Abstract
Purpose
The objective of this study was to investigate whether spent mushroom substrate (SMS) amendment was an appropriate way to reduce di(2-ehylhexyl) phthalate (DEHP) and di-n-butyl phthalate (DnBP) contents in soil and whether SMS could reduce DnBP accumulation in bok choy (Brassica rapa subsp. chinensis).
Materials and methods
Microcosm and pot experiments were carried out to study the influence of spent Agaricus bisporus substrate application on DnBP and DEHP dissipation in soils and plant uptake of DnBP. Variations in soil pH and enzyme activities were determined. The concentrations of phthalate esters (PAEs) in soils, bok choy, and atmosphere were examined with gas chromatography or gas chromatography–mass spectrometry.
Results and discussion
Adding sterilized or non-sterilized SMS can increase soil pH and urease activity, and non-sterilized SMS can promote soil laccase activity. The results show that the dissipation of DEHP is accelerated after incubation with SMS for 25 days; however, little effect can be found with continuing incubation due to low DEHP bioavailability. In this research, SMS amendment exhibits no effect on DnBP dissipation in soils and DnBP accumulation in bok choy. It was proposed that atmospheric deposition of DnBP might be the main source of DnBP in bok choy in the study, since equivalent amounts of DnBP were detected in the vegetables grown in soils with or without DnBP spiking.
Conclusions
This study indicates that the application of SMS as an organic fertilizer is less likely to affect the fate of PAEs in soils, and proper strategies should be conducted to reduce PAE levels in atmosphere to control PAE contamination in vegetables.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Phthalate esters (PAEs) are now ubiquitously detected in environments and foods, such as air, water, soils, sediments, sewage sludge, fertilizers, and vegetables. They are mainly used to increase the flexibility and durability of plastic products, and the content of PAEs are in the range of 10–60% in finished plastics (Gómez-Hens and Aguilar-Caballos 2003). Since PAEs are only physically bound to plastic polymers, these compounds can be easily released into the environment. Long-term exposure of PAEs can affect the human developmental and reproductive systems (Swan 2008; Wittassek et al. 2011). Some PAEs have been listed as priority pollutants by the US Environmental Protection Agency (Richards and Shieh 1986), including dimethyl phthalate (DMP), diethyl phthalate (DEP), di-n-butyl phthalate (DnBP), butylbenzyl phthalate (BBP), di(2-ehylhexyl) phthalate (DEHP), and di-n-octyl phthalate (DnOP).
Over the last three decades, there have been increasing concerns on PAE contamination in agricultural soils. The usage of agricultural plastic films, pesticides, fertilizers, biosolids, and wastewater irrigation is considered its main source, together with atmosphere deposition (He et al. 2015; Chen et al. 2017). The PAE contamination in soils can lead to reduced soil enzyme activities (Chen et al. 2013) and microbial diversity (Wang et al. 2016), as well as PAE accumulation in vegetables, which poses great threat to human health (Chen et al. 2017). The study of Wang et al. (2015) showed that total concentration of the six priority PAEs ranged from 0.40 to 6.20 mg kg−1 in soils and 0.51 to 7.16 mg kg−1 in vegetables, respectively, from different plastic film greenhouses in Nanjing, China. The concentration of DEHP in cotton fields was reported as high as 128.7 mg kg−1 in Xinjiang, China (Guo and Wu 2011). The level of DBP was up to 29.4 mg kg−1 in greenhouse soils in Handan, China (Xu et al. 2008). Thus, for the sake of human health and the ecosystem quality, more efforts are needed to develop agricultural practices, which can accelerate the dissipation of PAEs in soils and control PAE uptaking by vegetables.
It has been reported that addition of natural organic material (NOM) or biochar originated from agriculture residues would affect the fate of organic pollutants in soils or soil-plant systems (Zhang et al. 2013; Kästner and Miltner 2016). Little work has been carried out on the migration and transformation of PAEs after organic amendment. Zhao et al. (2016) reported that the application of 6.25% manure compost improved the degradation of DBP in soil. He et al. (2016) reported that biochar application at the dosage of 2% caused a decrease of DEHP accumulation in bok choy grown in the soil with a low organic carbon content (0.35%), but conflicted results were obtained in another soil with a higher organic carbon content(2.2%). The study also showed that the addition of biochar could greatly slow down DEHP degradation in these two soils. There are different NOM amendments, which can be applied to soils to improve soil properties, such as green waste compost, vermicompost, animal manure, crop residues, sawdust, and spent mushroom substrate (SMS) (Scotti et al. 2015). It is necessary to get more information regarding organic amendment-associated agricultural practices that would affect the dissipation of PAEs and plant accumulation. The work would be helpful in improving soil sustainability and reducing the risks of contaminated vegetables.
The common organic amendment SMS is the organic residue after mushroom harvest, which usually contains decomposed straw, manure, bean dregs, and gypsum. Due to the rapid development of the mushroom production industry in the world, great amount of SMS is generated annually. The annual production of SMS was > 13.2 million tons in 2010 in China (Gao et al. 2015), making the disposal of SMS an environmental issue. Improper storage/disposal of SMS can cause severe environmental pollution (Guo and Chorover 2006). On the other side, SMS is a valuable source, which can supply mineral nutrients, providing organic matter, microorganisms, and extracellular enzymes as soil amendment (Walsh et al. 2013). SMS application in horticulture and agriculture has been a common practice in many countries, and its utilization in contaminated soils has been investigated (Jordan et al. 2012). Previous studies have shown that SMS can facilitate soil microbial activities, plant growth, heavy metal immobilization, and the removal of organic pollutants, including PAHs, petroleum hydrocarbons, and pesticides (Chiu et al. 2009; Li et al. 2012; Jia et al. 2017). A recent study shows that spent mushroom compost (at an application rate of 40 or 80 ton ha−1) can decrease the translocation of heavy metals from plant roots to the shoots (Frutos et al. 2017). However, little information is available concerning the effects of SMS application on the migration and transformation of PAEs.
The overall objective of this study was to investigate PAE dissipation behaviors in different agricultural soils and the impacts of SMS as a soil conditioner on PAE dissipation and plant accumulation. In this study, a popular leafy vegetable, bok choy (Brassica rapa subsp. chinensis) was used as a target plant, which can be cultivated all over the year and can be consumed at different stages of maturity. The contribution of atmospheric PAE uptake in bok choy was also examined.
2 Materials and methods
2.1 Materials
Two loamy clay soils were collected from agricultural fields of Nanjing and Changshu, Jiangsu Province, China, respectively. After removing stones and plant debris, soils were air-dried, ground, and sieved (< 2 mm). Fresh SMS from Agaricus bisporus cultivation was obtained from a local mushroom production industry, which mainly contained decomposed wheat straw, chicken manure, and soybean dregs. The fresh SMS was air-dried, ground, and sieved (< 2 mm). Basic properties of the two soils and SMS are shown in Table 1. Seeds of bok choy were purchased from Mingda Seed Sales Shop, Nanjing, China.
PAEs and the internal standard namely benzyl benzoate (purity > 99%) were purchased from Sigma-Aldrich Co., USA, and they were all at the analytical grade. Acetone, n-hexane, and methanol were obtained from Tedia Company Inc., USA, and they were at the HPLC grade.
2.2 Experimental design
To examine the dissipation of four mostly occurred PAEs (DEP, di-iso-butyl phthalate (DiBP), DnBP, and DEHP, as shown in Table 2) in soils, each compound was spiked into two agricultural soils at 100 mg kg−1 and mixed well. To prepare the contaminated soils, 1 mL of individual PAE (1 g L−1 dissolved in acetone) was added to 2 g soil (dry weight (dw)). The mixture was stirred with a stainless spoon and then placed in a fume hood for 1 h to allow solvent to evaporate. The mixture was diluted with 8 g of original soil to obtain a final PAE concentration of 100 mg kg−1 soil. Soil microcosms were prepared in 40-mL brown glass bottles containing 10 g dw contaminated soil. Each treatment had three replicates. To conduct abiotic control experiments, soils were sterilized at 121 °C for 30 min three times. All treatments were incubated at 25 °C in the dark, and soil moisture was kept at about 70% of water holding capacity by adding sterilized deionized water every 3 days. After 42 days, soil samples were taken, freeze-dried, and stored in paper bags at − 20 °C until analysis.
To examine the impact of SMS on DEHP dissipation in soils, microcosms were prepared by adding 60 g prepared soils into 100-mL ceramic pots at an initial DEHP concentration of 200 mg kg−1. The original soil was from Changshu, Jiangsu Province, China. Air-dried SMS (sterilized or non-sterilized) was added at two levels, 2 and 4% (equal to 45 and 90 ton ha−1, respectively, dw). The soil without amendment was included as a control. The final concentrations of NH4 +-N (35.9 mg kg−1), NO3 −-N (12.2 mg kg−1), available P (53.9 mg kg−1), and available K (153.1 mg kg−1) were the same in all microcosms by supplementation with mineral salt solutions. Thus, the nutritional differences among the treatments were mainly from organic part. After incubation for 25 and 50 days at 25 °C in the dark, soil samples from microcosms were taken; some of which were immediately analyzed for enzyme activities and soil pH, while the others were freeze-dried, ground, and extracted for DEHP analysis.
To examine the influence of SMS on the bioaccumulation of DnBP in bok choy, 820 g (dw) DnBP-premixed soil (from Changshu, Jiangsu Province, China) was mixed thoroughly with 2% of non-sterilized SMS in 1.5-L ceramic pots, and the initial concentration of DnBP was 50 mg kg−1. The pots without SMS amendment were conducted as control experiments. Before cultivation, all pots were fertilized with urea, Ca(H2PO4)2·H2O and KCl at a dose of N 0.4 g kg−1, P2O5 0.16 g kg−1, and K2O 0.24 g kg−1.After germination for 7 days, six seedlings of bok choy were planted at equal spacing in each pot. The pots were randomly placed on the bench in a greenhouse, which were watered once every day. After 15 and 30 days, soils and plants were taken for analysis. Soil samples were divided into top soil (top 1 cm), bulk soil, and rhizosphere soil (the soil attached to plant roots). Plant shoots and roots were separated, rinsed with deionized water carefully, and dried with tissue paper before further preparation. Freeze-dried soil and plant samples were used for subsequent analysis.
2.3 Extraction of soil and vegetable samples
Freeze-dried soil samples (2 g) or plant samples (0.25 g) were weighed into 20-mL glass centrifuge tubes, in which 100 μL internal standard benzyl benzoate (2 g L−1 in hexane) (Liu et al. 2010) was added. Then, samples were extracted for 1 h with 10 mL mixture of acetone:hexane (1:1, v/v) at 30 °C in an ultrasonic water bath. After centrifugation at 3000g for 5 min, the organic supernatant was collected, and 0.2 g of anhydrous sodium sulfate was added to remove any moisture. The extracts from soil samples and plant samples were concentrated till 2.0 mL with a rotary evaporator at 40 °C. To reduce the interference of pigments, a cleanup procedure was conducted for plant extracts: 50 mg graphitized carbon black with 6.25 mg anhydrous magnesium sulfate was added to the concentrated plant extract; after vortexing for 2 min, the extract was allowed to settle for 30 min at ambient temperature. The extracts from plant or soil samples were filtered through 0.22-μm syringe filters into 2-mL sample vials.
For analyses of PAE profiles in greenhouse atmosphere, air samples were collected with a medium-volume air sampler (TH-150F, Tianhong, China) at 100 L min−1for 48 h for particles and at 1 L min−1 for 3 h for total air, respectively. The particles on the glass fiber paper (absorbent for particulate PAEs) were extracted with acetone:hexane (1:1, v/v). Glass fiber filters are usually used for collection of airborne particulates (Sharma and Maloo 2005) and methanol has been used as adsorbent for PAEs in air (Wang et al. 2010). The extracts and methanol were concentrated to 2 mL before GC-MS analysis. Clean glass fiber filter paper and methanol were also analyzed as blanks.
The concentrations of PAEs were determined with an Agilent 7890A gas chromatography (Agilent, USA) and a Shimadzu GC-MS QP2010 Ultra system (Shimadzu, Japan) as described by Chen et al. (2017). In brief, helium was used as the carrier gas at a flow rate of 1.2 mL min−1. The initial column temperature was 70 °C, which was held for 1 min. The temperature increased to 140 °C at a rate of 20 °C min−1, held for 2 min, and then increased to 280 °C at a rate of 10 °C min−1, held for 5 min.
2.4 Evaluation of DEHP bioavailability in soil
The bioavailability of DEHP was evaluated by measuring the rapidly desorbing fraction of DEHP from soil using a modified method of Ling et al. (2009). Briefly, 1 g of contaminated soil was mixed with 11 mL of 0.01 M CaCl2 solution in 20-mL glass centrifuge tubes. NaN3 (0.1%, w/v) was used to inhibit microbial growth. After shaking the tubes at 150 rpm for 36 h on a reciprocal shaker, the mixture was centrifuged at 4000g for 15 min and 10 mL of the supernatant was extracted with 10 mL hexane at 200 rpm for 1 h. Then, the hexane phase was concentrated to 1 mL and analyzed using GC-MS as described above. Tubes without soil under the same extraction procedure were included as blank controls.
2.5 Soil characterization
To determine soil pH, 5 mL deionized water was added to 2 g fresh soil sample, which was kept by continuous shaking for 15 min and left to settle for 30 min (Rousk et al. 2009). The pH value of the supernatant was measured using a pH meter (Orion 5-star, Thermo Scientific, USA). Soil urease activity was measured following a modified method of Kandeler and Gerber (1988). In brief, 1 g fresh soil was incubated with 0.5 mL of urea (80 nM) for 2 h at 37 °C in the dark. The production of ammonium was determined on a Shimadzu UV-Vis 2700 spectrophotometer (Shimadzu, Japan) at 690 nm, and the activity was equivalent to NH4 +-N (μg g−1 h−1). Soil laccase activity was determined using a modified method of Bourbonnais and Paice (1990). In brief, 3 g fresh soil was extracted at pH 7.0, 25 °C in 6 mL 0.05 M phosphate buffer for 20 min. After centrifugation at 4500g for 10 min, 0.15 mL supernatant (enzyme extract) was taken and added into a tube which contained 2.55 mL citrate-phosphate buffer at pH 5.0 and 30 mM ABTS. Laccase activity was determined with a Shimadzu UV-Vis 2700 spectrophotometer (Shimadzu, Japan) at 420 nm.
2.6 Statistical analysis
The results were expressed as means ± one standard deviations of three replicates on a dry weight basis, except for the data related to the roots on day 15 (roots were pooled for analysis). Statistical analyses including the analysis of variance (ANOVA) with a multiple comparison test (Tukey’s honest significant difference test at p < 0.05) were performed using SPSS Statistics 16.0 (IBM, USA).
3 Results and discussion
3.1 Dissipation behaviors of PAEs in two agricultural soils
The dissipation of four commonly detected PAEs in Nanjing yellow brown soil and Changshu gray fluvo-aquic soil displayed the same pattern, following the order of DEP > DiBP > DnBP > DEHP (Table 3). More than 93% of DEP and DiBP were rapidly degraded in both soils within 42 days. The degradation of DnBP was slightly slower, being 90.4% in the yellow brown soil and 81.8% in the gray fluvo-aquic soil. The degradation of DEHP was much slower than the other three chemicals, 51.8% in the yellow brown soil and 29.7% in the gray fluvo-aquic soil. The results are in accordance with previous studies that long-chain PAEs are more difficult to degrade than short-chain PAEs (Wang et al. 2000; Chang et al. 2004) and the long-chain PAE DEHP can be persistent in soil systems (Cartwright et al. 2000). This is probably because long-chain PAEs have lower water solubility or higher hydrophobicity (thus a lower bioavailability) than short-chain ones. The detection of short-chain PAEs such as DEP in soils in many surveys (Chen et al. 2017) might be a result of continuous inputting and/or adverse biodegradation conditions due to drought or low-temperature stress.
Biodegradation was the major route for PAE dissipation in this study. Non-biological removal of PAEs (1.6–3.9%) was shown to be negligible in both soils except DEP. Similar results are found in the study by Xu et al. (2008) that DnBP and DEHP removal in sterile soils was below 3% after 30 days of incubation at 20–30 °C. In the present study, high DEP removal (12.3–13.3%) was observed in sterilized soil, which might be due to higher vapor pressure of DEP (Table 2).
Although partial degradation of PAE has been reported for pure cultures (Engelhardt and Wallnofer 1978; Chatterjee and Dutta 2008), no accumulation of reported metabolites of PAEs (such as monoester phthalate, phthalic acid, protocatechuate, and benzoic acid) was observed in biotic experiments (data not shown), suggesting a complete degradation of PAEs in the two soils. Considering the metabolic versatility of soil microorganisms, phthalate metabolites generated might have been utilized rapidly.
3.2 Influence of SMS on DEHP dissipation
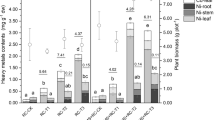
The gray fluvo-aquic soil had slower DEHP degradation rate when compared with the other soil, and thus, the gray fluvo-aquic soil was used to study whether SMS application was helpful in the removal of DEHP. After the amendment of SMS for 50 days, urease activity increased 1.54–3.51 times (p < 0.05) compared with that in control microcosms (Fig. 1a). The results was consistent with the report of Jia et al. (2017).The application of non-sterilized SMS significantly promoted soil laccase activity but not in sterilized SMS microcosms (Fig. 1b). The results demonstrated that SMS in this study contained considerable extracellular ligninolytic enzymes but sterilization processes would make these enzymes inactive. The observation of a decline in laccase activity from 0 to 50 days suggested that soil conditions were not favorable for maintaining the activity of laccase or the growth of laccase-producing fungi. Soil acidity is an important factor affecting the availability of nutrients and organic pollutants. In this study, soil pH increased after the addition of SMS (Fig. 1c).
Urease activity (a), laccase activity (b), pH value (c), and DEHP degradation (d) in unamended control soil and soils amended with sterilized (SSMS) or non-sterilized (SMS) spent mushroom substrate, on days 25 and 50. For each sampling time, bars with the same letter are not significantly different at p < 0.05
The amendment of SMS could accelerate DEHP dissipation. The results showed that only 12.4% of DEHP was degraded in the control soil after 25-day incubation (Fig. 1d). Significantly higher degradation (21.3–22.9%) was found in soils amended with 4% SMS (sterilized or non-sterilized). However, no significant difference of degradation rates between control (29.0 ± 2.1%) and treatments (31.3–35.2%) was found after 50-day incubation. The improved DEHP removal in SMS-amended soils during early times might be related to an increase in DEHP bioavailability (as shown in Fig. 2), as a result of altered soil pH and dissolved organic matter (DOM) content after SMS application. Previous studies have shown that the adsorption of PAEs to soils decreases with increasing solution pH (Yang et al. 2013), and DOM from organic wastes can facilitate the transport of DEHP in soil (de Jonge et al. 2002). In addition, increased microbial activities and modified microbial communities (due to a biostimulation effect of sterilized SMS or the biostimulation/bioaugmentation effects of non-sterilized SMS) might be the other reasons to enhance DEHP degradation in SMS-amended soils. For example, García-Delgado et al. (2015) reported that sterile SMS could efficiently stimulate the growth of soil native microorganisms due to an input of organic matter while non-sterile SMS could also introduce PAH-degrading bacteria into soil, leading to enhanced dissipation of PAHs under both conditions. Similarly, enhanced dichlorophen dissipation was found in the study of Jia et al. (2017), which was attributed to soil microbial activities promoted by SMS amendment. In the current study, the lack of a significant difference between sterilized and non-sterilized SMS microcosms reflected a main role of biostimulation in DEHP dissipation. In the present study, there was no correlation between DEHP degradation and soil laccase activity. This result was in conflict with the study of Kim et al. (2008), in which PAE degradation by fungi was closely related to laccase activity. In other researches, esterase was suggested to play an important role in PAE degradation (Hwang et al. 2012; Ahuactzin-Pérez et al. 2016). However, the determination of esterase activity in soil samples is still problematic and is not included in this study.
The beneficial effect of SMS on the degradation of DEHP weakened over time, probably because the bioavailability of DEHP became a limiting factor for biodegradation. The desorption of DEHP decreased markedly after 25-day incubation in SMS-amended soil (Fig. 2). This could be explained by a stronger sorption of DEHP onto soil through penetrating into micropores of soil organic matter and minerals with increasing contact time, and by a decrease in residual amount of DEHP due to biodegradation. Similarly, García-Delgado et al. (2015) found that the bioavailable fraction of fluoranthene decreased after an incubation for 63 days and the decrease was more obvious in SMS-amended soils than in the control soil.
3.3 Influence of SMS on the dissipation of DnBP and plant bioaccumulation
DnBP was used to study the fate of PAE in soil-plant systems, as in soils DnBP is thought to be easier absorbed by plant roots than DEHP which is more recalcitrant but less water soluble (He et al. 2015). The gray fluvo-aquic soil from Changshu was used. DnBP concentration showed no sufficient difference among the top soil, bulk soil, and rhizosphere soil on each sampling day. After incubation for 15 days, the concentration of DnBP decreased from 52.3 to <3 mg kg−1in all soil samples (Fig. 3a). The degradation of DnBP in this experiment was faster when compared with other studies. For example, 60–70% of DnBP was removed from soils after 15 days of incubation (Xu et al. 2008). The degradation of DnBP during the latter 15 days was quite limited, and the residual DnBP content was 1.38–2.02 mg kg−1 by the end of incubation (Fig. 3b). This could be due to that the remaining DnBP had very low bioavailability or the synthesis of degradative enzymes was not induced at low concentrations of DnBP. The application of SMS in soils had little effect on the dissipation of DnBP in this experiment. This is possibly because DnBP degradation in the soil under investigation could occur rapidly, requiring no additional fertilizer. On the other hand, the effects of SMS on the degradation of organic pollutants may be pollutant-dependent; thus, no benefit on the degradation of DnBP was observed. Marín-Benito et al. (2012) reported that the dissipation of four fungicides in soil was differently affected by SMS amendment. Compared to the unamended soil, the amended soil had higher, lower, and similar degradation rates for pyrimethanil, penconazole, and metalaxyl, respectively.
DnBP concentrations in contaminated soil and the original soil (with or without SMS), and DnBP accumulation in the root and shoot of bok choy (Brassica rapa subsp. chinensis) from the corresponding soils, on day 15 (a) and day 30 (b). For each sampling time, bars with the same letter are not significantly different at p < 0.05
Bioaccumulation of DnBP was observed in bok choy in this study. The concentration of DnBP in the shoot (3.66–3.69 mg kg−1) was higher than that in the root (2.20–2.86 mg kg−1) and in soils (1.99–2.22 mg kg−1) after growing for 15 days (Fig. 3a). The concentrations of DnBP in the shoots growing for 30 days were lower than those growing for 15 days (Fig. 3b), which could be a result of plant metabolism. Zhang et al. (2017) also observed a lower PAE concentration in bok choy at later growth stage than earlier growth stage. Although it was reported that biochar amendment decreased PAE accumulation in bok choy (He et al. 2016), SMS application appeared to have no effect on the uptake of DnBP by bok choy in this study. One possible reason could be that SMS did not cause a significant decrease in the bioavailable fraction of DnBP while biochar could immobilize soil PAE by strong adsorption.
Figure 3a shows that high concentration of DnBP was detected in the shoot (3.22–3.47 mg kg−1) and root (2.09–2.31 mg kg−1) in the control experiment with no DnBP spiking. The results suggested that atmospheric DnBP was the main source of DnBP in bok choy in this study, and thus, soil conditioners had less effects on plant bioaccumulation. The concentration of DnBP in air was 422.0 ± 76.5 ng m−3 (Table 4), and the total air concentration of DiBP, DnBP, and DEHP in the greenhouse was 1683 ng m−3, which was much higher than that in the open air near the greenhouse (< 90 ng m−3). There were plastic pots/buckets/trays in the greenhouse, which can release PAEs to the atmosphere (Li et al. 2016), and studies have shown that plants can take up vapor-phase PAE (Dueck et al. 2003).
Analysis of other PAEs showed that vegetables grown in soils with or without DnBP spiking had similar PAE profiles and concentrations, and PAE concentrations in bok choy was not affected by SMS amendment during a 30-day cultivation period (Table 5). This further indicates that PAE uptake in plant was primarily through PAEs in air. There have been reports showing that no linear correlation exists between vegetable and soil PAE concentrations in greenhouses (Wang et al. 2015; Chen et al. 2017), which suggest PAEs in air are important contamination sources for PAEs in plants. In addition, Wang et al. (2010) reported that DEHP contents in five different vegetables were positively correlated with DEHP levels in air, indicating that atmosphere deposition was the principal pathway for DEHP accumulation in these vegetables. Although in other studies PAE concentrations in vegetables have been found to positively correlate with soil contamination levels (Zeng et al. 2006; Cai et al. 2008), the residual concentrations in soil were as high as 16.6 mg kg−1, which were more than five times as those in this research. It seems that in soils with high PAE concentrations and low dissipation rate, the contribution from soil PAEs to plant bioaccumulation would be higher. However, in greenhouses where plastic films and pots are widely used, PAE exposure through the atmosphere cannot be neglected, and the efficiency of using soil conditioner to minimize PAE accumulation in vegetables needs to be verified.
4 Conclusions
This study showed that the degradation of four common PAEs in two agricultural soils was mainly through a biological process, and DEHP could be persistent in soil. When utilized as a soil conditioner, SMS improved soil enzyme activities and accelerated the dissipation of DEHP within 25 days. When soil DnBP levels were low, air deposition was the main source of DnBP in bok choy and SMS amendment had little effect on DBP accumulation in bok choy. The shoot tended to accumulate more PAEs than the root, which was proposed due to plant uptake through atmosphere. This work provided useful information for controlling PAE contamination in soil and plant.
References
Ahuactzin-Pérez M, Tlecuitl-Beristain S, García-Dávila J, González-Pérez M, Gutiérrez-Ruíz MC, Sánchez C (2016) Degradation of di (2-ethyl hexyl) phthalate by Fusarium culmorum: kinetics, enzymatic activities and biodegradation pathway based on quantum chemical modeling pathway based on quantum chemical modeling. Sci Total Environ 566:1186–1193. https://doi.org/10.1016/j.scitotenv.2016.05.169
Bourbonnais R, Paice MG (1990) Oxidation of non-phenolic substrates: an expanded role for laccase in lignin biodegradation. FEBS Lett 267:99–102
Cai QY, Mo CH, Zeng QY, QT W, Férard JF, Antizar-Ladislao B (2008) Potential of Ipomoea aquatica cultivars in phytoremediation of soils contaminated with di-n-butyl phthalate. Environ Exp Bot 62(3):205–211. https://doi.org/10.1016/j.envexpbot.2007.08.005
Cartwright CD, Thompson IP, Burns RG (2000) Degradation and impact of phthalate plasticizers on soil microbial communities. Environ Toxicol Chem 19(5):1253–1126. https://doi.org/10.1002/etc.5620190506
Chang BV, Yang CM, Cheng CH, Yuan SY (2004) Biodegradation of phthalate esters by two bacteria strains. Chemosphere 55(4):533–538. https://doi.org/10.1016/j.chemosphere.2003.11.057
Chatterjee S, Dutta TK (2008) Complete degradation of butyl benzyl phthalate by a defined bacterial consortium: role of individual isolates in the assimilation pathway. Chemosphere 70(5):933–941. https://doi.org/10.1016/j.chemosphere.2007.06.058
Chen H, Zhuang R, Yao J, Wang F, Qian Y (2013) A comparative study on the impact of phthalate esters on soil microbial activity. B Environ Contam Tox 91(2):217–223. https://doi.org/10.1007/s00128-013-1033-4
Chen N, Shuai W, Hao X, Zhang H, Zhou D, Gao J (2017) Contamination of phthalate esters in vegetable agriculture and human cumulative risk assessment. Pedosphere 27(3):439–451. https://doi.org/10.1016/S1002-0160(17)60340-0
Chiu SW, Gao T, Chan CSS, Ho CKM (2009) Removal of spilled petroleum in industrial soils by spent compost of mushroom Pleurotus pulmonarius. Chemosphere 75(6):837–842. https://doi.org/10.1016/j.chemosphere.2008.12.044
Dueck TA, Van Dijk CJ, David F, Scholz N, Vanwalleghem F (2003) Chronic effects of vapour phase di-n-butyl phthalate (DBP) on six plant species. Chemosphere 53(8):911–920. https://doi.org/10.1016/S0045-6535(03)00580-0
Engelhardt G, Wallnofer PR (1978) Metabolism of di-normal-butyl phthalate and mono-normal-butyl phthalate by soil bacteria. Appl Environ Microbiol 35(2):243–246
Frutos I, García-Delgado C, Cala V, Gárate A, Eymar E (2017) The use of spent mushroom compost to enhance the ability of Atriplex halimus to phytoremediate contaminated mine soils. Environ Technol 38(9):1075–1084. https://doi.org/10.1080/09593330.2016.1217938
Gao W, Liang J, Pizzul L, Feng XM, Zhang K, del Pilar Castillo M (2015) Evaluation of spent mushroom substrate as substitute of peat in Chinese biobeds. Int Biodeterior Biodegrad 98:107–112
García-Delgado C, D’Annibale A, Pesciaroli L, Yunta F, Crognale S, Petruccioli M, Eymar M (2015) Implications of polluted soil biostimulation and bioaugmentation with spent mushroom substrate (Agaricus bisporus) on the microbial community and polycyclic aromatic hydrocarbons biodegradation. Sci Total Environ 508:20–28. https://doi.org/10.1016/j.scitotenv.2014.11.046
Gómez-Hens A, Aguilar-Caballos MP (2003) Social and economic interest in the control of phthalic acid esters. TrAC Trends Anal Chem 22(11):847–857. https://doi.org/10.1016/S0165-9936(03)01201-9
Guo M, Chorover J (2006) Leachate migration from spent mushroom substrate through intact and repacked subsurface soil columns. Waste Manag 26(2):133–140. https://doi.org/10.1016/j.wasman.2004.12.024
Guo D, Wu Y (2011) Determination of phthalic acid esters of soil in south of Xinjiang cotton fields. Arid Environ Monit 25:76–79
He L, Gielen G, Bolan NS, Zhang X, Qin H, Huang H, Wang H (2015) Contamination and remediation of phthalic acid esters in agricultural soils in China: a review. Agron Sustain Dev 35:519–534
He L, Fan S, Müller K, Hu G, Huang H, Zhang X, Li X, Che L, Wang H (2016) Biochar reduces the bioavailability of di-(2-ethylhexyl) phthalate in soil. Chemosphere 142:24–27
Hwang SS, Kim HY, Ka JO, Ka HG (2012) Changes in the activities of enzymes involved in the degradation of butylbenzyl phthalate by Pleurotus ostreatus. J Microbiol Biotechnol 22(2):239–243. https://doi.org/10.4014/jmb.1107.07050
Jia Z, Deng J, Chen N, Shi W, Tang X, Xu H (2017) Bioremediation of cadmium-dichlorophen co-contaminated soil by spent Lentinus edodes substrate and its effects on microbial activity and biochemical properties of soil. J Soils Sediment 17:315–325
de Jonge H, de Jonge LW, Blicherb BW, Moldrup P (2002) Transport of di(2-ethylhexyl)phthalate (DEHP) applied with sewage sludge to undisturbed and repacked soil columns. J Environ Qual 31(6):1963–1971. https://doi.org/10.2134/jeq2002.1963
Jordan SN, Holland LB, Linnane SU (2012) Spent mushroom compost management and options for use. Environmental Protection Agency STRIVE Report Series No 74 http://wwwepaie/pubs/reports/research/waste/strivereport74html. Assessed 18 July 2015
Kandeler E, Gerber H (1988) Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol Fertil Soils 6:68–72
Kästner M, Miltner A (2016) Application of compost for effective bioremediation of organic contaminants and pollutants in soil. Appl Microbiol Biotechnol 100(8):3433–3449. https://doi.org/10.1007/s00253-016-7378-y
Kim Y, Yeo S, Song HG, Choi HT (2008) Enhanced expression of laccase during the degradation of endocrine disrupting chemicals in Trametes versicolor. Microbiology 46:402–407
Li X, Wu Y, Lin X, Zhang J, Zeng J (2012) Dissipation of polycyclic aromatic hydrocarbons (PAHs) in soil microcosms amended with mushroom cultivation substrate. Soil Biol Biochem 47:191–197. https://doi.org/10.1016/j.soilbio.2012.01.001
Li C, Chen J, Wang J, Han P, Luan Y, Ma X, Lu A (2016) Phthalate esters in soil, plastic film, and vegetable from greenhouse vegetable production bases in Beijing, China: concentrations, sources, and risk assessment. Sci Total Environ 568:1037–1043. https://doi.org/10.1016/j.scitotenv.2016.06.077
Ling W, Ren L, Gao Y, Zhu X, Sun B (2009) Impact of low-molecular-weight organic acids on the availability of phenanthrene and pyrene in soil. Soil Biol Biochem 41(10):2187–2195. https://doi.org/10.1016/j.soilbio.2009.08.003
Liu H, Liang H, Liang Y, Zhang D, Wang C, Cai H, Shvartsev SL (2010) Distribution of phthalate esters in alluvial sediment: a case study at JiangHan Plain, Central China. Chemosphere 78(4):382–388. https://doi.org/10.1016/j.chemosphere.2009.11.009
Marín-Benito JM, Andrades MS, Sánchez-Martín MJ, Rodríguez-Cruz MS (2012) Dissipation of fungicides in a vineyard soil amended with different spent mushroom substrates. J Agric Food Chem 60(28):6936–6945. https://doi.org/10.1021/jf301322h
Net S, Sempéré R, Delmont A, Paluselli A, Ouddane B (2015) Occurrence, fate, behavior and ecotoxicological state of phthalates in different environmental matrices. Environ Sci Technol 49(7):4019–4035. https://doi.org/10.1021/es505233b
Richards DJ, Shieh WK (1986) Biological fate of organic priority pollutants in the aquatic environment. Water Res 20(9):1077–1090. https://doi.org/10.1016/0043-1354(86)90054-0
Rousk J, Brookes PC, Bååth E (2009) Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl Environ Microbiol 75(6):1589–1596. https://doi.org/10.1128/AEM.02775-08
Scotti R, Bonanomi G, Scelza R, Zoina A, Rao MA (2015) Organic amendments as sustainable tool to recovery fertility in intensive agricultural systems. J Soil Sci Plant Nutr 15:333–352
Sharma M, Maloo S (2005) Assessment of ambient air PM10 and PM2.5 and characterization of PM10 in the city of Kanpur, India. Atmos Environ 39(33):6015–6026. https://doi.org/10.1016/j.atmosenv.2005.04.041
Swan SH (2008) Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res 108(2):177–184. https://doi.org/10.1016/j.envres.2008.08.007
Walsh G, Grogan H, Kellegher T, Plunkett M, Lalor S (2013) Spent mushroom compost–nutrient content for application to agricultural crops. Teagasc Technol Updates Project No 6355. http://www.teagasc.ie/publications/. Accessed 18 July 2015
Wang JL, Chen LJ, Shi HC, Qian Y (2000) Microbial degradation of phthalic acid esters under anaerobic digestion of sludge. Chemosphere 41:1245–1248
Wang JW, QZ D, Song YQ (2010) Concentration and risk assessment of DEHP in vegetables around plastic industrial area. Chin J Environ Sci 31:2450–2455
Wang L, Xu X, Lu X (2015) Phthalic acid esters (PAEs) in vegetable soil from the suburbs of Xianyang city, Northwest China. Environ Earth Sci 74(2):1487–1496. https://doi.org/10.1007/s12665-015-4141-0
Wang Z, Liu S, Xu W, Hu Y, Hu Y, Zhang Y (2016) The microbiome and functions of black soils are altered by dibutyl phthalate contamination. Appl Soil Ecol 99:51–61. https://doi.org/10.1016/j.apsoil.2015.11.024
Wittassek M, Koch HM, Angerer J, Brüning T (2011) Assessing exposure to phthalates—the human biomonitoring approach. Mol Nutr Food Res 55(1):7–31. https://doi.org/10.1002/mnfr.201000121
Xu G, Li F, Wang Q (2008) Occurrence and degradation characteristics of dibutyl phthalate (DBP) and di-(2-ethylhexyl) phthalate (DEHP) in typical agricultural soils of China. Sci Total Environ 393(2-3):333–340. https://doi.org/10.1016/j.scitotenv.2008.01.001
Yang F, Wang M, Wang Z (2013) Sorption behavior of 17 phthalic acid esters on three soils: effects of pH and dissolved organic matter, sorption coefficient measurement and QSPR study. Chemosphere 93:82–89
Zeng QY, Mo CH, Cai QY, QT W (2006) Possible pathways through which phthalic acid esters (PAEs) are accumulated in radish plants (Raphanus Sativus). Acta Scientiae Circumstantiae 26:10–16
Zhang X, Wang H, He L, Lu K, Sarmah A, Li J, Bolan NS, Pei J, Huang H (2013) Using biochar for remediation of soils contaminated with heavy metals and organic pollutants. Environ Sci Pollut Res 20(12):8472–8483. https://doi.org/10.1007/s11356-013-1659-0
Zhang T, Zhou CY, Chen SS, Zhou JX, Bai B (2017) Accumulation and metabolism of phthalates in Brassica campestris ssp. chinensis Makino. Acta Agricultureae Shanghai 33:86–90
Zhao HM, Du H, Feng NX, Xiang L, Li YW, Li H, Cai QY, Mo CH (2016) Biodegradation of di-n-butylphthalate and phthalic acid by a novel Providencia sp. 2D and its stimulation in a compost-amended soil. Biol Fertil Soils 52:65–76
Funding
This research was funded by the National Key Basic Research Program of China (No. 2014CB441105), the National Natural Science Foundation of China (No. 21377136), the Research Instrument Development Program of the Chinese Academy of Sciences (YZ201638), and the 135 Research Program of the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Kitae Baek
Rights and permissions
About this article
Cite this article
Zhu, F., Zhu, C., Chen, N. et al. Will spent mushroom substrate application affect the dissipation and plant uptake of phthalate esters?. J Soils Sediments 18, 1579–1589 (2018). https://doi.org/10.1007/s11368-017-1876-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-017-1876-0