOM, and the sixth segment for 80 DOM, respectively (Fig. 2b). The maximal concentration of atrazine for 160 DOM was 1.33-fold higher than that of the control.
3.3 Effect of TX-100 and DOM on leaching of atrazine
In soil column experiments, the 0.01 M CaCl2 (control), surfactant (TX-100), and DOM solutions were used as percolating solutions separately. The same leaching volume (1000 mL) was run in all different leaching experiments. The peak amount of atrazine was higher and the leached volume was less using TX-100 and DOM solution as percolating solution than the control using only 0.01 M CaCl2 (Table 3 and Figs. 3a and 4a). This result showed an overall effect of increase in the total amounts leached from the soil columns with percolating solutions of surfactant and DOM. The peak concentration was detected with 1.5 CMC as a percolating solution, almost 2.19-fold over the control. The content of atrazine was enhanced with the concentration of TX-100 and DOM (Fig. 3b; Fig. 4b). The cumulative content of atrazine was 299.4 μg for control, 367.4 μg for 0.5 CMC, 378.9 μg for 1.0 CMC, 374.4 μg for 1.5 CMC, 325.8 μg for 80 DOM, and 342.3 μg for 160 DOM, indicating that addition of surfactant and DOM increased atrazine movement in the soil columns.
The leaching columns were dissected into 5-cm segments, and the amount of atrazine left in soil was determined. There were different patterns of atrazine distribution in the columns with different treatments (Fig. 3c; Fig. 4c). The atrazine content in each section was shown in the following order: control > 0.5 CMC and control > 80 DOM > 160 DOM. Addition of surfactant and DOM reduced atrazine retention in soil column, suggesting that the presence of surfactant and DOM enhanced the migration of atrazine in the soil profile. However, there was little atrazine remaining in soil columns leached with 1.0 CMC and 1.5 CMC solutions. The data of atrazine residues in soil section were omitted (Fig. 3c).
3.4 Effect of TX-100 and DOM on uptake of atrazine
To get an insight into the mobility of atrazine from soil to plant, the content in soil and the uptake of atrazine in maize tissues were determined. The contents of atrazine in maize in the presence of surfactant were higher than the control, while those in maize supplied with DOM were lower than the control (Fig. 5(A, B)). A positive (for surfactant) or negative (DOM) concentration-dependent manner was observed. In contrast, the contents of atrazine with surfactant were lower than the control and those with DOM were higher than the control in soil. However, the amount of atrazine in soil decreased with increasing surfactant but increased with the increasing DOM (Fig. 5(C)). Under 1.5 CMC, 80 DOM, and 160 DOM treatments, the concentration of atrazine in soil and maize tissues significantly differed from the control. Bioconcentration factors (BCFs) and translocation factors (TFs) for atrazine in shoots and roots of maize were presented in Table 4. It is showed that BCFs increased and TFs decreased with the increasing concentration of TX-100, while BCFs decreased and TFs increased with the increasing concentration of DOM.
Effect of TX-100 and DOM on atrazine accumulation in maize tissues (A, B) and soil (C). Maize was cultured in soil containing atrazine at 1.0 mg kg−1 with TX-100 and DOM for 10 days. CK: soil containing only atrazine. The data are means of three replications. Different letters indicate significant difference between the treatments and the control (p < 0.05)
4 Discussion
Our results showed that application of DOM and surfactant obviously reduced atrazine sorption and significantly increased atrazine desorption from the soil. The reduced sorption may be attributed to the complex formation of DOM with organic chemicals or competition for sorption sites. Similar results were reported that the process occurred at the soil/solution interface, such as competition of DOM for sorption sites (Celis et al. 1998; Gao et al. 1998; Nelson et al. 1998; Flores-Céspedes et al. 2002; Spark and Swift 2002; Song et al. 2008; Zhang et al. 2010). Moreover, the sorption of DOM may modify the charge characteristics of soil and thus reduce the affinity of soil to pesticides (Zhou et al. 2000). Also, it was possible that DOM promoted the solubility of pesticides in soil solution by exchanging adsorbed atrazine on soil. The PAH desorption was also found when DOM from pig manure and manure compost was applied to soil (Cheng and Wong 2006). These results indicated that supply with DOM reduced the amount of atrazine adsorbed to the soil and increased the amount of atrazine desorption as well.
Application of surfactant to sorption–desorption of atrazine led to a pattern similar to that of DOM. The surfactant might have two effects on pesticide sorption in soil: (1) surfactant adsorbed onto sediments, thus providing greater affinity for pesticide sorption, and (2) the surfactant dispersed in water and promoted pesticide dissolution (Li et al., 2007b; Pan et al. 2009). Since TX-100 is a nonionic surfactant, the second effect was favored. The surfactant tended to be adsorbed onto the soil and bound to the adsorbed atrazine, which may form more stable surfactant–herbicide complex rather than sorption of herbicide in soil. The sorption capacity of soil for nonionic surfactants would be expected to decrease when the degree of hydrophilicity was increased (Paria and Khilar 2004). Overall, both surfactant and DOM reduced sorption and enhanced desorption of atrazine, which is implicated in the interaction of pesticide–DOM and pesticide–surfactant. Alternatively, surfactant and DOM might exchange with adsorbed atrazine and compete for sorption sites.
Soil thin-layer chromatography (TLC) was used for measuring the mobility of atrazine in soil microstructures. The transport of atrazine increased using DOM and surfactant as developing solvent compared with distilled water. A similar result was described previously for prometryne, chlorotoluron, and isoproturon on soil TLC using surfactant or DOM developing solvents (Cao et al. 2007; Song et al. 2008; Chen et al. 2010; Ding et al. 2011). Polarizable pesticide molecule was adsorbed onto the surfactant micelle–water interface and dissolved in aqueous phases, thus resulting in increased mobility of pesticide at the TX-100 concentrations above the CMC. It is likely that DOM might occupy the sorption sites and a portion of DOM dissolved into water would increase the solubility of pesticide in aqueous phase (Jiang et al. 2008). DOM is structurally and functionally similar to surfactant; it can enhance the solubility of compounds through DOM–pesticide interaction (Cho et al. 2002; Flores-Céspedes et al. 2002). In the column leaching experiment, the presence of surfactant and DOM in the leachate could facilitate the movement of pesticide, confirming the interaction between pesticides and DOM or surfactant (Flores-Céspedes et al. 2002; Jiang et al. 2008; Song et al. 2008; Zhang et al. 2010). Competition between the pesticide and DOM for sorption sites may contribute to this enhancement (Li et al. 2005; Zhang et al. 2010). Our analysis was consistent with the previous TLC experiment, which also reflected the mobility of atrazine in microcosm and macrocosm of soil with surfactant and DOM (Ravanel et al. 1999). The uptake of atrazine in maize was further examined. This could allow us to understand the transfer of atrazine to maize within the soil–water–plant system and estimate the toxic effect of atrazine on maize in the presence of DOM and surfactant. Roots always accumulated relatively more atrazine than leaves regardless of DOM and surfactant treatments, possibly due to the fact that roots are directly exposed to toxicants (Li et al. 2012). We found that accumulation of atrazine in maize tissues was positively related to the concentration of surfactant, but negatively associated with the concentration of DOM in both roots and shoots. The effect of DOM on the uptake of atrazine at the low level (1 mg/kg) was similar to the previous study in which the lower level of some other organic chemicals was also found in the presence of DOM (Haitzer et al. 1998; Song et al. 2008). This suggested that application of DOM was able to reduce the accumulation of atrazine. The mechanism underlying the reduced atrazine accumulation in maize plants is unclear. It was possible that atrazine may interact with DOM and form complex DOM. However, it is impossible that the pesticide–DOM complex could pass through the cellular plasma membrane into the root cells because it is too polar or too large in size (Song et al. 2007; Zhou et al. 2007). We observed the increased accumulation of atrazine in maize plants in the presence of the surfactant. This may be the result that surfactants were dispersed in the soil solution, which promoted the dissolvability of atrazine and thus increased the uptake of atrazine by maize. Finally, to avoid the effect of biological magnification to the calculated uptake amount of atrazine, the uptake of atrazine was separately calculated on the base of individual plant and unit weight of maize. The results from two calculations turned out consistent, indicating the methods used in the study were reliable.
5 Conclusions
Application of DOM and surfactant decreased atrazine sorption and increased desorption in soil. This could be a result of the increased mobility of the pesticide in soil, as evidenced by soil column leaching and soil thin-layer experiments. Uptake of atrazine into maize plants was also affected by the presence of surfactant and DOM, but a contrasting correlation was showed. In general, supply DOM reduced the content of atrazine, whereas addition of surfactant increased the uptake of atrazine. Thus, our study provided evidence that the DOM and surfactant were able to enhance the transfer of atrazine in both soil and plant.
References
Abu-Zreig M, Rudra RP (2002) Modelling of atrazine transport in the presence of surfactants. J Environl Sci Health B 37(1):15–32
Arias-Estévez M, Soto-González B, López-Periago E, Cancho-Grande B, Simal-Gándara J (2005) Atrazine sorption dynamics in acid-surface soils. Bull Environ Contam Toxicol 75:264–271
Blume E, Bischoff M, Moorman TB, Turco RF (2004) Degradation and binding of atrazine in surface and subsurface soils. J Agri Food Chem 52:7382–7388
Cao J, Guo H, Zhu HM, Jiang L, Yang H (2008) Effects of SOM, surfactant and pH on the sorption–desorption and mobility of prometryne in soils. Chemosphere 70:2127–2134
Celis R, Barriuso E, Houot S (1998) Sorption and desorption of atrazine by sludge-amended soil: dissolved organic matter effects. J Environ Qual 27:1348–1356
Chen G, Lin C, Chen L, Yang H (2010) Effect of size-fractionation dissolved organic matter on the mobility of prometryne in soil. Chemosphere 79:1046–1055
Cheng KY, Wong JWC (2006) Combined effect of nonionic surfactant Tween 80 and DOM on the behaviors of PAHs in soil-water system. Chemosphere 62:1907–1916
Cho HH, Park JW, Liu CK (2002) Effect of molecular structure on the solubility enhancement of hydrophobic organic compounds by environmental amphiphiles. Environ Toxicol Chem 21:999–1003
Deng JC, Jiang X, Wang DC, Lu X, Gao HJ, Wang F (2005) Research advance of environmental fate of herbicide atrazine and model fitting in farmland ecosystem. Acta Ecol Sin 25(12):3359–3367
Ding Q, Wu HL, Xu Y, Guo LJ, Liu K, Gao HM, Yang H (2011) Impact of low molecular weight organic acids and dissolved organic matter on sorption and mobility of isoproturon in two soils. J Hazard Mater 190:823–832
Flores-Céspedes F, González-Pradas E, Fernández-Pérez M, Villafranca-Sánchez M, Socias-Viciana M, Urena-Amate MD (2002) Effects of dissolved organic carbon on sorption and mobility of imidacloprid in soil. J Environ Qual 31:880–888
Gao H, Zepp RG (1998) Factors influencing photoreactions of dissolved organic matter in a coastal river of the southeastern United States. Environ Sci Technol 32:2940–2946
Gao JP, Maguhn J, Spitzauer P, Kettrup A (1998) Sorption of pesticides in the sediment of the Teufelsweiher pond (southern Germany). II: competitive adsorption, desorption of aged residues and effect of dissolved organic carbon. Water Res 32:2089–2094
Haitzer M, Höss S, Traunspurger W, Steinberg C (1998) Effects of dissolved organic matter (DOM) on the bioconcentration of organic chemicals in aquatic organisms-a review. Chemosphere 37:1335–1362
Huo SL, Xi BD, Yu HC, He LS, Fan SL, Liu HL (2008) Characteristics of dissolved organic matter (DOM) in leachate with different landfill ages. J Environ Sci-Chinese 20:492–498
Jiang L, Huang J, Liang L, Zheng PY, Yang H (2008) Mobility of prometryne in soil as affected by dissolved organic matter. J Agricul Food Chem 56:11933–11940
Kalbitz K, Solinger S, Park JH, Michalzik B, Matzner E (2000) Controls on the dynamics of dissolved organic matter in soils: a review. Soil Sci 165(4):277–304
Li SN, Sun Y, Yang T, Huangpu WG (2007a) Relationship between mobility factors (R f) of two hydrophobic termiticides and selected field and artificial soil parameters. Sci Total Environ 388:206–213
Li JH, Zhou BX, Shao JH, Yang QF, Liu YQ, Cai WM (2007b) Influence of the presence of heavy metals and surface-active compounds on the sorption of bisphenol A to sediment. Chemosphere 68:1298–1303
Li K, Xing BS, William AT (2005) Effect of organic fertilizers derived dissolved organic matter on pesticide sorption and leaching. Environ Pollut 134:187–194
Li XY, Wu T, Huang HL, Zhang SZ (2012) Atrazine accumulation and toxic responses in maize Zea mays. J Environ Sci 24:203–208
Ma HZ, Allen HE, Yin YJ (2001) Characterization of isolated fractions of dissolved organic matter from natural waters and a wastewater effluent. Water Res 35:985–996
Mandelbaum RT, Allan DL, Wackett LP (1995) Isolation and characterization of a pseudomonas sp. that mineralizes the s-triazine herbicide atrazine. Appl Environ Microbiol 61(4):1451–1457
Nelson SD, Letey J, Farmer WJ, Williams CF, Ben-Hur M (1998) Facilitated transport of napropamide by dissolved organic matter in sewage sludge-amended soil. J Environ Qual 27:1194–1200
OECD (2000) OECD guidelines for the testing of chemicals. Adsorption/desorption using a batch equilibrium method. OECD Test Guideline, 106. OECD Publications, Paris
Pan G, Jia CX, Zhao DY, You C, Chen H, Jiang GB (2009) Effect of cationic and anionic surfactants on the sorption and desorption of perfluorooctane sulfonate (PFOS) on natural sediments. Environ Pollut 157:325–330
Paria S, Khilar KC (2004) A review on experimental studies of surfactant adsorption at the hydrophilic solid-water interface. Adv Colloid Interf Sci 110:75–95
Ravanel P, Liégeois MH, Chevallier D, Tissut M (1999) Soil thin-layer chromatography and pesticide mobility through soil microstructures. J Chromatogr A 864:145–154
Raveton M, Ravnel P, Serre AM, Nurit F, Tissut M (1997) Kinetics of uptake and metabolism of atrazine in model plant systems. Pestic Sci 49:157–163
Rodriguez-Escales P, Sayara T, Vicent T, Folch A (2012) Influence of soil granulometry on pyrene desorption in groundwater using surfactants. Water Air Soil Pollut 223:125–133
Scott C, Jackson CJ, Coppin CW, Mourant RG, Hilton ME, Sutherland TD, Russell RJ, Oakeshott JG (2009) Catalytic improvement and evolution of atrazine chlorohydrolase. Appl Environ Microbiol 75(7):2184–2191
Shi R, Lv JG, Feng JM (2011) Assessment of pesticide pollution in suburban soil in South Shenyang, China. Bull Environ Contam Toxicol 87:567–573
Singh N, Megharaj M, Kookana RS, Naidu R, Sethunathan N (2004) Atrazine and simazine degradation in Pennisetum rhizosphere. Chemosphere 56:257–263
Solomon K, Baker DB, Richards RP, Dixon KR, Klaine SJ, Point TWL, Kendall RJ, Weisskopf CP, Giddings JM, Giesy JP, Hall LW, Williams WM (1996) Ecological risk assessment of atrazine in north American surface waters. Environ Toxicol Chem 15(1):31–76
Song NH, Chen L, Yang H (2008) Effect of dissolved organic matter on mobility and activation of chlorotoluron in soil and wheat. Geoderma 146:344–352
Song NH, Yin XL, Chen GF, Yang H (2007) Biological responses of wheat (Triticum aestivum) plants to the herbicide chlorotoluron in soils. Chemosphere 68:1779–1787
Spark KM, Swift RS (2002) Effect of soil composition and dissolved organic matter on pesticide sorption. Sci Total Environ 298:147–161
Su YH, Zhu YG (2006) Bioconcentration of atrazine and chlorophenols into roots and shoots of rice seedlings. Environ Pollut 139:32–39
Torrents A, Jayasundera S (1997) The sorption of nonionic pesticide onto clays and the influence of natural organic carbon. Chemosphere 35:1549–1565
Udiković-Kolić N, Scott C, Martin-Laurent F (2012) Evolution of atrazine-degrading capabilities in the environment. Appl Microbial Biotechnol 96:1175–1189
Voelker BM, Sedlak DL, Zafiriou OC (2000) Chemistry of superoxide radicals in seawater: reactions with organic Cu complexes. Environ Sci Technol 34:1036–1042
Zhang JJ, Lu YC, Yang H (2014) Chemical modification and degradation of atrazine in Medicago sativa through multiple pathways. J Agricul Food Chem 62(40):9657–9668
Zhang JJ, Gao S, Xu JY, Lu YC, Lu FF, Ma LY, Su XN, Yang H (2017) Degrading and phytoextracting atrazine residues in Rice (Oryza sativa) and growth media intensified by a phase II mechanism modulator. Environ Sci Technol 51(19):11258–11268
Zhang R, Cui J, Zhu HM, Yang H (2010) Effect of dissolved organic matters on napropamide availability and ecotoxicity in rapeseed (Brassica napus). J Agricul Food Chem 58:3232–3240
Zhou LX, Yang H, Shen QL, Wong MH, Wong JWC (2000) Fraction and characterization of dissolved organic matter derived from sewage sludge and compost sludge. Environ Technol 21:765–771
Zhou ZS, Huang SQ, Guo K, Mehta SK, Zhang PC, Yang ZM (2007) Metabolic adaptations to mercury-induced oxidative stress in roots of Medicago sativa L. J Inorgan Biochem 101:1–9
Zsolnay Á (2003) Dissolved organic matter: artefacts, definitions, and functions. Geoderma 113:187–209
Funding
This study received financial support from the National Key Research and Development Project of China (No. 2016YFD0200201).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Heike Knicker
Electronic supplementary material
ESM 1
(DOC 46 kb)
Rights and permissions
About this article
Cite this article
Tian, B.B., Zhou, J.H., Xie, F. et al. Impact of surfactant and dissolved organic matter on uptake of atrazine in maize and its mobility in soil. J Soils Sediments 19, 599–608 (2019). https://doi.org/10.1007/s11368-018-2095-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-018-2095-z