Abstract
Purpose
Soil contamination with heavy metals, such as Cd and Pb, has caused severe health and environmental risks all over the world. Possible eco-friendly solutions for Cd and Pb immobilization were required to reduce its mobility through various cost-effective amendments.
Materials and methods
A laboratory incubation study was conducted to assess the efficiency of biochar (BC), zeolite (ZE), and rock phosphate (RP) as passivators for the stabilization of Cd and Pb in paddy soil as well as soil microbial biomass. Various extraction techniques were carried out: a sequential extraction procedure, the European Community Bureau of Reference (BCR), toxicity characteristic leaching procedure (TCLP) test, and single extraction with CaCl2. The impact of passivators on soil pH, dissolved organic carbon (DOC), and microbial biomass (carbon, nitrogen, and phosphorus) was examined in the metal contaminated soil.
Results and discussion
The results showed that the exchangeable portion of Cd in soil was significantly reduced by 34.8, 21.6, and 18.8% with ZE, RP, and BC at a 3% application rate, respectively. A similar tendency of reduction in Pb soluble portion was observed by ZE (9.6%), RP (20%), and BC (21.4%) at a 3% application rate. Moreover, the TCLP leachate of Cd and Pb was apparently reduced by 17 and 30.3% with BC at a 3% application dose, respectively, when compared to the control. Soil pH, nutrients, and microbial biomass C, N, and P were significantly increased with the addition of BC, RP, and ZE passivators.
Conclusions
The results showed that the incorporation of BC, ZE, and RP significantly reduced the Cd and Pb mobility in paddy soil as well as enhanced soil nutrients and microbial biomass. Overall, among all the amendments, rice straw derived-BC performed better for Cd and Pb immobilization in paddy soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The rapid industrial development, in favor of urbanization, has caused the excessive release of potentially toxic pollutants into the environment. The pollutants include heavy metals which are highly toxic, persistent, and cannot be easily degraded into simple compounds (Bogusz et al. 2015). A discharge of toxic heavy metals, for instance, cadmium (Cd) and lead (Pb), is a cause of global environmental problem. They are easy to be transferred into the food chain and ultimately pose a threat to human beings (Kumari et al. 2014). The excessive use of Cd and Pb in modern industries such as mining, smelting, and fertilizer and pesticides production is the direct or indirect discharge of their effluents into the environment, which causes contamination in soil and groundwater (Bogusz et al. 2015). These industrial activities are necessary for the growth of civilization; therefore, the amelioration of Cd and Pb co-contaminated soil is an important task to reduce their mobility within the soil. For this purpose, various remediation techniques have been performed, such as (physical and chemical approaches and phytoextraction) (Kumpiene et al. 2008). On the other side, in situ remediation option has exerted a substantial attention now in these days, due to low-cost remediating agents through various mechanisms (i.e., precipitation, adsorption, and co-precipitation) with chemical amendments (Ahmad et al. 2017).
Immobilization of heavy metal with alkaline and mineral amendments is also a promising technique for in situ remediation due to long-term stabilization and cost-effective applicability (Ok et al. 2011). Recently, the use of several organic (compost, plant residues, and biochar) and inorganic (clay minerals and phosphate) fertilizers has been shown to be excellent amendments for soil (Bashir et al. 2018a, b). Biochar (BC) is a carbonaceous alkaline material that is usually derived from organic biomass under pyrolysis at high temperatures in the absence of oxygen (O2) (Lehmann et al. 2011; Bashir et al. 2018a). Biochar can ameliorate the toxicity of heavy metals and also improves soil quality (Ahmad et al. 2017; Hussain et al. 2017; Herath et al. 2017). Compared to the several organic amendments, BC is a novel remediating approach and considered a cost-effective amendment to remediate heavy metal pollution (Frišták et al. 2015). Application of BC can increase soil microbial growth, activities, and community composition that in turn can affect nutrient cycling, plant growth, and greenhouse gas emission, as well as soil organic carbon mineralization (Lehmann et al. 2011; Bashir et al. 2018a). Biochar incorporation into soil could increase organic matter mineralization and C sequestration as well as increase microbial biomass (Zhou et al. 2017).
The chemical amendment like zeolite (ZE) belongs to an aluminosilicate mineral family that has high adsorptive ability for anions and other molecules due to its high CEC (Inglezakis et al. 2002). Oste et al. (2002) and Bashir et al. (2018b) indicated that in situ chemical immobilization of heavy metal mainly Cd by zeolite is a good technique to stabilize metals in the polluted soils. Moreover, they suggested that the addition of zeolite could increase heavy metal immobilization through adsorption and ion exchange mechanism. The previous study reported that the addition of zeolite to a polluted soil could reduce heavy metal mobility, increased soil microbial activity, litter decomposition, and basal respiration (Kiikilä et al. 2001).
Among the alkaline amendments, phosphate minerals especially, rock phosphate (RP) can significantly reduce heavy metal mobility in soil (Islam et al. 2010). The application of rock phosphate as a P source to contaminated soils has the ability to reduce the toxicity of potential elements and thereby, increase carbon and phosphorus turnover by increasing their transformation and mineralization (Li et al. 2015). In addition, P source amendments have a noticeable effect on soil pH, which can also influence microbial growth (Li et al. 2015). However, direct evidence for the transfer of available C and nutrients from biochar and P source amendments to microorganisms is still lacking, but they mainly provide a natural habitat to microorganisms due to their porous structure and excessive release of nutrients (Kolb et al. 2009; Lehmann et al. 2011). The most likely mechanism involved is the significant increment of C and P mineralization which directly increases substrate for soil microbial growth (Kolb et al. 2009; Huang et al. 2016).
The environment-friendly reuse of industrial byproducts and valuable amendments in the agriculture is well considered as a sound substitute for both environmentally and economically. Recently, there is an increasing demand for novel and cost-effective amendments to use them as soil passivators for heavy metal remediation in contaminated soils (Ok et al. 2011). Shaheen et al. (2015) examined the effectiveness of variety of low-cost alkaline amendments as well as clay minerals to eliminate exchangeable toxic elements from polluted soils and water.
However, various remediating agents, while economical and environmentally friendly, have not been broadly used for Cd and Pb in acidic paddy contaminated soils. The increasing demand for newly, friendly, and applicable methods towards the immobilization of multi-contaminated soil has led to being a progressive evolution of organic and inorganic materials having the high adsorptive capacity. Many findings have been focused for heavy metal immobilization with chemical agents. In the current study, comparative effect of organic and inorganic immobilizing materials such as biochar, natural zeolite, and rock phosphate for geochemical fractionation of Cd and Pb in paddy polluted soil was observed. Moreover, their efficiency on microbial biomass and nutrient status in metal contaminated soil was also measured.
2 Materials and methods
2.1 Soil collection and characterization
The soil used in this study was collected at 0–20 cm depth from a paddy field beside a Pb mining area located in Linxiang city, Hunan Province, China. Stones, plant residues, and earthworms were detached from soil samples and then mixed to get a composite sample. The soil was then transferred to the laboratory, where the samples were air-dried for 2 weeks and ground to pass through a 2-mm mesh size. Selected physico-chemical properties of pre-experimental soil were analyzed before the onset of the experiment. A pH meter (Mettler Toledo Delta 320) and EC meter (DDS-307A) were used for pH and EC measurement, respectively (Shaaban et al. 2013). Organic matter content was determined using wet oxidation with H2SO4·K2Cr2O7 (Lu 2000). Soil textural analysis was performed using the pipette method (Gee et al. 1986), and soil cation exchange capacity (CEC) was determined by ammonium acetate method at pH 7. Soil macro elements such as N, P, and K were determined by Lu (2000). Soil total metal contents of Cd and Pb were determined using atomic adsorption spectrophotometer (AAS), after digesting with anHCl–HNO3–HClO4 mixture. The selected physical and chemical properties of soil were as follows: silt 48%, clay 16% and sand 36%, pH 5.9, organic matter 40.6 g kg−1, and CEC 8.65 cmol (+) kg−1. The total Cd and Pb contents in the studied soil were 4.9 and 1073 mg kg−1, respectively.
2.2 Biochar production and other passivators
Rice straw samples were collected from the experimental station of Huazhong Agricultural University, Wuhan, China, after harvest of the rice crop. Rice straw was washed, cleaned, and air-dried for 2 weeks and then chopped to pass through a 10-mm mesh sieve. Rice straw was placed in a porcelain crucible, covered with an air tight lid before pyrolysis under limited oxygen conditions at 500 °C for 2 h, and then prepared as described previously by Yuan et al. (2011). The temperature was raised with 20 °C per minute and kept constant at 500 °C for 2 h and then the product (biochar) was cooled at room temperature and ground to pass through 60 mesh size (0.25-mm pore size). The selected basic biochar properties were as follows: BET-SA 39 m2 g−1, yield 47%, ash contents 49%, C 54% and N 1.6%, PO4−3 8.02 mg g−1, CO3−2 10.3 mg g−1, Ca2+ 9.69 mg g−1, Mg2+ 2.32 mg g−1. The surface area of BC was determined by BET method at 77 K using an (Autosorb-1, Quantachrome, USA). Natural rock phosphate and zeolite were used, obtained from a local company Zhongxiang Ltd., Hubei Wuhan, Province, China. Zeolite has the following properties such as CEC (40 cmol kg−1), surface area 20 m2 g−1, CaO 4.5%, and SiO2 69%.
2.3 Incubation study
Incubation experiment was conducted in plastic beakers with each beaker containing 200 g of air-dried naturally contaminated paddy soil. The experiment was statistically arranged with seven treatments and three replications as follows: (1) control (CK), (2) rice straw (BC) 1.5%, (3) rice straw (BC) 3%, (4) zeolite (ZE) 1.5%, (5) zeolite (ZE) 3%, (6) rock phosphate (RP) 1.5%, and (7) rock phosphate (RP) 3% (w/w). Biochar, zeolite, and rock phosphate were applied to soils at 1.5 and 3%, respectively, and mixed thoroughly. All soil samples were incubated for 3 months while maintaining 70% (w/v) moisture at 25 °C. The beakers were covered with plastic lids, each of which contained a hole to reduce gaseous exchange and water loss. After an incubation period, the moist soil was collected from each experimental unit for soil biochemical analysis and the remaining soil was air-dried and ground to pass through 0.054-mm sieve for further chemical analysis.
2.4 BCR sequential extraction
Metal partitioning was determined according to the European Community Bureau of Reference (BCR) sequential extraction method (Rauret et al. 1999) which was described in detail by Rizwan et al. (2016). Briefly, step 1—acid-soluble fraction; 20 ml of acetic acid (0.11 M L−1) was added into in a 50-ml polyvinyl centrifuge tube already containing 0.5 g soil, and then shaken for 16 h to reach equilibrium. After that, the suspension was centrifuged at 4000 revolutions per minute (rpm) for 20 min. The supernatant was stored at 4 °C for further analysis. The soil pellet was washed with distilled water and used for further extraction (reducible fraction). Step 2—subsequently, 20 ml of 0.1 M hydroxylamine hydrochloride (acidified at pH 2) for Fe/Mn-bound metal fraction was added in the residual soil pellet of step 1, and then shaken and centrifuged and the filtrate was stored at 4 °C. The soil pellet was washed with distilled water and used for further extraction. Step 3—the water used that washed the remaining soil residue in step 2 was carefully used in the third step (oxidizable proportion). Five milliliters of 30% (m/v) H2O2 was added into soil pellet and allowed standing for 1 h at 85 °C on water bath. Another 5 ml of 30% (m/v) H2O2 was used to digest sample at 85 °C. Then, the soil was extracted with 25 ml of 1 M of ammonium acetate at pH 2 as described in step 1. After the third step, residual washed and air-dried soil was digested with the mixture of (3:1) HCl–HNO3 acids to extract the final residual fraction. After digestion, samples were diluted to make 25 ml volume and analyzed for Cd and Pb using atomic absorption spectrophotometer (AA-240FS Varian, USA).
2.5 Solubility and bioavailability of Cd and Pb
The solubility product of Cd and Pb was estimated from each experimental unit by toxicity characteristic leaching procedure (TCLP) method USEPA 1311 (USEPA 1992). Briefly, 1.0 g of test soil was taken in a 50-ml centrifuge tube and extracted with 20 ml of un-buffered glacial acetic acid solution (pH 2.88) for 18 h.
Similarly, the CaCl2 extractable soil Cd and Pb concentration were determined after the incubation study as described by Houben et al. (2013). Briefly, 2.00-g test soil sample was extracted with 20 ml of 0.01 M CaCl2 in 50-ml centrifuge plastic tubes and then shaken on an orbital shaker for 2 h at 25 °C. Then, Cd and Pb concentration in the extract was determined using AAS.
2.6 Soil microbial biomass C, N, and P
Soil microbial biomass was estimated after fumigation extraction method (Wu et al. 1990). Briefly, 10 g of fumigated and non-fumigated soil was extracted with 50 ml 0.5 M K2SO4 solution and then filtered through 0.45 membrane filter paper. Total organic carbon and nitrogen in the extracts were analyzed using an automatic TOC analyzer (Shimadzu Corp. Japan). Soil microbial biomass was calculated using the following equations. Soil microbial biomass carbon (mg kg−1) = (CF − CNF) × 0.45, microbial biomass N (mg kg−1) = (NF − NNF) × 0.54 (Brookes et al. 1985), and microbial biomass P (mg kg−1) = (PF − PNF) × 0.4 (Brookes et al. 1982). Similarly, soil dissolved organic carbon (DOC) was extracted from 5 g moist soil with an addition of 25 ml distilled water and shaken for 1 h. after shaking samples were centrifuged and filtered through 0.45 membrane filter paper. The water soluble concentration was determined by using a TOC analyzer (Shimadzu Corp. Japan).
2.7 Statistical analysis
The results of the soil heavy metal extraction techniques were expressed as standard deviation (SD) and means. One-way analysis of variance (ANOVA) and Duncan’s multiple range tests (p < 0.05) were used to analyze the data. All statistical analyses were performed using Microsoft Office Excel 2013.
3 Results
3.1 Amendment effect on soil pH
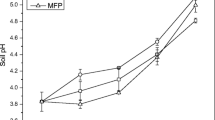
The efficiency of passivators on soil pH after 90-day incubation is presented in Fig. 1. The soil pH increased significantly (p < 0.05) with the addition of amendments. Addition of rock phosphate and biochar increased soil pH and higher values with RP application. Soil pH reached to 6.6 to 6.9 in 1.5 and 3% RP-treated soil, while 6.3 to 6.6 in 1.5 and 3% BC-treated soil, respectively. There was not any significant change in soil pH when soil was treated with zeolite.
3.2 BCR fraction of soil Cd and Pb
To understand the effect of passivators on Cd and Pb, among their geochemical distribution is presented in Figs. 2 and 3. The exchangeable metal concentrations prominently decreased with the addition of biochar, rock phosphate, and zeolite. The extent of reduction in metal solubility was different among three metals and chemical amendments. The acid-soluble contents of metals were significantly decreased with the addition of BC and higher reduction with an increase in application dose from 1.5 to 3% in multi-contaminated soil. Application of rice straw BC dramatically reduced the acid-soluble Cd (27.5–34.8%) and Pb (13.6–21.4%), respectively, when compared to the control soil (Figs. 2 and 3). The maximum portions of these metals transformed into reducible phase because of BC addition. The proportion of Cd and Pb in reducible phase was increased by 6.5 and 11.74%, respectively, in BC at 3% application rate as compared to control. However, the residual portion of all three metals significantly increased with the incorporation of biochar. Particularly, the biochar-amended soil presented prominent increase in the residual portion of Cd and Pb by 52.8–72.92% and 53–65.3% with 1.5 and 3% application rate, respectively, as compared to control.
The addition of zeolite significantly (p < 0.05) decreased exchangeable Cd concentration by 9–19%, but increased from 33.8 to 55.5% in most stable residual form relative to the control soil. The exchangeable Pb also decreased (6.3–9.5%) in zeolite-treated soil, but on the contrary, its residual fraction increased by 20.9–42% as compared to control.
Application of rock phosphate (either 1.5 or 3%) significantly (p < 0.05) decreased exchangeable form of Cd and Pb concentrations from 17.4 to 21.6% and 12.6 to 20%, respectively, when compared to the control. The residual portion of Cd and Pb was increased in soil with the following order of amendments: BC > RP > ZE > control.
3.3 TCLP and CaCl2 extractable Cd and Pb
The contents of TCLP extractable Cd and Pb in treated and untreated soil are shown in Figs. 4 and 5. The concentration of Cd and Pb in TCLP extract was significantly (p < 0.05) increased with the increasing rate of BC, RP, and ZE amendments. Specifically, biochar prominently reduced the leachability of Cd and Pb in the soil about 14.7–16.9% and 24.8–30%, respectively, as compared to the control. In the same way, rock phosphate also reduced the concentration of leachable Cd and Pb from 7.4 to 9.27% and 14.6 to 21%, respectively. However, the application of zeolite was comparatively less effective in reduction of TCLP extractable Cd and Pb as compared to other amendments. Overall, our results suggested that biochar significantly decreased the concentration of Cd and Pb in TCLP extract. In general, the following amendments’ order played a key role in the reduction of leachable Cd and Pb: BC > RP > ZE > control.
The CaCl2 extractable Cd and Pb were also decreased among all the amendments (Figs. 6 and 7). The greater reduction occurred in biochar-treated soil. The application of BC at 1.5 and 3% significantly decreased Cd by 28–32% and Pb by 33.6–40.4%, respectively, when compared to the control. A similar trend was observed with the addition of RP at 1.5 and 3% which reduced Cd by 24–27% and Pb 26.3–28.86%, respectively, over control. However, ZE offered a slight reduction in Cd by 20.5–23% and Pb 17.24–21.58% at 1.5 and 3% application rate, respectively.
3.4 Amendment effect on DOC and nutrients
The significant (p < 0.05) effect of amendments on dissolved organic carbon in incubation study is shown in Fig. 8. Soil DOC was increased among all the amendments and their application rates when compared to control. DOC was increased by 54.6, 26.8, and 28.4% for BC-, ZE-, and RP-amended soil, respectively, at 3% application rate, over control.
The significant (< 0.05) changes in soil available nutrient N, P, and K contents among all the amendments and their application rate in paddy acid soils are shown in Table 1. The greater increase in soil N, P, and K was observed at 3% application rate of BC, ZE, and RP. The obvious increase in available soil N contents by 27, 12.6, and 11.6% for BC-, ZE-, and RP-amended soil as compared to control was observed. The similar tendency was observed for available P by 40, 16.23, and 25% for BC-, ZE-, and RP-amended, respectively, over control soil. Compared to the control, soil available K was increased by 84, 18.4, and 10.13% for BC-, ZE-, and RP-amended soil, respectively.
3.5 Soil microbial biomass
The effect of amendments on soil microbial biomass in incubated soil is shown in Table 2. The results showed that after 90-day incubation period, the soil microbial biomass carbon (Cmic) was significantly (p < 0.05) increased by the increasing rate of BC, RP, and ZE. The highest soil Cmic was observed about 63.25, 23.21, and 21.11% for BC-, RP-, and ZE- amended soil, respectively, at 3% application rate as compared to the control.
Similarly, soil microbial biomass phosphorous (Pmic) was increased by 28.7 and 47.5% for BC- and RP-amended soil at 3% application rate as compared to untreated soil. Conversely, the slight increment was also observed in ZE-treated soil as compared to the control after the incubation period. The similar tendency was observed for soil microbial biomass nitrogen (Nmic) by the application of BC, ZE, and RP. Particularly, soil Nmic was increased by 65, 43, and 49.7% for BC-, RP-, and ZE-amended soil, respectively, at 3% application rate as compared to the control.
4 Discussion
4.1 Effect of amendments on soil pH and metal fractions
The application of alkaline amendments to metal contaminated soils is an emerging solution for in situ heavy metal immobilization in acidic soils (Lu et al. 2014). All the amendments except zeolite showed significant increment in soil pH due to their liming effect and higher alkalinity (Zhu et al. 2004; Lu et al. 2014). Generally, BC contains plenty of Ca+, Mg+, and K+ which replaces H+ in the soil solution and increases soil pH (Houben et al. 2013). During pyrolysis of organic substance at elevated temperature, biochar surface results in generation of hydroxides, carbonates, a wide variety of functional groups, and high mineral ash contents causing the liming effect which might induce the increment in soil pH (Yuan et al. 2011). Similarly, RP has low surface area but has high pH and Ca+ contents due to the presence of the greater amount of calcium carbonates and phosphate ions in RP, which could increase soil pH and promote metal phosphate through precipitation (Zhu et al. 2008). The hydrolysis and dissolution of these alkaline substances could increase soil pH and induce Cd precipitation as CdCO3 and Pb as Pb5 (PO4)3OH (Zhu et al. 2008; Cao et al. 2011; Bashir et al. 2018c). However, zeolite belongs to the alumino-silicate family, it has high CEC and surface area, due to these properties, zeolite might be able to trap metal ions in its structure and enhance isomorphic substitution (Oste et al. 2002; Wen et al. 2016).
Soil pH has a significant effect on immobilization of heavy metals. The concentrations of Cd and Pb, which were obtained by sequential extraction of BCR, were obviously changed by the addition of amendments. The application of biochar significantly enhanced soil pH, which could change the soil Cd and Pb concentration from acid-soluble fraction to reducible, oxidizable, and more stabilized residual form. Soil pH had a prominent effect on heavy metal speciation, due to their dissolution and precipitation and providing modified pH-dependent charges on soil organic matter (Adriano et al. 2004). Compared to the control, acid-soluble Cd concentration prominently reduced up to 34.8% with the increasing rate of BC at 3%, which was consistent with the results of Shaheen and Rinklebe (2015) as they demonstrated that the reduction of Cd (4–60%) and Pb (6–87%) solubility occurred within corporation of biochar in co-contaminated soil. A recent study reported by Naggar et al. (2018) found that the application of rice hull-derived biochar significantly reduced Cd, Zn, Cu, and Ni soluble portion when compared to non-treated biochar soil. It is important to mention in our results the highest concentration of Cd and Pb in reducible and oxidizable portion, which might be pointed out the heavy metal precipitation with Fe and Mn ions or complexed with soil organic matter after biochar addition into contaminated soil (Mohamed et al. 2015; Rizwan et al. 2016). A recent study reported by Cui et al. (2016) found that addition of wheat straw biochar showed remarkable increment in reducible and oxidizable portion of Cd and Pb in paddy soil. The increase in oxidizable and residual fractions of Cd and Pb not only due to surface functional groups (hydroxyl, carboxylic, phenolic) but also might resulted because of precipitation with CO2−3 and PO3−4 on biochar surface (Bashir et al. 2018c). According to our findings, biochar significantly increased residual portion of Cd and Pb as compared to the control soil. These were in consistence with those of Park et al. (2011) as they found that incorporation of green waste-derived biochar prominently increased the sum of organic-bound and residual fractions of Cd about 9.39–25.0%.
Another mechanism of heavy metal reduction might be the adsorption and surface complexation of Cd and Pb with biochar surface functional groups (Jiang et al. 2012; Bashir et al. 2018c). It can be demonstrated that addition of biochar in contaminated soil induces affinity for metal cations adsorption on its surface. Biochar has micro porous structure, high CEC, plenty of surface functional groups, and highest C/O ratio as well as lower zeta potential that plays an important role to increase surface adsorption, which might be the reasons for heavy metal stabilization in highly polluted soils (Jiang et al. 2012).
The acid-soluble Cd and Pb concentration decreased with increasing RP and ZE mineral amendments. In our present study, results were attributed that high concentration of Cd and Pb decreased from their soluble fraction and easily transformed into less available portion. The addition of RP increased soil pH, which might dissociate metal ions and promote the formation of metal phosphate. These results were in accordance with those of Zhu et al. (2004), who concluded that application of RP into soil increased soil pH due to the highest amount of CaCO3, which could induce dissolution of heavy metals and promote metal phosphate complex formation. It can be demonstrated that addition of RP could increase soil pH (Fig. 1) that enables to reduce heavy metal mobility or the exchangeable portion of metals (Figs. 2 and 3), which might enhance the precipitation of Cd and Pb with oxides, carbonates, and phosphates (Bolan et al. 2014).
The addition of natural zeolite to the soil also changed the metal fractionation. The acid-soluble fraction of Cd and Pb were decreased significantly (p < 0.05) by increasing ZE application rate, which resulted in the reduction of metal toxicity. Similarly, results were suggested by Wen et al. (2016) as they indicated that ZE was the best stabilization option for Cd removal about 26.7% in acid soluble as well as showed positive trend to enhance reducible and residual fraction of Cu, Zn, and Pb by decreasing their soluble fraction. Zhang et al. (2016) also confirmed that ZE addition caused an increase in soil porosity as well as the surface area that were the main reasons for ZE ability to increase heavy metal adsorption.
4.2 Effect of amendments on Cd and Pb solubility
The addition of BC, RP, and ZE greatly declined TCLP extractable Cd and Pb either with 1.5 or 3% application rate. The greater decline in TCLP extractable Cd and Pb in the studied soil might result in the considerable raise in soil pH (Fig. 1). Earlier research has also demonstrated that TCLP extractable Cd, Pb, Cu, and Zn were markedly lowered with biochar application to contaminated soil and higher reduction was observed with increasing biochar application rate (Lu et al. 2014).
The decrease in concentration of TCLP extractable Pb was relatively higher as compared to Cd in biochar-amended soil, which might be resulted from the precipitation of Pb into Pb phosphate mineral. Our results were in line with the findings of Xu et al. (2013) as they examined that the immobilization of Pb was more as compared to Cd due to the precipitation with PO4−3 or CO3−2 ions on biochar surface instead of complexation with surface functional groups or delocalized by π-electron. Liang et al. (2014) also reported that biochar application prominently reduces the leaching of Cd, Pb, and Zn about 98.1 and 62.7%, in TCLP and CaCl2 extraction. They stated that the increase in soil pH following biochar incorporation could be one of the main reasons for the reduction of heavy metal mobility.
The TCLP extract of Cd and Pb were slightly decreased with increasing amount of RP. This might be due to the high surface area or adsorption sites on rock phosphate for Cd and Pb. A similar finding was observed in the previous study reported by Liang et al. (2011) indicating that the application of phosphate fertilizers with sepiolite decreased Cd about 51.9% and Pb 55.3% in the contaminated soil. Similar results were described by Cao and Harris (2010), who also confirmed the reduction in TCLP leachate of Pb by the application of rock phosphate; moreover, this reduction was below the limit about 5 mg L−1 set by USEPA. Application of natural ZE to highly metal contaminated soil resulted the reduction in metal solubility and leaching concentration with increasing application dose. There are two major factors, pH and CEC, that affect ZE ability to stabilize heavy metals (Oste et al. 2002). In addition to the increase in soil pH, efficient binding sites on the ZE surface also contribute to immobilizing heavy metals. The results are in line with Oste et al. (2002) as they indicated that ZE application effectively provided binding sites for heavy metal stabilization because of low proton competition in soil, and provides better response towards Cd and Pb stabilization.
4.3 Amendment effect on soil biochemical properties
Application of alkaline amendments such as biochar, zeolite, and rock phosphate has the significant effect on soil organic matter that may play vital role to increase soil nutrient status. In the present study, we assumed that addition of biochar into acidic soil could ameliorate the acidity of soil and increase carbon sequestration in soil that may have the ability to reduce carbon dioxide release and increase organic matter contents in soils (Lehmann et al. 2011; Lu et al. 2014). In the present study, we could suggest that the significant increment in soil nutrient might be possible due to the dissolution of biochar organic carbon and mineral elements. When biochar was incorporated into the moist soil, the desorption of several cations, anions, and organic compounds could take place (Lehmann et al. 2011), which may have the ability to improve nutrient status, soil microbial dynamic, and increased organic matter decomposition in soil.
Chemical amendments including zeolite and rock phosphate also have influence on soil nutrient status as compared to control. The increment in soil pH might reduce the presence of Fe and Al ions in soil solution and improved soil P, N, and K availability. Particularly, a zeolite has an anhydrous tectosilicate structure, due to this, it can easily alter nutrient status and water retention due to isomorphic substitution and CEC property. Several previous studies have confirmed that zeolite addition could improve soil nutrient status as well as soil quality and plant growth by improving pH of acidic soils (Oste et al. 2002; Querol et al. 2006). It could demonstrate that application of RP with other chemical fertilizers exerted significant influence of soil organic carbon and available P and also induce significant changes in biochemical activities (Meena and Biswas 2015). Similar results were observed by Zin et al. (2005) indicating that RP has the ability to induce significant changes in soil pH, which cause increment in soil nutrient status and CEC.
Soil microorganisms play a vital role in terrestrial ecosystems because they facilitate nutrient cycling, organic matter disintegration, soil fertility, and productivity (Murrieta et al. 2006). In the present study, the differences in soil microbial biomass carbon, phosphorus, and nitrogen were observed among all amendments in Cd and Pb contaminated soil. According to our results, soil microbial biomass decreased significantly in metal contaminated soil, which might be due to an unfavorable environmental condition for microbes.
Application of biochar significantly increased soil microbial biomass carbon (MBC). The increase in soil MBC was possibly accomplished due to the liable fraction of organic carbon added through biochar, which might promote soil microbial activity. It has been reported that addition of biochar enhances carbon contents and nutrient release in soil, which provides energy source for soil microorganisms. The higher MBC in biochar-amended soil is supported by Lehmann et al. (2011) as they reported that biochar addition to soil increased microbial biomass C. This was attributed to macro and micro porous structure, excessive nutrient, and carbon contents of biochar, which provide a suitable microorganism’s habitat. According to our previous study (Bashir et al. 2018a), it was described that addition of biochar as a carbon source could increase C and N mineralization in soil, which might provide substrate for microorganisms in polluted soil. Stewart et al. (2013) observed similar results as they reported that addition of biochar in acidic soils increased soil pH and enhanced soil carbon decomposition rate as well as soil aeration because of sufficient oxygen supply. Similar results were observed by Zhou et al. (2017) concluding that biochar was a very stable source of carbon that has high efficiency for C sequestration in soil. Moreover, they explained that biochar additions with crop residues also influence on nitrogen mineralization and reduce nitrogen loss by increasing 25% net nitrogen and carbon in soil.
The application of minerals in the heavy metal polluted soils has the strongest influence on soil microbial processes, such as substrate generation, nutrient acquirement, and biofilm formation as well as cell adhesion (Brown et al. 2008). The release of essential nutrients, i.e., C, N, P, and Fe from mineral surface, could promote the nutrient status and microbial activity by enhancing substrate and mineralization as well as reduce the toxicity of heavy metals and organic compounds (Vaughan et al. 2002). According to our obtained findings, the application of clay mineral especially ZE addition might promote microbial activity as well as enhance the soil organic matter mineralization process, because clay mineral reduces heavy metal mobility towards microbes (Usman et al. 2004). Our results suggested that addition of ZE significantly reduced heavy metal mobility in soil which might be the effective reason to promote organic matter degradation and mineralization as well as enhance litter decomposition.
Application of RP to metal contaminated acidic soil showed considerable increase in microbial activity and promising increase in carbon as well as nutrient cycle. In our results, we found a positive effect of RP application in metal contaminated soil by increasing microbial biomass phosphorus. According to obtained findings, application of RP into acidic soil might increase soil pH and liable pool of P, which indicated that its application might increase phosphorus use efficiency in soil and enhance microbial P due to mineralization of native carbon and P pool in soil. These results are aligned with those of Huang et al. (2016) as they indicated that addition of high dose of phosphorus could increase dissolved organic carbon and P availability in soil, which promotes microbial biomass due to significant increase in soil pH. Similar results were observed with those of Meena and Biswas (2015), who examined that application of rock phosphate enriched with compost at 5 t ha−1 specifically increase microbial biomass P (MBP) about 5.62 and 4.28 mg kg−1 soil.
5 Conclusions
The effects of three passivators (biochar, zeolite, and rock phosphate) on Cd and Pb immobilization in polluted soil were observed using BCR, TCLP, and CaCl2 extraction techniques. Among all the amendments, RP was the most efficient at increasing the soil pH. Increasing the BC application rates was considered to be a more efficient passivator to stabilize Cd and Pb in co-contaminated soil because BC resulted in a large reduction in the acid-soluble portion of Cd and Pb and induce significant increase in residual portion. Overall, the order followed by the impact of amendments on the immobilization of Cd and Pb in contaminated soil was therefore BC > RP > ZE. Biochar also has the potential to decrease the leachability and availability of Cd and Pb in soil. Moreover, the maximum soil microbial biomass was also observed with BC amendment as compared to RP and ZE passivators.
Based on our results, the addition of biochar to Cd and Pb polluted soil could be more effective in enhancing the percentage of Cd and Pb removal from polluted soil under acidic conditions. Furthermore, the immobilization of Cd and Pb by these applied amendments in heavy metal polluted soils need to be evaluated under field conditions.
References
Adriano DC, Wenzel WW, Vangronsveld J, Bolan NS (2004) Role of assisted natural remediation in environmental cleanup. Geoderma 122:121–142
Ahmad M, Lee SS, Lee SE, Al-Wabel MI, Tsang DCW, Ok YS (2017) Biochar-induced changes in soil properties affected immobilization/mobilization of metals/metalloids in contaminated soils. J Soils Sediments 7:717–730
Bashir S, Hussain Q, Akmal M, Riaz M, Hu HQ, Ijaz SS, Iqbal M, Abro S, Mehmood S, Ahmad M (2018a) Sugarcane bagasse-derived biochar reduces the cadmium and chromium bioavailability to mash bean and enhances the microbial activity in contaminated soil. J Soils Sediments 18:874–886
Bashir S, Zhu J, Fu Q, Hu HQ (2018b) Cadmium mobility, uptake and anti-oxidative response of water spinach (Ipomoea aquatic) under rice straw biochar, zeolite and rock phosphate as amendments. Chemosphere 194:579–578
Bashir S, Zhu J, Fu Q, Hu HQ (2018c) Comparing the adsorption mechanism of Cd by rice straw pristine and KOH modified biochar. Environ Sci Pollut Res. https://doi.org/10.1007/S11356-018-1292-Z
Bogusz A, Oleszczuk P, Dobrowolski R (2015) Application of laboratory prepared and commercially available biochars to adsorption of cadmium, copper and zinc ions from water. Bioresour Technol 196:540–549
Bolan N, Kunhikrishnan A, Thangarajan R, Kumpiene J, Park J, Makino T, Kirkham MB, Scheckel K (2014) Remediation of heavy metalloids contaminated soils—to mobilize or to immobilize. J Hazard Mater 266:141–166
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Brookes PC, Powlson DS, Jenkinson DS (1982) Measurement of microbial biomass phosphorus in soil. Soil Biol Biochem 14:319–329
Brown GE, Trainor TP, Chaka AM (2008) Geochemistry of mineral surfaces and factors affecting their chemical reactivity. In: Nilsson A, Pettersson LGM, Norskov JK (eds) Chemical bonding at surfaces and interfaces. Elsevier, Amsterdam, pp 457–509
Cao X, Ma L, Liang Y, Gao B, Harris W (2011) Simultaneous immobilization of lead and atrazine in contaminated soils using dairy-manure biochar. Environ Sci Technol 45(11):4884–4889
Cao XD, Harris W (2010) Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour Technol 101:5222–5228
Cui L, Pan G, Li L, Bian R, Liu X, Yan JL, Quan G, Ding C, Chen T, Liu Y, Liu Y, Yin C, Wei C, Yang Y, Hussain Q (2016) Continuous immobilization of cadmium and lead in biochar amended contaminated paddy soil: a five-year field experiment. Ecol Eng 93:1–8
Frišták V, Pipíška M, Lesny J, Soja G, Friesl-Hanl W, Packová A (2015) Utilization of biochar sorbents for Cd2+, Zn2+, and Cu2+ ions separation from aqueous solutions: comparative study. Environ Monit 9:14–40
Gee GW, Bauder JW, Klute A (1986) USA. Particle-size analysis. Methods of soil analysis. Part 1. Phys Mineral Method 9:383–411
Herath I, Iqbal MCM, Al-Wabel MI, Abdul JA, Ahmad M, Usman ARA, Ok YS, Vithanage M (2017) Bioenergy-derived waste biochar for reducing mobility, bioavailability, and phytotoxicity of chromium in anthropized tannery soil. J Soils Sediments 7:731–740
Houben D, Evrard L, Sonnet P (2013) Mobility, bioavailability and pH dependent leaching of cadmium, zinc and lead in a contaminated soil amended with biochar. Chemosphere 92:1450–1457
Huang J, Hu B, Qi K, Chen W, Pang X, Bao W, Tian G (2016) Effects of phosphorus addition on soil microbial biomass and community composition in a subalpine spruce plantation. Eur J Soil Biol 72:35–41
Hussain M, Farooq M, Nawaz A, Al-Sadi AM, Solaiman ZM, Alghamdi SS, Ammara U, Ok YS, Siddique KHM (2017) Biochar for crop production: potential benefits and risks. J Soils Sediments 17:685–716
Inglezakis VJ, Loizidou MD, Grigoropoulou HP (2002) Equilibrium and kinetic ion exchange studies of Pb+2, Cr+3, Fe+3 and Cu+2 on natural clinoptilolite. Water Res 36:2784–2792
Islam M, Chandra MP, Patel R (2010) Physico-chemical characterization of hydroxyapatite and its application towards removal of nitrate from water. J Environ Manag 91:1883–1891
Jiang TY, Jiang J, Xu RK, Li Z (2012) Adsorption of Pb(II) on variable charge soils amended with rice-straw derived biochar. Chemosphere 89:249–256
Kiikilä O, Perkiömäki J, Barnette M, Derome J, Pennanen T, Tulisalo E, Fritze H (2001) In situ bioremediation through mulching of soil polluted by a copper–nickel smelter. J Environ Qual 30:1134–1143
Kolb SE, Fermanich KJ, Dornbush ME (2009) Effect of charcoal quantity on microbial biomass and activity in temperate soils. Soil Sci Soc Am J 73:1173–1181
Kumari KG, Moldrup ID, Paradelo PM, De Jonge LW (2014) Phenanthrene sorption on biochar-amended soils: application rate, aging and physicochemical properties of soil. Water Air Soil Pollut 225:2105
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments: a review. Waste Manag 28:215–225
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC (2011) Biochar effects on soil biota—a review. Soil Biol Biochem 43:1812–1836
Li J, Li Z, Wang FM, Zou B, Chen Y, Zhao J, Mo QF, Li Y, Li X, Xia H (2015) Effects of nitrogen and phosphorus addition on soil microbial community in a secondary tropical forest of China. Biol Fertil Soils 51:207–215
Liang B, Yang X, He X (2011) Effects of 17-year fertilization on soil microbial biomass C and N and soluble organic C and N in loessial soil during maize growth. Biol Fertil Soils 47:121–128
Liang XF, Han J, Xu YM, Sun YB, Wang L, Tan X (2014) In situ field-scale remediation of Cd polluted paddy soil using sepiolite and palygorskite. Geoderma 9–18:235–236
Lu RK (2000) Methods of inorganic pollutants analysis. In: Soil and agro-chemical analysis methods. Agricultural Science and Technology Press, Beijing, pp 205–266
Lu W, Ding W, Zhang J (2014) Biochar suppressed the decomposition of organic carbon in a cultivated sandy loam soil: a negative priming effect. Soil Biol Biochem 76:12–21
Meena MD, Biswas DR (2015) Effect of rock phosphate enriched compost and chemical fertilizers on microbial biomass phosphorus and phosphorus fractions. African J Microbiol Res 9:1519–1526
Mohamed I, Zhang G, Li Z, Liu Y, Chen F, Dai K (2015) Ecological restoration of an acidic Cd contaminated soil using bamboo biochar application. Ecol Eng 84:67–76
Murrieta MSV, Garduño IM, Hernández OF, Govaerts B, Dendooven L (2006) C and N mineralization and microbial biomass in heavy-metal contaminated soil. Eur J Soil Biol 42:89–98
Naggar AE, Shaheen SM, Ok YS, Rinklebe J (2018) Biochar affects the dissolved and colloidal concentrations of Cd, Cu, Ni, and Zn and their phytoavailability and potential mobility in a mining soil under dynamic redox-conditions. Sci Total Environ 624:1059–1071
Ok YS, Lim JE, Moon DH (2011) Stabilization of Pb and Cd contaminated soils and soil quality improvements using waste oyster shells. Environ Geochem Health 33:83–91
Oste LA, Lexmond TM, Van Riemsdijk WH (2002) Metal immobilization in soils using synthetic zeolites. J Environ Qual 31:813–821
Park JH, Choppala GK, Bolan NS, Chung JW, Chuasavathi T (2011) Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 348:439–451
Querol X, Alastuey A, Moreno N, Alvarez-Ayuso E, Garcia-Sanchez A, Cama J, Ayora C, Simon M (2006) Immobilization of heavy metals in polluted soils by the addition of zeolitic material synthesized from coal fly ash. Chemosphere, 62:171–180
Rauret G, Lopez-Sanchez JF, Sahuquillo A, Rubio R, Davidson C, Ure A, Quevauviller P (1999) Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J Environ Monit 1:57–61
Rizwan MS, Imtiaz M, Chhajro MA, Huang G, Fu Q, Zhu J, Aziz O, Hu H (2016) Influence of pyrolytic and non-pyrolytic rice and castor straws on the immobilization of Pb and Cu in contaminated soil. Environ Technol 37:2679–2686
Shaaban M, Abid M, Qi-An P (2013) Short term influence of gypsum, farm manure and commercial humic acid on physical properties of salt affected soil in rice paddy system. J Chem Soc Pak 35:1034–1040
Shaheen SM, Rinklebe J (2015) Impact of emerging and low cost alternative amendments on the (im)mobilization and phytoavailability of Cd and Pb in a contaminated floodplain soil. Ecol Eng 74:319–326
Shaheen SM, Rinklebe J, Selim HM (2015) Impact of various amendments on the bioavailability and immobilization of Ni and Zn in a contaminated floodplain soil. Inter J Environ Sci Techn, 12:2765–2776
Stewart CE, Zheng JY, Botte J (2013) Co-generated fast pyrolysis biochar mitigates greenhouse gas emissions and increases carbon sequestration in temperate soils. GCB Bioenergy 5:153–164
USEPA (1992) Test methods for evaluating solid waste, physical/ chemical methods. US Environmental Pollution Agency USA, Washington, DC
Usman RA, Kuzyakov Y, Stahr K (2004) Effect of clay minerals on extractability of heavy metals and sewage sludge mineralization in soil. Chem Ecol 20:123–135
Vaughan DJ, Pattrick RAD, Wogelius RA (2002) Minerals, metals and molecules: ore and environmental mineralogy in the new millenium. Mineral Mag 66:653–676
Wen J, Yi Y, Zeng G (2016) Effects of modified zeolite on the removal and stabilization of heavy metals in contaminated lake sediment using BCR sequential extraction. J Environ Manag 178:63–69
Wu J, Joergensen RG, Pommerening B, Haussod R, Brookes PC (1990) Measurement of soil microbial biomass C by fumigation-extraction-an automated procedure. Soil Biol Biochem 22:1167–l169
Xu X, Cao X, Zhao L, Wang H, Yu H, Gao B (2013) Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ Sci Pollut Res 20:358–368
Yuan JH, Xu RK, Zhang H (2011) The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour Technol 102:3488–3497
Zhang HY, Li AM, Zhang W, Shuang CD (2016) Combination of Na-modified zeolite and anion exchange resin for advanced treatment of a high ammonia nitrogen content municipal effluent. J Colloid Interf Sci 468:128–135
Zhou H, Zhang D, Wang P, Liu X, Cheng K, Li L, Zheng J, Zhang X, Zheng J, Crowley D, Van Zwieten L, Pan G (2017) Changes in microbial biomass and the metabolic quotient with biochar addition to agricultural soils: a meta-analysis. Agric Ecosyst Environ 239:80–89
Zhu R, Yu R, Yao J, Mao D, Xing C, Wang D (2008) Removal of Cd2+ from aqueous solutions by hydroxyapatite. Catal Today 139:94–99
Zhu W, Chen S, Yang J (2004) Effects of soil amendments on lead uptake by two vegetable crops from a lead-contaminated soil from Anhui, China. Environ Int 30:351–356
Zin ZZ, Zulkifli H, Tarmizi AM, Hamadan AB, Khalid Raja ZRO (2005) Rock phosphate fertilizers recommended for young oil palm planted on inland soils. MPOB Information Series, ISSN 1511-7871. Malaysian Palm Oil Board, Ministry of Plantation Industries and Commodities, Malaysia
Funding
The study was financially supported by National Science and Technology Support Plan of China (2015BAD05B02).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Yong Sik Ok
Rights and permissions
About this article
Cite this article
Bashir, S., Shaaban, M., Hussain, Q. et al. Influence of organic and inorganic passivators on Cd and Pb stabilization and microbial biomass in a contaminated paddy soil. J Soils Sediments 18, 2948–2959 (2018). https://doi.org/10.1007/s11368-018-1981-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-018-1981-8