Abstract
Purpose
Fine sediment accumulates upstream of hydroelectric dams. To ensure that dams can operate properly, part of the sediment has to be dredged and land managed. In parallel, using topsoil from agricultural parcels for urban greening or land restoration is currently controversial because arable surface areas are decreasing. An alternative idea for protecting these natural resources consists in reusing fine dredged sediment to construct multifunctional soils. This agronomic use is only possible if sediment can provide acceptable physical and chemical properties for plant growth.
Materials and methods
Four dredged sediments with contrasted initial agronomic properties and one control soil were mixed or not with green waste compost (40% v/v) and used to construct triplicate 30-cm depth soils in lysimetric containers (1.11 × 0.71 m). The 30 constructed soils were exposed to the in situ conditions and sown with ryegrass (Lolium perenne). The evolution of soil chemical and physical properties and plant development were studied every 6 months for 18 months.
Results and discussion
Above- and below-ground biomass production of the constructed soils contrasted according to the sediment properties and to compost addition. A statistical approach identified eight soil parameters linked to biomass production. Among these parameters, soil structure, quantified from aggregate stability, played a fundamental role. A focus on physical properties confirmed that some sediments were only partially adapted to ryegrass support. Compost addition improved sediment physical properties over time, but caused temporary N deficiency during the first months after installation which limited shoot biomass production. Exogenous plant species developed on the constructed Technosols, especially on the soils where the lack of structure and N deficiency had the strongest effect.
Conclusions
All sediments were suitable for plant growth over the 18 months of the study. A few soil properties emerged as markers of the fertility of sediment-made Technosols. Among them, the soil structure was one of the most determining parameters. It can be assessed by measuring aggregate stability, macroporosity, the crustability index, and bulk density, while available nutrients (N, P, K) and pH seem sufficient to assess chemical fertility. The balance between the properties of the sediment-made Technosols and the needs of the plants seems to be an essential lever for the establishment of functional soil-plant systems for urban greening or for ecological restoration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
When the water speed slackens upstream of hydropower dams, natural sedimentation of elementary organic and mineral particles occurs, coming mainly from the watershed rocks and soil erosion. These sediments accumulate in the water reservoirs and can potentially reduce their water storage capacity. To avoid such accumulation, ensuring the correct operation of hydropower production and maintain dam safety, dam managers promote sediment transfer downstream to maintain sediment continuity in the river. Today, several dam managers have to manage large amounts of accumulated fine sediment composed of at least 50% of particles finer than 80 μm (Anger 2014). Part of this fine sediment has to be dredged, and there is currently no suitable way it can be valorized and recycled. The land management of fine sediment is a worldwide issue because it is also a matter of concern for harbor managers (Sturgis et al. 2001a, b; Sheehan et al. 2010; Macia et al. 2014) and waterway managers (Vervaeke et al. 2001; Daniels et al. 2007). To anticipate the land management of fine sediments, several alternative uses are investigated.
Damas and Coulon (2016) estimates that 3 million cubic meters of topsoil, coming mainly from agricultural soils, are needed for urban greening each year in France. In addition, about 100,000 ha of industrial, commercial, and military brownfields could require ecological reclamation in France (BRGM 2012).
Even if dams have been controversial in many parts of the world, using topsoil from agricultural land for urban greening or brownfield reclamation is also currently controversial because arable surface areas are decreasing. An alternative idea for protecting these non-renewable natural resources consists in reusing by-products and waste materials to construct functional soils (Séré et al. 2008). However, the agronomic use of such materials is legally conditioned by evidence of its agronomic interest and environmental safety. A large part of sediments are compatible with environmental requirements (INERIS 2010). Using fine sediment in soil construction for urban greening and brownfield ecological reclamation could be a way of valorizing it. But the use of fine dredged sediment for soil construction raises the question of its quality and capability to ensure plant growth, especially with the scarce literature data available.
Soil quality can be evaluated through many parameters taking into account their physical, chemical, and biological characteristics (Huinink 1998; Schjønning et al. 2004). However, few physical (fine soil granulometry, bulk density, aggregate stability) and chemical (pH, soil organic matter content) parameters can be used to efficiently monitor soil fertility (Scharenbroch and Catania 2012). Plant growth also depends on soil nutrient availability. Nitrogen deficiency can limit plant growth in urban ecosystems in the same way as in agricultural systems (Jim 1998). This limitation can also be caused by deficiencies in other macro-elements such as phosphorus (P) and potassium (K) which affect plant growth and health (Craul 1992; Tisdale et al. 1993; Kramer and Kozlowski 1979). Soil nutrient deficiency in an urban context cannot be treated in the same way as in an agricultural context because agricultural fertilization techniques are most often unsuited to urban contexts. Therefore, the constructed soil should have sufficient nutrient levels during installation, in line with the species to be planted and the management of the created spaces.
As soon as waterlogged sediments are transferred to upland conditions, the ripening process begins and slowly transforms them into an aerated soil (Pons and Zonneveld 1965). Several studies have already focused on dredged sediment for soil construction, and proved that it was rather suitable for most plants of ornamental or agricultural interest, especially in terms of soil chemistry (Sturgis et al. 2001a, b; Daniels et al. 2007; Sheehan et al. 2010; Haus 2011; Macia et al. 2014; Koropchak et al. 2015). However, certain sediments require, prior to their agronomical use, pretreatments to lower their salt content, especially in the case of marine sediments (Sheehan et al. 2010; Macia et al. 2014), to adjust their pH which can be too extreme, e.g., in the presence of pyrite or sulfate (Vermeulen et al. 2003; Sheehan et al. 2010; Macia et al. 2014), or to remove organic or metallic pollutants (Macia et al. 2014).
The massive structure and high bulk density of certain sediments could prevent fluid flows and partially inhibit plant germination and rooting (Sturgis et al. 2001b; Daniels et al. 2007). This suggests that dredged sediment physical fertility should be investigated with a view to using it in soils. Long-term sediment agronomic properties can be improved and guaranteed by adding composted organic matter. The input of large amounts of organic products concomitantly with perennial plant installation (i.e., up to 50% in volume) is commonly used by landscape management services in cities (Bacholle et al. 2006). Several studies showed that organic amendments had positive effects on the physical and chemical properties of cultivated soils by (i) decreasing dry bulk density, (ii) increasing the soil water-holding capacity (Carter and Stewart 1996; Celik et al. 2004), (iii) increasing hydraulic conductivity at saturation (Yang et al. 2011), (iv) improving the soil structure (Hati et al. 2007), and (v) supplying nutrients (Frossard et al. 2002).
Dam sediment is an interesting candidate for urban greening without using arable soil. As a consequence, the objectives of this study are (i) to assess the capability of hydropower dam sediments to support medium-term plant growth and (ii) to evaluate the impact of composted organic matter on the improvement of agronomic soil properties. In this prospect, an in situ 18-month experiment was conducted to compare the agronomic potentials of four sediments to allow ryegrass development. Sediments were amended with green waste compost or not, and their properties were compared to an arable soil.
2 Materials and methods
2.1 Parent materials
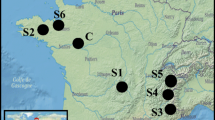
Four sediment samples—named S1 to S4—were taken from four different hydropower dams located in three mountainous terrains in France between December 2014 and March 2015 (Fig. 1). The sediments were chosen to maximize differences in texture and organic matter content, and also to be free of anthropogenic pollutant contamination so as to only focus on their agronomic potential. S1 and S2 were sampled in low-altitude mountainous terrains (the Massif Central and the Massif Armoricain, respectively), and S3 and S4 were sampled in high-altitude mountainous terrains (the Alps). The control soil (C) was topsoil (full A horizon, 20 cm thick) taken from an agricultural field near Angers, France, sampled in May 2015. It came from the topsoil horizon of a Fluvisol (IUSS Working Group WRB 2015) located on alluvial terraces of the Loire River. It is usually used by Angers City for vegetated soil construction and is available in large quantities. The main initial agronomic properties of these five materials are presented in Table 1.
After sampling, each sediment was spread in a 10- to 15-cm-thick layer over geotextile to be air-dried in the open air for 1 to 2 months. Sediments were manually homogenized according to the quarter technique (Schumacher et al. 1991). Then, they were sieved through a 40-mm screen to crush the larger clods formed during drying and homogenize them.
Green waste compost (Co) was used as an organic amendment. It was produced in the Ile-de-France region, near Paris. This compost was chosen because it complied with the French national standards of organic amendments (AFNOR 2006), because green waste compost is the main organic amendment used in France for vegetated soil construction (Bacholle et al. 2006), and because it had only undergone a 4-month maturation process to limit the risk of phytotoxicity due to too high electrical conductivity. The compost was not crushed, so that 460 g kg−1 of the particles was greater than 2 mm. Its main initial agronomical properties are presented in Table 2.
2.2 Constructed soils and experimental setup
A field experiment was carried out at the experimental platform of Agrocampus-Ouest in Angers (47° 28′ 49″N, 0° 36′ 32″W) from 1 October 2015 to 7 April 2017 (18 months). The climate is oceanic, with a mean annual temperature 12.3 °C, and an average annual rainfall of 693 mm. The five materials (sediments S1 to S4 + the control soil C) were studied either in the absence of amendment to assess the ability of the sediment-made soils to fulfill the vegetation support function on their own, or mixed with the green waste compost (Co) (mix ratio 60% material/40% compost (v/v)) to assess the impact of a large amount of exogenous organic matter on sediment-made soil fertility. This corresponded to an amount of 19.86 kg DM of compost added in each of the five materials. The mix ratio was chosen according to Grosbellet et al. (2011), Vidal-Beaudet et al. (2012), and Cannavo et al. (2014), who demonstrated that 40% (v/v) of green waste compost significantly improved the physical properties of constructed soil for urban greening. This represented 10 conditions. The main initial agronomic properties of the five compost-mixed soils are presented in Table 2.
The constructed soils were set up in 30 individual 535-L containers (area 1.11 × 0.71 m) in one homogeneous 0.3-m deep layer. This soil thickness was chosen to mimic the practices of urban green space services, who work with a soil thickness of 20 to 30 cm to establish a herbaceous stratum (UNEP 2012). The pure material soils (C, S1 to S4) were homogenized in a 1-m3 mixer and poured directly into individual containers to reach the bulk density of the 40-mm sieved and dried (mass water content ≤ 10%) materials without packing (Table 1). The mass of the required materials had previously been determined in laboratory by calculating bulk density according to the EN 13040 standard (CEN 1999) and the moisture content. The same method was used to determine the mass of the materials needed to construct compost-mixed soils (CCo, S1Co to S4Co). The compost and the materials were also homogenized in a 1-m3 mixer, and poured directly into individual containers. The bottom of the containers was drained by a 0.31-m layer of gravel separated from the constructed soil by a geotextile. Three containers were built for each of the 10 soil conditions. For each sampling date (6, 12, and 18 months after 1 October 2015), one container of each condition was destroyed to allow soil sampling. Ryegrass (Lolium perenne L. var. Escal) was sown in all the containers at a seed density of 200 kg ha−1 on the first day of the experiment. Perennial ryegrass is native to southern Europe, the Middle East, North Africa, and eastwards to central Asia. It can be used to prevent erosion and to stabilize soils, as well as to create a hardwearing turf for lawns and golf courses. Additionally, it is used for industrial or mining soil restoration (Norland and Veith 1995; Vangronsveld et al. 1996; Cordova et al. 2011). This species was also selected for its ability to colonize soils because it provides a homogeneous soil cover, has a fasciculate root system, and is commonly used to revegetate soils built from wastes or alternative materials (Séré et al. 2010; Vidal-Beaudet et al. 2018). Perennial ryegrass was also chosen for its agronomical requirements. It tolerates both acidic and alkaline soils with a pH range of 5.5 to 8.4. It is also very responsive to the nitrogen soil content (Hannaway et al. 1999).
2.3 Measurements
2.3.1 Soil physical analyses
The physical and chemical properties of the constructed soils were determined 6, 12, and 18 months after the beginning of the experiment. At each sampling date, one container per condition was destroyed to collect three undisturbed cores (diameter 5 cm, height 6 cm) at 5- and 25-cm depths to estimate bulk density. Soil water content was determined after oven-drying the samples at 105 °C for 48 h, and bulk density values were calculated from the dry soil mass and the cylinder volume (CEN 1999).
Three other undisturbed cores (diameter 7 cm, height 5 cm) were sampled per container at 15-cm depth. They were used to estimate the soil volumetric water content (m3 m−3) at saturation (θs) (− 0.1 kPa), at field capacity (θfc) (− 31.6 kPa), and at the wilting point (θwp) (− 1500 kPa) according to Richards (1947) and De Boodt et al. (1974). Available water (AW) for plants (mm of water cm−1 of soil) (Eq. (1)) and macroporosity (Pmacro) (m3 m−3) (Eq. (2)) were calculated as follows:
One kilogram of composite soil sample was collected in each container through the entire soil thickness in triplicate and was air-dried for physical analyses. The size distribution of the dry aggregates was determined by successive sieving of the dried samples with 5-, 3-, 2-, 1-, and 0.25-mm sieves. The dry sieved aggregate (DSA) of each of the six aggregate fractions (< 0.25, 0.25–1, 1–2, 2–3, 3–5, > 5 mm) was expressed as a function of the initial dry mass of the sample (g × 100 g−1). Aggregate stability of the 3–5-mm aggregates was measured as described by Le Bissonnais and Le Souder (1995), and expressed in mean weight diameter (MWD) (mm) of the three aggregate stability tests. The fraction of water stable aggregates (WSA) was determined following the wet-sieving method modified from the method of Haynes and Swift (1990), using 4 g of 2–3-mm-sized dry-sieved aggregates and an Eijkelkamp wet-sieving apparatus (0.25-mm mesh sieve). The coarse fraction made of > 0.25-mm particles (sand, organic fragments) possibly present in the stable aggregates was determined by mechanical aggregate destruction with a stencil brush, distilled water washing, and oven-drying (105 °C for 48 h). The WSA was calculated according to the equation proposed by Regelink et al. (2015) (Eq. (3)):
where WSA is the water-stable aggregate (g g−1) of the 3.15–2-mm fraction of the soil. It ranges between 0 and 1, 0 meaning that no aggregate was stable in water and 1 meaning that all aggregates were stable in water. minitial is the mass of the initial dry-sieved aggregates (3.15–2-mm fraction), mnon − stableis the mass of the water non-stable aggregate passed through the 0.25-mm mesh sieve, and mcp is the mass of the coarse particles (> 0.25 mm).
Five-hundred-gram composite soil samples were collected in each container through the entire constructed soil thickness in triplicate to determine the five classes of soil textures and solid densities, according to the NF X31–107 standard method (AFNOR 2003a) and the water pycnometer method (Blake 1965), respectively. The crustability index (CI) was calculated according to Rémy and Marin-Laflèche (1974) and Schvartz et al. (2005). This index estimates the probability for a slaking crust to appear at the soil surface according to its content in fine silt, coarse silt, clay and OM, and to the water pH. The risk of a slaking crust is insignificant if the CI is below 1.4, while it is very high if the CI is higher than 2.
2.3.2 Soil chemical analyses
One kilogram of composite soil samples was collected in each container through the entire constructed soil thickness in triplicate for chemical analyses. Samples were air-dried and 2-mm-sieved, and then used to measure the pH in water (pHwater) according to the NF ISO 10390 standard (1:5 soil:solution ratio) (AFNOR 2005), electrical conductivity (EC) according to the NF ISO 11265 standard (AFNOR 1995a), the organic matter content (OM) by loss of ignition (450 °C for 7 h) on previously oven-dried samples (105 °C for 48 h), total N and total C content by dry combustion at 1800 °C using a CN analyzer (Thermoquest® Device) according to the NF ISO 13878 standard (AFNOR 1998), the total calcium carbonate content (CaCO3) according to the NF ISO 10693 (AFNOR 2014), the Metson cation exchange capacity (CEC) according to the NF X31–130 standard (AFNOR 1999), and EDTA-extractable iron according to the NF X31–120 standard (AFNOR 2003b).
SOC was calculated by subtracting the CaCO3 carbon content from the total carbon content. Available phosphorus (POlsen) was determined by NaHCO3 extraction and spectrometric quantification according to the NF ISO 11263 method (AFNOR 1995b). N-NH4+ and N-NO3− were measured by KCl extraction following the NF ISO 14256–2 method (AFNOR 2007).
2.3.3 Monitoring of plant development
The above-ground plant biomass was manually collected every 3 months on all constructed soils. The plants were cut 8 cm from the ground to preserve their growth. At each sampling date (i.e., after 6, 12, and 18 months), the plant biomass of the destroyed containers was collected on the soil surface to measure the whole above-ground biomass produced. The collected biomass was oven-dried at 60 °C for 48 h, and expressed in kg of aerial dry matter biomass per square meter (kg DM m−2).
The below-ground biomass was only measured after 6, 12, and 18 months in triplicate on the destroyed containers. Soil cores (10-cm diameter, 10-cm height) were collected every 10 cm of the constructed soil. The nine soil cores per container were air-dried, and the root biomass was collected by dry sieving. Roots were then oven-dried at 60 °C for 48 h, and expressed in kg of root dry matter biomass per square meter per 30 cm of soil depth (kg DM 30 cm−1 m−2).
2.4 Statistical analyses
Statistical differences of accumulated biomass over 18 months among soil conditions were revealed using analysis of variance (ANOVA, α = 0.05) with three replicates per condition. Prior to the analysis, Shapiro-Wilk’s tests of normality (α = 0.05) were performed to validate the use of a parametric ANOVA test. Variance homoscedasticity was tested with Bartlett’s test (α = 0.05). When the effects were significant at a level of 0.05, means were tested with Tukey’s pairwise comparison test. The relationships between above- and below-ground biomass were assessed using single regression analysis. The significance thresholds for the coefficients and models were set at 0.05. To identify the soil parameters linked with above-ground biomass production, Pearson’s correlation matrices were set. Principal component analysis (PCA) was performed to identify relations between variables and to cluster similar constructed soils, using the “FactoMineR” package (Lê et al. 2008). It was performed on 30 samples (10 constructed soil conditions × 3 replicates). It was used only as a descriptor to detect any trends in our dataset, but the number of samples limited the interpretation of results. All calculations were performed with R 3.1.2 (R Core Team 2015).
3 Results and discussion
3.1 Initial agronomic properties
The initial materials had relatively close textures ranging from loam (C, S3) to silt loam (S1, S2, S4) according to the USDA classification (Table 1). Bulk densities were below 0.75 g cm−3 for S1 and S2, and 1.32 and 1.17 g cm−3 for S3 and S4, respectively. S1 and S2 particle densities were lower than the density of the control (2.59 g cm−3), and S3 and S4 particle densities were greater. S1 and S2 were carbonate-free and had the highest organic matter content (224.3 and 81.4 g kg−1, respectively) associated to high total nitrogen content (8.3 and 3.6 g kg−1, respectively) and a moderate C:N ratio, close to the control C:N ratio (11.7). Compared to the other materials, these acidic sediments (pHwater < 6) had a high cation exchange capacity (CEC) of 29.7 and 11.6 cmol+ kg−1, respectively. S3 and S4 had the lowest OM content (29.1 and 29.6 g kg−1, respectively), the lowest total nitrogen content (0.5 and 0.7 g kg−1, respectively), and a higher C:N ratio (22.4 and 13.4, respectively). Their pHwater ranged from 7.5 to 7.8, and they had a high CaCO3 content (250 and 244 g kg−1, respectively) and a very low CEC (< 2.2 cmol+ kg−1) although the OM content was around 30 g kg−1 (Table 1). The CEC of these two sediments may have been underestimated because of the high CaCO3 content. The initial agronomic properties of the sediments were linked to their origins. S1 and S2 were acidic and more organic sediments because they came from low-altitude crystalline mountainous terrains while S3 and S4 were alkaline and less organic sediments because they came from high-altitude alkaline mountainous terrains.
Green waste compost (0.46 g cm−3) addition decreased the bulk density of all materials by − 10.5% (S1) to − 34.2% (S4) (C: − 28.4%) (Table 2). With its 429 g kg−1 of OM, the compost also increased the OM content of the mixes from + 5.4% (S1) to + 89.4% (S4) (C: + 63.0%). It increased C:N ratios, which reached values ranging between 17.4 (S1) and 23.1 (S3) (C: 18.9). The compost buffered the pH of the mixes around its own pH (7.4) by increasing the acidic pH values of C, S1, and S2, and maintaining the basic pH values of S3 and S4. Green waste compost had satisfactory content of total N (12.1 g kg−1), total P (3.7 g kg−1), and total K (5.9 g kg−1) likely to improve the amount of available nutrients for plants in constructed soils.
3.2 Above-ground biomass production
Twelve days after sowing, ryegrass germination occurred in all soil conditions, ruling out a phytotoxic effect of the constructed soils. After 18 months, ryegrass had developed in contrasting ways according to the soil conditions (Fig. 2). The above-ground plant biomass collected over 18 months on the control soil (C) (1860 g DM m−2) was statistically identical to that measured on S1 (1870 g DM m−2). During the same period, S2 produced more above-ground biomass (2192 g DM m−2) than C, whereas S3 and S4 produced 2.4-fold less biomass than C (756 and 794 g DM m−2, respectively). As early as after 6 months, S3 and S4 biomass production values were lower than in C, but growth on these sediments seemed to increase during the second period (6–12 months). The ryegrass grown on S3 and S4 showed scattered symptoms of leaf end yellowing, whereas the ryegrass grown on C, S1, and S2 developed healthy green leaves.
Above-ground plant biomass collected over 18 months from the ten constructed soil conditions. Letters indicate significant differences among the biomass accumulation values of each condition (statistical significance determined at p < 0.05 according to Tukey’s HSD test). Vertical bars represent standard errors (n = 3)
The compost-mixed constructed soils produced less biomass over 18 months than the soils constructed from pure materials. These differences were − 5% for CCo (1758 g DM m−2), − 17% for S1Co (1554 g DM m−2), − 22% for S2Co (1704 g DM m−2), − 51% for S3Co (369 g DM m−2), and − 86% for S4Co (107 g DM m−2). They were mainly due to a slower growth during the first 6 months for CCo, S1Co, and S2Co. For S3Co and S4Co, there was lower biomass production throughout at least the first year (S3Co) or throughout the entire experiment (S4Co). During the first 6 months, symptoms of leaf end yellowing were observed on the five compost-mixed soils. They were scattered for CCo, S1Co, and S2Co, but more marked for S3Co and S4Co.
The leaf end yellowing symptoms could be a sign of a nitrogen deficiency possibly caused by an intrinsic lack of nitrogen in the constructed soils (Arvalis 2017a). Moreover, these symptoms may have been enhanced by compost addition or provoked by soil waterlogging which limits nitrogen uptake by plants (Arvalis 2017b). However, these yellowing symptoms may also have been caused by the lack of other elements, such as potassium or iron. Nevertheless, these symptoms were no longer visible after 12 months on any of the soil conditions, giving a comforting perspective of the ryegrass development capacity.
The potential annual yield of a temporary meadow mainly sown with ryegrass is 6.9 t DM ha−1 yr−1 in the same region and under the same climate as the experiment (Local Agriculture Department 2012) (Table 3). C (12.4 t DM ha−1 yr−1), S1 (12.5 t DM ha−1 yr−1), and S2 14.6 (t DM ha−1 yr−1) produced almost twice as much as the local reference. CCo, S1Co, and S2Co produced less biomass than their compost-free counterparts, but still produced more biomass than the reference (11.7, 10.8, and 11.4 t DM ha−1 yr−1, respectively). S3 (5.0 t DM ha−1 yr−1) and S4 (5.3 t DM ha−1 yr−1) produced 1.3-fold less biomass than the reference meadow. The plant biomass yields of S3Co and S4Co were far below the reference, with only 2.5 and 0.7 t DM ha−1 yr−1 produced.
3.3 Below-ground plant biomass production
The cumulative root biomass collected over 18 months on C was 79.9 kg DM 30 cm−1 m−2, and statistically equal to the root biomass of S1 (80.7 kg DM 30 cm−1 m−2) (Fig. 3). S2 produced 16% more root biomass than C, and S3 and S4 produced 37 and 47% less root biomass than C, respectively. Compost addition increased the root biomass of CCo (+ 41%), S1Co (+ 18%), and S2Co (+ 87%) as compared to the corresponding pure soils. But it had a negative impact on root biomass production in S3Co and S4Co, which had 58 and 75% less below-ground biomass than S3 and S4 after 18 months.
Below-ground plant biomass collected over 18 months from the ten constructed soil conditions. Letters show significant differences among the biomass accumulation values of each condition (statistical significance determined at p < 0.05 according to Tukey’s HSD test). Vertical bars represent standard errors (n = 3)
3.4 Relation between above- and below-ground biomass
A strong positive correlation was evidenced between above-ground and below-ground biomass values for the soils constructed from pure sediments and the control soil (R2 = 0.90) (Fig. 4). It corroborated a similar linear correlation found on four perennial ryegrass (Lolium perenne) groups (Crush et al. 2010). In the compost-mixed soils, the correlation between roots and aerial parts was less marked (R2 = 0.69) (Fig. 4). Leaving S2Co aside, the correlation was stronger (R2 = 0.87) and close to that of the pure soils. The impact of compost addition reduced the slope regression by 3-fold (i.e., 0.01 vs. 0.03 in the absence of compost addition). Therefore, organic matter addition led to greater root development than aboveground biomass production.
The root:shoot ratio of ryegrass was calculated after each sampling date. It was between 17.6 (S3Co after 18 months) and 850.8 (S3Co after 6 months). These ratios were far above the ratio calculated by Robin et al. (2010) after 89 days (0.43 to 0.54) and by Crush et al. (2010) after 115 days (0.92 to 0.99) on one plant. This large difference between ratios could be explained by the sowing density, nutrient availability, and the length of the experiment. We sowed ryegrass at a high density (200 kg ha−1), probably causing the plants to compete for resources, whereas Robin et al. (2010) and Crush et al. (2010) studied individual plants. The lack of light due to the dense aerial biomass may have limited shoot biomass production. Furthermore, the possible nutrient deficiency (mostly N, P) in several sediments may have promoted root growth, as demonstrated by Lopez-Bucio et al. (2003). Moreover, the normal pattern of perennial ryegrass root dynamics involves continual formation of new roots (Robin et al. 2010). Our measurement method did not distinguish living roots from dead ones, so that the living and dead roots accumulated over time led to the large amounts of below-ground biomass we recorded. That is why we decided to focus preferentially on above-ground biomass to identify the soil factors involved in plant development.
3.5 Explanatory factors of plant development
We first tried to determine which soil properties were the main drivers of above-ground biomass production whatever the material considered (sediment or control soil). In this prospect, all sampling dates were taken into account. Then, we investigated the specific soil physical and chemical properties that influenced plant development in a given material, with or without compost addition. The above-ground plant biomass production of the constructed soils was significantly correlated to eight soil parameters (three physical and five chemical parameters) according to Pearson’s correlation matrix (p value < 0.05) among the 41 soil parameters we tested (Table 4). They are presented and discussed in the next two sections.
3.5.1 Soil physical factors
Aggregate stability, which is an indicator used to quantify the soil structure (Six et al. 2000), evaluated by MWD (R ≥ 0.76) and by WSA (R ≥ 0.69) was positively correlated to plant biomass at the three measurement dates (Table 4). MWD and WSA were correlated with each other (R ≥ 0.92). According to Arshad and Coen (1992) and Six et al. (2000), aggregate stability is a soil quality indicator used to quantify the soil structure. As for the soil structure, it is one of the major parameters affecting plant production by promoting rooting and root exploration, by allowing water storage and by allowing fluids (water and air) and fauna to move through the soil (Pagliai and Vignozzi 2002). The statistical approach suggested that the soil structure was involved in its fertility. Consequently, to focus on the soil structure and try to better understand the differences in biomass production, five soil physical properties of the constructed soils are presented in Table 5.
In the absence of compost addition, S1 and S2 had the lowest bulk densities (0.84 and 1 g cm−3 after 18 months, respectively) as compared to C, S3, and S4. These densities were lower than the bulk density threshold known to limit root growth due to high compaction (i.e., 1.55 g cm−3 for a silt loam soil; Hanks and Lewandowski 2003). By contrast, after 6 months C, S3, and S4 exceeded this threshold (> 1.60 g cm−3, according to their texture), which restricted root growth (Table 5). S3 and S4 bulk densities were still above that threshold after 18 months. This may partly explain differences in biomass production (Figs. 2 and 3). Compost addition decreased bulk densities whatever the constructed soil. Several studies showed that exogenous organic matter addition decreased bulk density while increasing soil porosity (Nyamangara et al. 2001; Celik et al. 2004; Barral et al. 2007; Grosbellet et al. 2011). This could facilitate root growth and explain part of our results. Even though S3Co and S4Co bulk densities were lower than the restricting root threshold proposed by Hanks and Lewandowski (2013), ryegrass biomass was lower than the biomass collected on S3 and S4. This suggests that other parameters were involved in plant growth.
S3 and S4 (MWD < 0.19 mm), and to a lesser extent S3Co and S4Co (MWD < 0.84 mm), had a very low aggregate stability, indicating a high risk of crustability (MWD < 0.65 mm) according to Le Bissonnais (2016) (Table 5). This was confirmed by a CI greater than 2 for S3 and S4 whatever the time after sowing, showing a very high risk of crustability (Rémy and Marin-Laflèche 1974) (Table 5). For the other constructed soils, MWD greater than 1.5 mm indicated stable or very stable aggregates (Le Bissonnais 2016) and so a limited risk of crustability. This was confirmed by a CI lower than 1.24.
Urban soils dedicated to greening applications should have soil AW and macroporosity (MP) values greater than 1 mm cm−1 and 0.2 m3 m−3, respectively (Dexter 1988; Pagliai and Vignozzi 2002). Except for S2Co and S3Co at month 18, all constructed soils had satisfactory AW values ranging from 1.07 to 1.59 mm cm−1 18 months after sowing. For S2Co and S3Co, a reorganization of the pore space due to compost addition may have promoted the formation of macropores (Grosbellet et al. 2011) which limited the AW. Moreover, OM mineralization during the experiment led to a strong decrease of the OM content in S2Co (54.5 g kg−1) and S3Co (24.4 g kg−1) after 18 months. Thus, the positive effect of OM on AW was lost. Using a more mature compost could provide a more stable OM and limit rapid mineralization. Constructed soil macroporosity at month 6 was lower than 0.2 m3 m−3 in C, S2, S3, and S4 (0.1, 0.15, 0.07, and 0.05 m3 m−3, respectively). Compost addition improved these values, which became greater than the acceptable threshold or remained close to it. After 18 months, macroporosity increased in all conditions, even if it remained lower than 0.2 m3 m−3 in C, S3, and S4.
Although S3 and S4 had interesting available water values, their macroporosity was very low. In addition, a slaking crust limited the air and water flows. These physical properties led to partial asphyxia of ryegrass roots, particularly during the first months after sowing (rainy winter period). These waterlogging conditions may also have limited nitrogen uptake by plants and caused the leaf end yellowing symptoms observed in the first months. This result corroborates the hypothesis put forward by Sturgis et al. (2001a, b), who suggested that the massive structure and high bulk density of sediments prevented fluid flow and partially inhibited plant germination and rooting.
Compost addition limited the slaking crust risk, reduced AW, and increased S3Co and S4Co macroporosity, which induced a lower soil waterlogging. Despite the improvement of physical properties induced by compost input, biomass production on compost-mixed soils, especially S3Co and S4Co, remained low. This suggests that other parameters, in particular chemical ones, are involved in biomass production, as indicated by the previously presented statistical approach.
3.5.2 Soil chemical factors
The clay content, which was strongly correlated to aggregate stability (R > 0.90), was also positively correlated (R ≥ 0.87) to shoot biomass production (Table 4). EDTA-extractable iron was positively correlated (R ≥ 0.82) to biomass production at the three measurement dates. These two parameters provide plant-favorable properties to soils because both of them directly contribute to the cationic exchangeable capacity, an essential parameter of plant nutrition. But they are also involved in aggregate stability (Tisdall and Oades 1982; Amézketa 1999; Bronick and Lal 2004), as suggested by the correlations between clay content and MWD (R ≥ 0.94) and clay content and WSA (R ≥ 0.88), and the correlations between Fe EDTA and MWD (R ≥ 0.78) and Fe EDTA and WSA (R ≥ 0.64).
Mineral nitrogen was positively correlated to biomass production (Table 4). After 6 months, nitrate and ammonium nitrogen were significantly correlated to biomass production (R = 0.68 and R = 0.69, respectively). But after 12 and 18 months, only ammonium nitrogen was significantly correlated (R ≥ 0.69). We supposed that anions were consumed or partially washed out. Mineral nitrogen is an important driver of plant growth because it is a component of plant cell amino acids, nucleic acids, proteins, and enzymes (Boussadia et al. 2010). Furthermore, mineral N availability may be counterbalanced by its high solubility that makes it easily leachable or denitrified (Lopez-Bucio et al. 2003). Available phosphorus plays an essential role in plant growth, yet it was not significantly correlated to above-ground biomass production. However, it was significantly positively correlated to below-ground biomass production (0.81 ≥ R ≥ 0.75) at the three time points (data not shown). The pH and total calcium carbonate content were also significantly but negatively correlated to biomass production (R ≤ − 0.89 and R ≤ − 0.86, respectively) (Table 4). As the CaCO3 content was significantly positively correlated to pH (R ≥ 0.87), it probably mainly explained the pH, which was adapted to ryegrass development or not.
Six chemical parameters were significantly involved in biomass production by the constructed soils. Among these parameters, Fe EDTA and clay content (due to its CEC and reactivity with OM) explained part of biomass production, but mainly because of their involvement in aggregate stability. Fe EDTA could also reflect a risk of iron deficiency that could lead to iron chlorosis. Nitrate and ammonium nitrogen were summed into mineral nitrogen to simplify the data. As the pH and the CaCO3 content were highly correlated, only the pH was presented in that part. Finally, available P and exchangeable K were presented too because these two nutrients play a major role in plant development (Craul 1992). Thus, four chemical parameters were selected to try to better understand the differences in biomass production among conditions (Table 6).
The pH values of the compost-free constructed soils after 6 months were close to or included in the optimal pH range for ryegrass growth (5.5 to 7.5) and strictly included in the pH range tolerated by ryegrass to develop (5.1 to 8.4) (Hannaway et al. 1999). But after 18 months, without compost addition, S1 and S2 grew acidic, whereas with compost addition S3 and S4 grew alkaline. Indeed, the pH values of S1 (4.87) and S2 (4.64) were below the tolerated pH (< 5.1) theoretically expected to limit plant growth (Table 6). However, aerial biomass production on these two sediments was similar to production on C, indicating no significant effect of too acidic pH values. Maybe an effect would be visible beyond 18 months. Compost addition improved the pH values of all conditions, which complied with pH guidelines for ryegrass growth. Eighteen months after sowing, S3 and S4 pH values were higher than the optimal basic pH (> 7.5) but still below the basic pH limit (< 8.4). The lower biomass production measured in S3 and S4 could be partly explained by these high pH values.
Lower biomass production could also be explained by mineral nitrogen deficiency. Nmin was lower than 1.86 mg kg−1 in S3 and S4, whereas it was higher than 4 mg kg−1 in the other soils. Koenig and Isaman (2010) evaluated that a constructed soil for urban greening should have more than 20 mg kg−1 of mineral NO3−-N to promote satisfactory conditions for plant growth. Only S1 after 6 months reached this threshold (Table 6). All the other constructed soils had less nitrogen than required after 6 and 18 months. Thus, the particularly low nitrogen content in S3, S4, S3Co, and S4Co (< 2 mg kg−1) suggest an important risk of nitrogen deficiency, which could explain part of their lower biomass production.
Compost (C:N ratio 17:7) addition may have induced nitrogen deficiency in the compost-mixed soils (Jakobsen 1995). This could explain the lower biomass production measured in the compost-mixed soils as compared to the pure-material soils and the leaf end yellowing observed in the first 6 months.
Lower biomass production could also be explained by iron chlorosis induced by iron deficiency. EDTA-extractible Fe showed that available Fe was satisfactory in all the constructed soils, which limited the risk of iron chlorosis. Compost addition led to an average decrease of Fe EDTA (− 49% at 6 months) mainly because S1 and S2 had high content of Fe EDTA.
N deficiency was associated to the particularly low P and K content in S3 and S4 (i.e., lower than 0.02 and 0.03 mg kg−1 for P and K, respectively). These values are lower than the minimal agronomic thresholds which allow biomass production without additional fertilization (0.05 and 0.12 g kg−1 for P and K, respectively) (Arvalis 2009; Comifer 1995). Both C and S2 also presented lower K content than the acceptable threshold after 6 and 18 months, but K deficiency did not affect plant growth for these conditions (Figs. 2 and 3). Finally, compost addition improved contents of P and K, and also increased available contents of Ca, Mg, Na, Bo, and Zn while decreasing the available Cu content (data not shown), but plant biomass in S3Co and S4Co remained lower than in S3 and S4.
3.6 Grouping of constructed soils
In order to have an integrated overview of the results, a PCA was undertaken (Fig. 5). The previously identified explanatory factors of plant development (bulk density, aggregate stability (MWD, WSA), crustability index, available water, macroporosity, clay content, mineral nitrogen content, available P and K contents, EDTA-extractable Fe, pH, CaCO3 content) seemed to be well adapted to assess the capacity of the sediment-made soils to fulfill the vegetation support function. Dimension 1 of the PCA explained 68.9% of the observed variability (Fig. 5b). It was mainly composed of physical properties such as aggregate stability, macroporosity, and bulk density, but also of the CaCO3 content and ammonium N. Dimension 2 explained rather less variability (15.0%) and was mainly composed of water and nutrient availabilities (Fig. 5b). The principal component analysis performed on the selected soil parameters and on plant biomass after 18 months distinguished four groups of sediment-made soils (Fig. 5a). S1 and S2 were characterized by high shoot biomass production. They were well supplied with mineral nitrogen, iron and available water, and had stable aggregates, low bulk density, and an acidic pH. C has similar properties to these soils. Compost addition to these sediments led to the formation of another group of sediment-made soils that included S1Co and S2Co. Their low bulk density and their good nutrient supply (N, P, and K) facilitated rooting. CCo had similar properties to these soils. Although the PCA distinguished two groups of soils among S3, S4, S3Co, and S4Co, they were close to each other. They were characterized by basic pH values, high CaCO3 content, strong bulk densities, and a weak aggregate stability leading to a significant risk of slaking crust. These soils produced the lowest amounts of ryegrass biomass but they were strongly colonized by exogenous plant species throughout the 18 months of the experiment. This colonization suggests that ryegrass was not well adapted to these soils that were yet fertile enough to allow for the development of adapted plant species. The long-term evolution of plant development should be investigated. Several long-term studies have been carried out over up to 6 to 21 years after soil preparation and vegetation seeding or planting (Alday et al. 2012; Chen et al. 2016; Lei et al. 2016), in a soil restoration context. The organic matter content tended to increase owing to progressive colonization by plants and the subsequent increase in organic matter input towards the soil. Such an increase favored the soil water-holding capacity and nutrient reservoir (Lei et al. 2016).
Graphical displays of the first two dimensions of the principal component analysis (PCA) (graph of individuals (a) and graph of variables (b)) performed on the explanatory factors of plant development on the eight sediment-made soils after 18 months. C and CCo are illustrative individuals, and species richness (Species) is an illustrative variable. N-NO3− (N_nit), available water (AW), EDTA-extractable iron (Fe_EDTA), above-ground plant biomass (Shoot_B), mean weight diameter (MWD), water stable aggregate (WSA), N-NH4+ (N_amm), below-ground plant biomass (Root_B), available phosphorus (P_olsen), exchangeable potassium (K_ex), pH water (pH), total calcium carbonate content (CaCO3_t), bulk density (BD), crustability index (CI), and macroporosity (p_macro). Only variables with cos(θ)2 over 0.75 are shown
4 Conclusions
The objective of the present study was to assess the capacity of dam sediment to support plant growth, as a substitute of arable soil commonly and excessively used to create urban green spaces. Sediment medium-term agronomic properties were brought face to face to ryegrass growth, and we characterized the impact of green waste compost addition on plant growth.
All sediments were suitable for plant growth, but in contrasting ways. S1 and S2, which came from low-altitude mountainous terrains (the Massif Central and the Massif Armoricain), had satisfactory chemical and physical properties leading to a quick installation of a dense ryegrass cover that produced an important quantity of biomass, suggesting a good health state. Therefore, our results show that these two sediments could be used to set up green spaces or to ecologically reclaim degraded soil areas, such as brownfields.
The chemical and physical properties of soils S3 and S4 which came from high-altitude mountainous terrains (the Alps) were only partially adapted to ryegrass support. Their lack of structure led to limited water and air flows, which favored root asphyxia. In addition, their low available N, P, and K levels limited ryegrass development. However, exogenous wind-dispersed plant species developed spontaneously on these sediments during the experiment, proving that they can support better adapted plants, such as species which need a low trophic level and calcicolous conditions.
Green waste compost addition had contrasting effects on sediment fertility. Compost first had an initial negative effect leading to temporary N deficiency for the plants. But this effect eased after a maturation period whose length (6 to 18 months) depended on the sediment, and then, satisfactory chemical properties were found. In addition, exogenous OM increased the nutrient (P, K) stocks. Compost addition had beneficial effects on the physical properties. This suggests that exogenous OM addition is beneficial for sediment fertility, but its possible negative effect on N availability should be taken into account during soil construction. Therefore, a maturation period of the sediment-compost mixture or the addition OM with adequate fertilizers and pH regulators before planting or sowing could avoid nutrient deficiencies for plants.
According to our results and based on our constructed soils, few soil properties can be used to assess the fertility of sediment-made Technosols. Among them, the soil structure is one of the most determining parameters. It can be assessed from aggregate stability, macroporosity, the crustability index, and bulk density. Available nutrients (N, P, K) and pH values seem sufficient to assess chemical fertility.
These results are encouraging and need to be confirmed at a larger scale in real conditions and in the long term. Two larger experimental sites have been installed in the Alps to assess the properties of the sediment-made Technosols over time to follow the development of Alps local plant species and to confirm that these Technosols could be used for ecological restoration. These results will be aggregated with the results of the current field experiments to develop a decision-making tool that will help identify whether a sediment is suitable for vegetation support in green space setting up or ecological restoration, whether it requires an exogenous OM input and which plant species are best adapted to the sediment-made Technosol.
References
AFNOR (1995a) Qualité du sol - Détermination de la conductivité électrique spécifique. NF ISO 11265
AFNOR (1995b) Qualité du sol – Dosage du phosphore – dosage spectrométrique du phosphore soluble dans une solution d’hydrogénocarbonates de sodium. NF ISO 11263
AFNOR (1998) Qualité du sol - Détermination de la teneur totale en azote par combustion sèche. NF ISO 13878
AFNOR (1999) Qualité des sols - Méthodes chimiques - Détermination de la capacité d'échange cationique (CEC) et des cations extractibles. NF:X31–X130
AFNOR (2002) Qualité des sols – détermination des cations Ca2+, Mg2+, K+, Na+ extractibles par l’acétate d’ammonium – méthode par agitation. NF:X31–X108
AFNOR (2003a) Qualité du sol - Détermination de la distribution granulométrique des particules du sol - Méthode à la pipette. NF:X31–X107
AFNOR (2003b) Qualité des sols - Détermination du cuivre, du fer, du manganèse et du zinc - Extraction par l'acétate d'ammonium en présence d'EDTA. NF:X31–X120
AFNOR (2005) Qualité du sol - Détermination du pH. NF ISO 10390
AFNOR (2006) Amendements organiques - dénominations, spécifications et marquage. NF:U44–U051
AFNOR (2009) Qualité de l’eau – dosage d’éléments choisis par spectroscopie d’émission optique avec plasma induit par haute fréquente (ICP-OES). NF EN ISO 11885
AFNOR (2014) Qualité du sol - Détermination de la teneur en carbonate - Méthode volumétrique. NF ISO 10693
Alday JG, Marrs RH, Martinez-Ruiz C (2012) Soil and vegetation development during early succession on restored coal wastes: a six-year permanent plot study. Plant Soil 353:305–320
Amézketa E (1999) Soil aggregate stability: a review. J Sustain Agric 14:83–151
AFNOR (2007) Qualité du sol – dosage des nitrates des nitrites et de l’ammonium dans les sols bruts par extraction avec une solution de chlorure de potassium- partie 2 : méthode automatisée avec analyse en flux segmenté. NF ISO:14256–14252
Anger B (2014) Caractérisation des sédiments fins des retenues hydroélectiques en vue d'une orientation vers des filières de valorisation matière. Dissertation. In: University of Caen
Arshad MA, Coen GM (1992) Characterization of soil quality: physical and chemical criteria. Am J Altern Agric 7:25–32
Arvalis (2009) Teneurs-seuils PK par type de sol. ARVALIS : institut du végétal. https://www.arvalis-infos.fr/.../seuils_pk_regionaux_v7341752814230201015.pdf. Accessed 17/03/2009
Arvalis (2017a) Fiches accidents – Carence en azote: accidents physico-chimiques sur blé tendre, blé dur, triticale. Arvalis-infos. http://www.fiches.arvalis-infos.fr/fiche_accident/fiches_accidents.php?mode=fa&type_cul=1&type_acc=1&id_acc=56. Accessed 21/11/2017
Arvalis (2017b) Fiches accidents – Excès d’eau : accidents liés au climat sur blé tendre, blé dur, triticale. Arvalis-infos. http://www.fiches.arvalis-infos.fr/fiche_accident/fiches_accidents.php?mode=fa&type_cul=1&type_acc=5&id_acc=64. Accessed 21/11/2017
Bacholle C, Leclerc B, Coppin Y (2006) Utilisation des produits organiques en reconstitution de sol. Inventaire des pratiques en France. Etat de l’art des connaissances liées aux impacts de ces pratiques. ADEME, Angers
Barral MT, Bujan E, Devesa R, Iglesias ML, Velasco-Molina M (2007) Comparison of the structural stability of pasture and cultivated soils. Sci Total Environ 378:174–178
Blake GR (1965) Particle density. In: Black CA (ed) Methods of soil analysis: part 1: physical and mineralogical properties, including statistics of measurement and sampling. American Society of Agronomy, Madison, pp 371–373
Boussadia O, Steppe K, Zgallai H, Hadj SBE, Braham M, Lemeur R, Labeke MCV (2010) Effects of nitrogen deficiency on leaf photosynthesis, carbohydrate status and biomass production in two olive cultivars “Meski” and “Koroneiki”. Sci Hortic 123:336–342
BRGM (2012) Base de données nationale d’anciens sites industriels et activités de service BASIAS. http://basias.brgm.fr
Bronick CJ, Lal R (2004) Soil structure and management: a review. Geoderma 124:3–22
Cannavo P, Vidal-Beaudet L, Grosbellet C (2014) Prediction of long-term sustainability of constructed urban soil: impact of high amounts of organic matter on soil physical proerties and water transfer. Soil Use Manag 30:272–284
Carter MR, Stewart BA (1996) Structure and organic matter storage in agricultural soils. Lewis publishers. In: CRC press. USA, Boca Raton, FL
Celik I, Ortas I, Kilic S (2004) Effects of compost, mycchoriza, manure and fertilizer on some physical properties of a Chromoxerert soil. Soil Till Res 78:59–67
CEN (1999) EN 13040. Soil improvers and growing media. Sample preparation for chemical and physical tests, determination of dry matter content, moisture content and laboratory compacted bulk density. European Committee for Standardization, Bruxelles
Chen X, Duan Z, Tan M (2016) Restoration affect soil organic carbon and nutrients in different particle-size fractions. Land Degrad Dev 27:561–572
COMIFER (1995) Aide au diagnostic et à la prescription de la fertilisation phosphatée et potassique des grandes cultures. COMIFER
Cordova S, Neaman A, Gonzalez I, Ginocchio R, Fine P (2011) The effect of lime and compost amendments on the potential for the revegetation of metal-polluted, acidic soils. Geoderma 166:135–144
Craul PJ (1992) Soil fertility. In: Craul PJ (ed) Urban soil in landscape design. Wiley, New York, pp 157–185
Crush JR, Nichols SN, Ouyang L (2010) Adventitious root mass distribution in progeny of four perennial ryegrass (Lolium perenne L.) groups selected for root shape. New Zeal J Agr Res 53:193–200
Damas O, Coulon A (2016) Créer des sols fertiles: du déchet à la végétalisation urbaine. Chapter 1: Construire des sols pour végétaliser la ville 335p
Daniels WL, Whittecar GR, Carter III CH (2007) Conversion of Potomac River dredge sediments to productive agricultural soils. 2007 National Meeting of the American Society of Mining and Reclamation 183–199
De Boodt M, Verdonck O, Cappaert J (1974) Methods for measuring the water release curve of organic substrates. Acta Hort (37):2054–2062
Dexter AR (1988) Advances in characterization of soil structure. Soil Till Res 11:199–283
Grosbellet C, Vidal-Beaudet L, Caubel V, Charpentier S (2011) Improvement of soil structure formation by fractionation of coarse organic matter. Geoderma 162:27–38
Frossard E, Skrabal P, Sinaj S, Bangerter F, Traoré O (2002) Form and exchangeability of inorganic phosphate in composted solid organic wastes. Nutr Cycl Agroecosys 62:103–113
Hanks D, Lewandowski A (2003) Protecting urban soil quality: examples for landscape codes and specifications. USDA, 20p
Hannaway D, Fransen S, Cropper J, Teel M, Chaney M, Griggs T, Halse R, Hart J, Cheeke P, Hansen D, Klinger R, Lane W (1999) Perennial Ryegrass : Lolium perenne L. Oregon State University
Hati KM, Swarup A, Dwivedi AK, Misra AK, Bandyopadhyay KK (2007) Changes in soil physical properties and organic carbon status at the topsoil horizon of a vertisol of Central India after 28 years of continuous cropping, fertilization and manuring. Agric Ecosyst Environ 119:127–134
Haus NW (2011) Beneficial reuse of dredged materials in upland environments. Master of science in crop & soil environment sciences. Virginia Polytechnic institute
Haynes R, Swift R (1990) Stability of soil aggregates on relation to organic constituents and soil water content. J Soil Sci 41:73–83
Huinink JTM (1998) Soil quality requirements for use in urban environments. Soil Till Res 47:157–162
INERIS (2010) Qualité chimique des sédiments fluviaux en France: Synthèse des bases de données disponibles. Rapport d'étude n°INERIS-DRC-10-105335-04971A
IUSS Working Group WRB (2015) World Reference Base for soil resources 2014, update 2015 – p.115
Jakobsen ST (1995) Aerobic decomposition of organic wastes 2. Value of compost as a fertilizer. Resour Conserv Recycl 13:57–71
Jim C (1998) Soil characteristics and management in an urban park in Hong Kong. Environ Manag 22:683–695
Koenig R, Isaman V (2010) Topsoil quality guidelines for landscpaing. All Archived Publications. Paper 42. http://digitalcommons.usu.edu/extension_histall/42
Koropchak SC, Daniels WL, Wick A, Whittecar GR, Haus N (2015) Beneficial Use of Dredge Materials for Soil Reconstruction and Development of Dredge Screening Protocols. J Environ Qual, Special section: Soil in the city
Kramer PJ, Kozlowski TT (1979) Mineral nutrition and salt absorption. Physiology of woody plants 334–373
Le Bissonnais Y (2016) Aggregate stability and assessment of soil crustability and erodibility: I. Theory and methodology. Eur J Soil Sci 67(1):11–21
Le Bissonnais Y, Le Souder C (1995) Mesurer la stabilité structurale des sols pour évaluer leur sensibilité à la battance et à l’érosion. Etude et Gestion des Sols 2:43–55
Lê S, Josse J, Husson F (2008) FactoMineR: an R package for multivariate analysis. J Stat Softw 25:1–18
Lei H, Peng Z, Yigang H, Yang Z (2016) Vegetation and soil restoration in refuse dumps from open pit coal mines. Ecol Eng 94:638–646
Local Agriculture Department (2012) Référentiel de production des prairies en Pays-de-la-Loire. Chambre d’agriculture Pays-de-la-Loire et INRA
Lopez-Bucio J, Cruz-Ramirez A, Herrera-Estrella L (2003) The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol 6:280–287
Macia P, Fernandez-Costas C, Rodriguez E, Sieiro P, Pazos M, Sanroman MA (2014) Technosols as a novel valorization strategy for an ecological of dredged marine sediments. Ecol Eng 67:182–189
Norland MR, Veith DL (1995) Revegetation of coarse taconite iron ore tailing using municipal solid waste compost. J Hazard Mater 41:123–134
Nyamangara J, Gotosa J, Mpofu SE (2001) Cattle manure effects on structural stability and water retention of a granitic sandy soil in Zimbabwe. Soil Till Res 62:157–162
Pagliai M, Vignozzi N (2002) Soil pore system as an indicator of soil quality. In: Pagliai M, Jones R (eds) Sustainable soil management for environmental protection. Soil Physics Aspects. Catena Verlag, Reiskirchen, pp 71–82
Pons LJ, Zonneveld IS (1965) Soil ripening and soil classification. Initial soil formation in alluvial deposits and a classification of the resulting soils, Publication 13, International Institute for Land Reclamation and Improvement
R Core Team (2015) R: a language and environment for statistical computing. R foundation for statistical computing. http://www.R-project.org
Regelink IC, Stoof CR, Rousseva S, Weng L, Lair GJ, Kram P, Nikolaidis N, Kercheva P, Banwart M, Comans S, Comans RNJ (2015) Linkages between aggregate formation, porosity and soil chemical properties. Geoderma 248:24–37
Rémy JC, Marin-Laflèche A (1974) L’analyse de terre: réalisation d’un programme d’interprétation automatique. Ann Agron 25:607–632
Richards LA (1947) Pressure membrane apparatus: construction and use. Agric Eng 28:451–454
Robin AHK, Matthew C, Crush JR (2010) Time course of root initiation and development in perennial ryegrass – a new perspective. Proceedings of the New Zealand grassland Association 72:233–240
Scharenbroch BC, Catania M (2012) Quality attributes as indicators of urban tree performance. Arboric Urban For 38:214–228
Schvartz C, Muller JC, Decroux J (2005) Guide de la fertilisation raisonnée. In: La France Agricole (ed) 414p
Schjønning P, Elmholt S, Christensen BT (2004) Soil quality management - concepts and terms. In: Managing soil quality: challenges in modern agriculture. CAB International
Schumacher BA, Shines KC, Burton JV, Papp ML (1991) A comparison of soil sample homogenization techniques (chapter 4) in: Simmons MS (ed) Hazardous waste measurements. Lewis publishers Inc, Chelsea, pp 53–68
Séré G, Ouvrard S, Schwartz C, Renat JC, Morel JL (2008) Soil construction: a step for ecological reclamation of derelict lands. J Soils Sediments 8:130–136
Séré G, Schwartz C, Ouvrard S, Renat JC, Watteau F, Villemin G, Morel JL (2010) Early pedogenic evolution of constructed Technosols. J Soils Sediments 10:1246–1254
Sheehan C, Harrington J, Murphy JD (2010) A technical assessment of topsoil production from dredged material. Resour Conserv Recycl 54:1377–1385
Six J, Paustian K, Elliott ET, Combrick C (2000) Soil structure and organic matter: I Distribution aggregate-size classes and aggregate-associated carbon. Soil Sci Soc Am J 64:681–689
Sturgis TC, Lee CR, Banks HC (2001a) Evaluation of Toledo Harbor for manufactured soil. US Army Corps of Engineers, environmental laboratory
Sturgis TC, Lee CR, Banks HC, Burchell MR, Johnson K (2001b) Evaluation of manufactured soil using dredged material from New York/New Jersey Harbor Newton Creek site. US Army Corps of Engineers, environmental laboratory
Tisdale SL, Nelson WL, Beaton JD, Havlin JL (1993) Elements required in plant Nutrition In: Soil Fertility and Fertilizers, 5th edn Macmillan Publishing Company, Corey, pp 45–80
Tisdall JM, Oades JM (1982) Organic matter and water-stable aggregates in soils. J Soil Sci 33:141–163
UNEP (2012) Travaux des sols, supports de paysage : caractérisation, amélioration, valorisation et reconstitution. Règles professionnelles. P.C.1-R0
Vangronsveld J, Colpaert JV, Van Tichelen KK (1996) Reclamation of a bare industrial area contaminated by non-ferrous metals: Physico-chemical and biological evaluation of the durability of soil treatment and revegetation. Environ Pollut 94:131–140
Vermeulen J, van Dijk SG, Grotenhuis JTC, Rulkens WH (2003) Quantification of physical properties of dredged sediments during physical ripening. Geoderma 129:147–166
Vervaeke P, Luyssaert S, Mertens J, De Vos B, Speleers L, Lust N (2001) Dredged sediment as a substrate for biomass production of willow trees established using the SALIMAT technique. Biomass Bioenergy 21:81–90
Vidal-Beaudet L, Grosbellet C, Forget-Caubel V, Charpentier S (2012) Modelling long-term carbon dynamics in soils reconstituted with high quantities of organic matter. Eur J Soil Sci 63:787–797
Vidal-Beaudet L, Rokia S, Nehls T, Schwartz C (2018) Aggregation and availability of phosphorus in a Technosol constructed from urban wastes. J Soils Sediments 18:456–466
Yang X, Li P, Zhang S, Sun B, KXinping C (2011) Long-term fertilization effects on soil organic carbon, physical properties, and wheat yield of a loess soil. J Plant Nutr Soil Sci 174:775–784
Acknowledgments
We wish to thank Claudie Mazzega and Yvette Barraud-Roussel (technicians in the Environnement Physique de la Plante Horticole (EPHor) unit, – Agrocampus-Ouest) for their help in the laboratory work, Férréol Braud (technician in the Institut de Génétique Environnement et Protection des Plantes (IGEPP) – Institut National de la Recherche Agronomique (INRA), Agrocampus-Ouest, Université de Rennes 1) for his help in botanical survey, and Sébastien Menu (engineer in Development Direction – Division Production Ingénierie Hydraulique (DPIH) – Electricité de France (EDF)) for his advice and his revision of the manuscript. Experiments were conducted at the ImHorPhen (shared experimental facilities) with the assistance of R. Gardet.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Rongliang Qiu
Rights and permissions
About this article
Cite this article
Fourvel, G.J., Vidal-Beaudet, L., Le Bocq, A. et al. Fertility of Technosols constructed with dam sediments for urban greening and land reclamation. J Soils Sediments 19, 3178–3192 (2019). https://doi.org/10.1007/s11368-018-2077-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-018-2077-1