Abstract
Purpose
This study investigates the effects of surface liming on soil attenuation radiation properties. For this, measurements of soil chemical attributes (pH, organic carbon, H+Al, Al3+, Ca2+, and Mg2+) and attenuation radiation parameters (mass attenuation coefficient, μm, atomic and electronic cross sections, σa and σe, effective atomic number and electron density, Zeff and Nel) were carried out. This aim was motivated by the fact that possible μm variation might cause as well variation in the determination of soil physical properties.
Materials and methods
The studied soil, classified as a Dystrudept sity-clay, is located in South Brazil. The trial consisted of five stripes, one of them under pasture and the remaining under no-till system (NTS). Lime rates of 0, 10, 15, and 20 t ha−1 were broadcast on the NTS soil surface. Disturbed soil samples were collected 30 months after liming at the top (0–10 cm) and subsoil (10–20 cm) layers. Soil chemical attributes were characterized following standard experimental procedures. The soil oxide composition, obtained by EDXRF analysis, was used to calculate μm for 241Am and 137Cs photon energies with XCOM computer code. μm values were employed to calculate σa, σe, Zeff, and Nel and to predict variations in soil bulk density (ρ) and total porosity (φ).
Results and discussion
Surface liming notably increased contents of soil pH, Ca2+, and Mg2+ while reduced H+Al and Al3+ at the top soil layer, where μm, σa, σe, and Zeff were also increased with the lime rates. However, at the subsoil layer, liming neither lessened soil acidity nor induced remarkable changes in the attenuation parameters. When using 137Cs photon energy, incoherent scattering totally dominated over the radiation interaction processes whereas photoelectric absorption and coherent scattering substantially contributed when 241Am photon energy was used. Therefore, the increasing in soil attenuation parameters at the top soil layer was more accentuated considering 241Am than 137Cs photon energy. Variation in μm caused considerable variation in ρ and φ only for 241Am photon energy.
Conclusions
The findings regarding the effect of μm variation induced by liming on the determination of soil physical properties are extremely relevant because traditionally, in the soil science area, μm values are calculated without considering any chemical modification to which the soil can be submitted. Bearing in mind that ρ and φ are important parameters from the agricultural and environmental points of view, not representative measurements of μm can lead to biased values of ρ and φ.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The mass attenuation coefficient (μm) is an important parameter for characterizing the penetration and diffusion of gamma radiation in multi-element materials (Hubbell 1969). This parameter can be determined experimentally by nuclear techniques or theoretically by the knowledge of the soil chemical composition, with good agreement between these methodologies (Pires and Pereira 2014). The theoretical computation of μm for elements, compounds, or mixtures, in a wide energy range (1 keV to 100 GeV), can be easily accomplished with the help of computer codes specifically developed for this purpose, such as XCOM (Berger and Hubbell 1987) and its successor WinXCom (Gerward et al. 2004).
The aforementioned software programs take into consideration the absorption and scattering mechanisms (photoelectric absorption, Compton, pair production, and Rayleigh effects) by which the radiation is attenuated by the matter (Kaplan 1977). In the case of composite materials, the processes of radiation interaction are related to the effective atomic number and electron density (Zeff and Nel), which are in turn dependent on the molecular, atomic, and electronic cross sections (σm, σa, σe) (Han and Demir 2009). All of these attenuation parameters can be predicted based on μm and the soil chemical composition, as reported in Medhat (2011) and Un and Sahin (2012).
A great number of studies regarding measurements of soil parameters such as water retention curve, water content, bulk density, and porosity have been performed by adopting attenuation of photons as an accurate, convenient, and non-destructive technique (Pires et al. 2005; Demir et al. 2008; Pires et al. 2009; Costa et al. 2013). However, it is important that any measurements of soil physical properties are representative of this porous media. Hence, when it comes to addition of soil amendments, such as lime, even though the variation in μm is likely to be slight, biased results may be obtained if such variation is not considered.
Liming is the most agriculture-employed practice to correct soil acidity. The use of Ca amendments such as limestone has been proven to increase soil pH and decrease exchangeable Al, increasing crop yield as a result (Mora et al. 1999). In addition, lime applications are particularly known to increase soil organic matter content in the long term (Haynes and Naidu 1998).
Besides changing the soil acidity, liming is also likely to promote variation of soil elemental composition. Dolomite limestone, for instance, generally contains 30.4% CaO, 21.8% MgO, and 47.8% CO3 (Jones 2003). Thus, after solubilization, there might remain increased contents of CaO and MgO due to the non-reacted part of lime products. Here, these changes in the soil elemental composition can lead to modifications in μm.
The current research aims to investigate the effects of surface liming, 30 months past the lime application, on soil chemical attributes and soil attenuation properties, at the top and subsoil layers. This study also evaluates how possible differences in mass attenuation coefficients would promote changes in the soil bulk density and total porosity, considering the most common radioactive sources employed in soil physics (241Am and 137Cs).
2 Materials and methods
2.1 Soil sampling
The soil samples come from an experiment installed in May 2012, in a familiar rural site located in the Southeast region of Paraná State, Brazil (25°28′S, 50°54′W and 821 m a.s.l). The soil was classified as a Dystrudept silty-clay (Soil Survey Staff 2013).
The experimental area was designed in five stripes, one of them under pasture, considered as reference (Ref.), and the remaining under no-till system (NTS). Lime rates of 0 t ha−1 (C0), 10 t ha−1 (C10) 15 t ha−1 (C15), and 20 t ha−1 (C20) were applied on the NTS soil surface, without disturbing the soil. The lime used had 285 and 200 g kg−1 of CaO and MgO, and 100.6, 74.7, and 75.1% neutralizing power, reactivity, and total neutralizing relative power, respectively.
Thirty months after lime application, during bean flowering, four different locations per stripe were chosen to collect the disturbed soil samples, so that spatial variability was appropriately covered, at 0–10 cm (A) and 10–20 cm (B) soil layers. More details about the history of crop rotation adopted for the experiment under study can be found in Auler et al. (2017).
2.2 Soil analyses
2.2.1 Chemical attributes
Prior to chemical analysis, the soil samples were dried in forced air circulation oven (40 °C/48 h) and ground to pass through a 2-mm sieve. Soil organic carbon (OC) was determined by the Walkley-Black method according to Brazilian guidelines reported in van Raij et al. (2001). Soil pH (active soil acidity) was determined in a 0.01 mol L−1 CaCl2 suspension (1:2.5 soil/solution, v/v).
The potential acidity (H+Al) was determined by a SMP buffer procedure and exchangeable Al3+, Ca2+, and Mg2+ were extracted with neutral 1 mol L−1 KCl (1:10 soil/solution, v/v), according to standard methods described in Pavan et al. (1992). Exchangeable Al3+ (exchangeable acidity) was determined by titrating with 0.025 mol L−1 NaOH solution, Ca2+ and Mg2+ by titrating with 0.025 mol L−1 EDTA.
2.2.2 X-ray fluorescence technique
Semi-quantitative elemental analyses of the soil samples were performed through an energy dispersive X-ray fluorescence (XRF) spectrometer model EDX-720 (Shimadzu), equipped with an Rh X-ray tube. The equipment voltage varied from 5 to 50 kV and its tube current from 1 to 1000 μA. The system detector was a Si(Li) semi-conductor cooled with liquid N at − 196 °C. Standard procedures of calibration were performed whenever necessary (Pires et al. 2016).
Three measurement repetitions were carried with sample portions (~ 2 g containing equal aliquots from the four original samples for each stripe and soil layer) powdered and reduced to diameters lower than 45 μm which were placed into proper sample analysis cups covered with Mylar film (6 μm thickness). The measuring time for each sample was 100 s in Na-Sc (15 kV) and Ti-U (50 kV) energy bands. Measurements were performed under 30 Pa pressure and the spectral output was acquired in terms of oxides.
2.2.3 Attenuation parameters
The computer code XCOM, available in a web version supported by the National Institute of Standards and Technology, was used to compute the soil mass-absorption coefficients, μm = μ/ρ (cm2 g−1), where μ is the linear absorption coefficient (cm−1) and ρ is the soil bulk density (g cm−3). For that, the oxide composition obtained from XRF analyses was taken as input data.
Photon energies related to the most common radioisotopes used as sources for γ-ray attenuation experimental investigations were selected for this study: 59.5 keV (241Am) and 661.6 keV (137Cs) (Corey et al. 1971; Reginato 1974; Ferreira and Pires 2016).
Contents of soil pH, OC, H+Al, Al3+, Ca2+, and Mg2+ were plotted against values of μm and a second-degree polynomial mathematical function was used to fit the data. Additionally, analyses of linear correlation were performed between values of μm and oxide mixtures aiming to determine if μm variations can be mostly explained by variations in the proportions of particular soil oxides.
For each studied photon energy, XCOM also provided the contribution of the incoherent scattering (is), coherent scattering (cs), photoelectric absorption (pa), and pair production (pp) in μm (Berger and Hubbell 1987):
The μm values were also used to determine the total molecular cross section (σm) as follows (Akça and Erzeneoğlu 2014):
where \( M={\sum}_{\mathit{\mathsf{i}}}{n}_i{A}_i \) is the molecular weight of the compound, NA is Avogadro’s number, A i is the atomic weight of the ith element and n i is the number of formula units in the molecule.
The average atomic and electronic cross sections (σa and σe) were in turn obtained through (Han and Demir 2009):
where \( {\sum}_{\mathit{\mathsf{i}}}{n}_i \) is the total number of formula units; f i = n i /∑ j n j and Z i are fractional abundance and atomic number of the constituent element, n j is the number of atoms of the constituent element, and ∑ j n j is the total number of atoms present in the molecular formula.
The effective atomic number (Zeff) was obtained by relating the Eqs. (2), (3), and (4) (Han and Demir 2009; Akça and Erzeneoğlu 2014):
Finally, the effective electron number or electron density (number of electrons per unit mass) was calculated by (Medhat et al. 2014):
Values of μm along with percentage contributions of cs, is and pa, σa, σe, Zeff, and Nel were plotted as function of soil lime treatments (C0, C10, C15, and C20) and Ref., for 241Am and 137Cs. In addition, the variation of μm, Zeff, and Nel (plus the behavior of Zeff versus Nel), as function of the photon energy (40–661.6 keV), was graphically presented with power, logarithmic, and linear fittings.
2.2.4 Prediction of soil physical properties
When a gamma-ray beam of incident intensity I0 (cps) interacts with a soil of thickness x, the transmitted intensity I (cps) through the absorber follows the Beer-Lambert law (Pires and Pereira 2014):
Therefore, the values of μm obtained in the present study, together with fixed values of I (62,035 cps), I0 (506,458 cps), and x (6.027 cm), which were extracted from a previous study performed with a soil of similar elemental composition (Pires et al. 2014), were used to predict the soil bulk density (ρ) and total porosity (φ) as follows:
where ρp is the soil particle density which was determined with an Helium gas multipycnometer (model MVP-D160-E, Quantachrome Instruments). The average ρp values obtained for layers A and B were 2.38 and 2.47 g cm−3, respectively.
3 Results and discussion
Comparing C10, C15, and C20 to Ref. and C0 (Table 1), it is notable that surface liming increased contents of soil pH, Ca2+, and Mg2+, while reduced H+Al and Al3+ at layer A. Nevertheless, at layer B, chemical attributes did not undergo as pronounced variations. The less successful effect of liming to correct soil acidity at the subsurface layer is due to the slow natural mobility of lime through the soil depth related to the low solubility of agricultural limestones (Ernani et al. 2004). At both soil layers, no effect of management alone can be inferred from the results since only slight changes occurred between Ref. (pasture area) and C0 (no-till system without liming), regarding all chemical attributes.
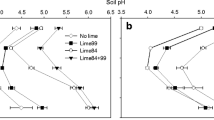
An investigation concerning effects of surface and incorporated liming on physical-hydrical attributes of the same soil under study in this work, after 18 months of lime application, is found in Auler et al. (2017). For each application manner, the mentioned investigation considered only 0 and 15 t ha−1 (calculated to raise the base saturation at the 0–20-cm soil layer to 70%) lime rates, respectively equivalent to C0 and C15. Twelve months after this evaluation, totalizing 30 months of lime application, most of the chemical attributes undergone greater changes for C15 in relation to C0 at the top soil layer (Fig. 1a). This is an indicative that variations in soil chemical attributes after the 30-month period is not only due to crop rotation, whose effects are exclusively seen in C0, but it is still strongly related to lime reaction.
Comparison of soil chemical attributes after 18 (Auler et al. 2017) and 30 months of lime application at a 0–10 cm (A) and b 10–20 cm (B) soil layers. For this comparison, only C0 and C15 were considered
At layer B, from 18 to 30 months, C0 and C15 (Fig. 1b) presented much more subtle effects than at layer A (Fig. 1a). Besides, at this soil depth, only reduction of H+Al content is more pronounced for C15 in relation to C0, which indicates that soil acidity components were not remarkably ameliorated at the subsurface layer in the course of time. Similar results regarding effects of liming on soil chemical attributes, over a period of 4 years, at the 0–5, 5–10, 10–20, and 20–40 cm soil depths are found in Caires et al. (2006).
Rheinheimer et al. (2000) developed a study considering lime rates of 0.0, 2.0, 8.5, and 17 Mg ha−1 superficially applied and incorporated to a soil from south Brazil. These authors reported that the soil acidity correction at the top layer was proportional to the lime rate, independent of the application manner. However, greater values of soil pH, OC, and Ca2+ as well as lower values of H+Al and Al3+ were observed for C15 instead of C20, at layer A (Table 1). This indicates that the highest lime rate did not promote further soil chemical amelioration beyond the intermediary rate.
Among soil constituent oxides, SiO2, Al2O3, and Fe2O3 are predominant (Garrison 2008) (Table 2). Considering that CaO was not identified for neither Ref. and C0 at layer A nor for any treatment at layer B, it is reasonable to say that this particular oxide is not inherent to this soil. Inceptisols classified as Dystrudept, such as the soil under investigation, are indeed base-poor due to its acid parent material (Hudnall 2011). Therefore, the appearance of CaO at layer A is most likely exclusively due to liming and, as it would be expected, its contribution increases with the increase in the lime rate. In virtue of the slow mobility of lime through the soil depth, CaO have not reached the soil subsurface layer in sufficient amount to be detected by XRF technique.
It is known that low energy photons have more pronounced interaction with the matter in comparison to the high energy ones (Rangacharyulu 2014). For this reason, lower values of μm were obtained using the higher photon energy of 137Cs (Fig. 2b). The μm values shown in Fig. 2 are in agreement to the findings of Pires et al. (2016) (241Am: 0.26–0.30 cm2 g−1 and 137Cs: 0.0764–0.0765 cm2 g−1) concerning different Brazilian hardsetting soils. As can be seen from Fig. 2, μm increases with the increasing in the lime rate at layer A, considering both sources. At layer B, though, μm values kept practically constant among treatments regardless the photon energy.
Chemical attributes from layer A (Table 1) plotted against data of μm (calculated for 241Am and 137Cs photon energies) for Ref. and lime treatments (Fig. 2) resulted in curves which were in most cases well fitted by a second-degree polynomial mathematical model (Fig. 3). For both sources, better adjustments were found for contents of soil pH, H+Al, and Ca2+, which indicates that μm variation is in fact related to the effects of liming.
Among all chemical parameters evaluated, OC seem to play a less important role in the increasing of μm (Fig. 3). The soil OC is an indirect parameter to evaluate the content of soil organic matter but it measures only the carbon contribution (Baldock and Broos 2011), which, for being a light element, weakly influences μm. At layer B, neither polynomial nor linear adjustments fitted well to the data, suggesting that variation on soil chemical attributes and μm cannot be associated at this soil depth.
Linear correlation analysis between soil constituent oxides (Table 2) and μm values obtained for each soil treatment (Fig. 2) also provides evidence that, at layer A, the increase in μm is more strongly related to the increasing amount of CaO in the soil (Table 3). At this same layer, the correlation coefficients from 241Am and 137Cs photon energies are more alike in comparison to layer B. This happens probably because μm variation at layer B (Fig. 2) presents an inverse behavior when comparing results for the two different energies, i.e., while μm slightly diminishes for 241Am, it slightly increases for 137Cs. Therefore, at layer B, the decrease in μm for the 241Am photon energy is mostly due to the reduction in Fe2O3, which is in line with results of Pires et al. (2016), and the increase in μm for 137Cs is governed by the increase in SiO2 (Table 3).
It is notable from Fig. 4 that the incoherent scattering is the dominant process by which the radiation interacts with matter, mainly for 137Cs (~ 99.5% of the radiation interaction). Incoherent scattering in fact dominates over the remaining processes when the incident photon energy is in an intermediary energy range ~ 100 keV to ~ 1 MeV (Kucuk et al. 2013). On the other hand, considering the lower characteristic photon energy of 241Am, the incoherent scattering contribution drops to about half of the total contribution (Fig. 4c) and substantial percentages of photoelectric absorption (Fig. 4e) and coherent scattering (Fig. 4a) take place.
Percentage contributions of coherent scattering, incoherent scattering, and photoelectric absorption to the computation of the mass attenuation coefficients (μm) as function of the soil treatments (Ref, C0, C10, C15, and C20) at 0–10 cm (A) and 10–20 cm (B) soil layers for 241Am (a, c, e) and 137Cs (b, d, f) photon energies
Incoherent scattering cross section is weakly influenced by the chemical composition of the target because it has linear dependence on Z. The photoelectric and coherent cross sections, on the other hand, are more strongly dependent on the atomic number of the constituent elements (Z4–5 and Z2–3, respectively) (Medhat et al. 2014). Thus, taking into account the variations in chemical composition among treatments, the greater changes in μm found for 241Am in comparison to 137Cs (Fig. 2) are justified by the differences regarding the contributions of scattering and absorption processes between the considered photon energies (Fig. 4).
The assumption that the original atoms in a given molecule or compound could be replaced by an equivalent number of average atoms, each of which having the same average atomic number, leads to the idea of the effective atomic number (Zeff) (Manohara et al. 2010). Zeff depends not only on the atomic numbers of the various composite material elements and its relative proportion but also on the number of elements present in it (Mudahar and Sahota 1988; Baltaş and Çevik 2008). A demonstration of the dependence of Zeff on the chemical composition of amino acids is found in the study of Manohara and Hanagodimath (2007).
The current soil under study has in its composition elements with atomic number varying from 8 (O) to 40 (Zr) and, as expected, Zeff lies within this interval (Fig. 5e, f). However, Zeff is inversely dependent on the sum over the fractional abundance (f) of each soil constituent element (see Eqs. 4 and 6) and the elements that present higher f are O (8), Si (14), and Al (13) (Table 4). For this reason, Zeff is closer to the inferior limit of the Z variation in the soil.
As the variety of soil constituent elements increase when Ca starts being computed (Table 4) due to the lime rates, at layer A, Zeff also increases (Fig. 5e, f). Thus, at this layer, the increase of photoelectric absorption (Fig. 4e, f) and consequent decrease in incoherent scattering (Fig. 4c, d) contributions with lime treatments can be related to the increase in Zeff (Önder et al. 2012). This is because of the much stronger dependence on Z of photoelectric absorption in comparison to the incoherent scattering (Kaplan 1977). At layer B, it can be considered from the plot scale that Zeff kept rather constant among treatments and consequently slighter changes are seen for percentages of radiation interaction.
From Fig. 5, one can see that the trend behavior of all attenuation parameters (σa, σe, Zeff, and Nel) is practically the same for the two photon energies. Nevertheless, the variation of these parameters is smaller for 137Cs than for 241Am due to the total dominance of incoherent scattering in the former and broader contribution of photoelectric absorption in the last (Fig. 4). Both atomic and electronic cross sections increase with the increasing in the lime rate, meaning that the limed soil samples present a higher probability to attenuate the gamma-ray photons, in accordance with the variation in μm (Fig. 2) (Eisberg 1885).
Figure 6 portraits how μm, Zeff, and Nel vary from 40 to 661.6 keV. It is possible to see that over this energy range, μm goes from about 0.60 to under 0.10 cm2 g−1 in a power law (Fig. 6a, b; Table 5) and the variation due to treatments is more evident at lower energies, as seen also in Fig. 2.
Variation of mass attenuation coefficients (μm), effective atomic number (Zeff), and effective electron density (Nel) as function of the photon energy (E) (40–661.6 keV) with power and logarithmic adjustments. Nel variation as function of Zeff, with linear adjustment, over the same energy range at 0–10 cm (A) (a, c, e, g) and 10–20 cm (B) (b, d, f, h) soil layers
For all treatments, Zeff undergoes a sharp increase from 40 to 200 keV and then its increasing turns smoother, which is well represented by a logarithmic function (Fig. 6c, d; Table 5). The Zeff behavior described here is very similar to the one presented in Medhat et al. (2014), within the same energy interval, for eight soils from the Southeast and South of Brazil. Among treatments, though, differences in Zeff are not magnified at low or high energy regions, but on the contrary remain almost the same over the entire range (Fig. 6c, d).
Just like Zeff, Nel varies logarithmically with the energy, as seen in Fig. 6e, f and Table 5. Several studies such as those performed by Baltaş and Çevik (2008), Medhat (2011), and Marashdeh et al. (2015) report that Zeff and Nel present indeed similar dependence on photon energy. This was confirmed by plotting these two important attenuation parameters against each other (Fig. 6g, h), which resulted in a linear relation (Table 5). It is worth to mention that the plot scales in Fig. 6 were zoomed in relation to Fig. 5 in order to better visualize variations in Nel and Zeff.
Finally, it is notable that although the variation in μm among treatments is subtle, when using 247Am photon energy, it causes relevant variation in ρ and φ, in the sense that a higher μm gives lower ρs and higher φ (layer A) and vice versa (layer B) (Table 6). On the other hand, μm values obtained with 137Cs photon energy practically did not change ρs and φ at neither of the soil layers. This occurs due to the weak interaction of the photon with the soil for this specific energy, which explains the similarities in μm values (Pires and Pereira 2014; Pires et al. 2016).
With these results in mind, one should be aware that using a unique attenuation coefficient, in order to determine ρs and φ of a soil submitted to different lime rates, may over or underestimate these important soil physical properties, especially when the chosen energy source is 247Am.
These findings are relevant because traditionally, in the soil science area, μm values are calculated without considering any chemical modification to which the soil can be submitted. As the soil bulk density and its total porosity are important parameters from the agricultural and environmental points of view, not representative measurements of μm can lead to biased values of ρs and φ as shown (Pires and Medhat 2016).
4 Conclusions
-
1.
Thirty months after liming application in the soil surface, substantial improvements of chemical attributes were achieved at the top soil layer, reflecting reduction in soil acidity. However, this procedure was not efficient to correct soil acidity at the subsoil;
-
2.
Liming increased the soil attenuation parameters (mass attenuation coefficient, atomic and electronic cross sections, effective atomic number and electron density) at the surface layer but practically did not change them at the subsurface;
-
3.
The increasing in the soil attenuation parameters was more accentuated when using the 241Am photon energy in comparison to 137Cs due to the higher contribution of photoelectric absorption in the former;
-
4.
The determination of soil physical properties such as bulk density and total porosity is more influenced by variation of mass attenuation coefficients computed with 241Am than 137Cs photon energy.
References
Akça B, Erzeneoğlu SZ (2014) The mass attenuation coefficients, electronic, atomic, and molecular cross sections, effective atomic numbers, and electron densities for compounds of some biomedically important elements at 59.5 keV. Sci Technol Nucl Install 2014:1–8
Auler AC, Pires LF, dos Santos JAB, Caires EF, Borges JAR, Giarola NFB (2017) Effects of surface-applied and soil-incorporated lime on some physical attributes of a Dystrudept soil. Soil Use Manag 33:129–140
Baldock JA, Broos K (2011) Soil organic matter. In: Huang PM, Li Y, Sumner ME (eds) Handbook of soil sciences properties and processes—part II: soil chemistry. CRC Press, Boca Raton, pp 11.1–11.52
Baltaş H, Çevik U (2008) Determination of the effective atomic numbers and electron densities for YBaCuO superconductor in the range 59.5–136keV. Nucl Instrum Methods Phys Res Sect B Beam Interact Mater Atoms 266:1127–1131
Berger MJ, Hubbell JH (1987) XCOM: photon cross sections on a personal computer, NBSIR 87–3597. National Bureau of Standards, USA
Caires EF, Barth G, Garbuio FJ (2006) Lime application in the establishment of a no-till system for grain crop production in southern Brazil. Soil Tillage Res 89:3–12
Corey JC, Peterson SF, Wakat MA (1971) Measurement of attenuation of 137Cs and 241Am gamma rays for soil density and water content determinations. Soil Sci Soc Am J 35:215
Costa JC, Borges JAR, Pires LF (2013) Soil bulk density evaluated by gamma-ray attenuation: analysis of system geometry. Soil Till Res 129:23–31
Demir D, Ün A, Özgül M, Şahin Y (2008) Determination of photon attenuation coefficient, porosity and field capacity of soil by gamma-ray transmission for 60, 356 and 662keV gamma rays. Appl Radiat Isot 66:1834–1837
Eisberg R (1885) Cross sections for photon absorption and scattering. In: Quantum physics of atoms, molecules, solids, nuclei, and particles, 2nd edn. Wiley, New York, pp 48–54
Ernani PR, Ribeiro MFS, Bayer C (2004) Chemical modifications caused by liming below the limed layer in a predominantly variable charge acid soil. Commun Soil Sci Plant Anal 35:889–901
Ferreira TR, Pires LF (2016) Soil porosity distribution representative elementary area analyzed through gamma-ray computed tomography. Int Agrophysics. https://doi.org/10.1515/intag-2016-0016
Garrison S (2008) The chemistry of soils, second. Oxford University Press, New York
Gerward L, Guilbert N, Jensen KB, Levring H (2004) WinXCom—a program for calculating X-ray attenuation coefficients. Radiat Phys Chem 71:653–654
Han I, Demir L (2009) Determination of mass attenuation coefficients, effective atomic and electron numbers for Cr, Fe and Ni alloys at different energies. Nucl Instrum Methods Phys Res Sect B Beam Interact Mater Atoms 267:3–8
Haynes RJ, Naidu R (1998) Influence of lime, fertilizer and manure applications on soil organic matter content and soil physical conditions: a review. Nutr Cycl Agroecosyst 51:123–137
Hubbell JH (1969) Photon cross sections, attenuation coefficients, and energy absorption coefficients from 10 keV to 100 GeV, NSRDS-NBS 29. National Bureau of Standards, USA
Hudnall WH (2011) Classification of soils: Inceptisols. In: Huang PM, Li Y, Sumner ME (eds) Handbook of soil sciences properties and processes—part V: pedology, 2nd edn. CRC Press, Boca Raton, pp 63–71
Jones JBJ (2003) Agronomic handbook: management of crops, soils and their fertility. CRC Press, Boca Raton
Kaplan I (1977) Nuclear physics, 2nd edn. Addison-Wesley Press, Reading
Kucuk N, Tumsavas Z, Cakir M (2013) Determining photon energy absorption parameters for different soil samples. J Radiat Res 54:578–586
Manohara SR, Hanagodimath SM (2007) Studies on effective atomic numbers and electron densities of essential amino acids in the energy range 1keV–100GeV. Nucl Instrum Methods Phys Res Sect B Beam Interact Mater Atoms 258:321–328
Manohara SR, Hanagodimath SM, Thind KS, Gerward L (2010) The effective atomic number revisited in the light of modern photon-interaction cross-section databases. Appl Radiat Isot 68:784–787
Marashdeh MW, Al-Hamarneh IF, Abdel Munem EM et al (2015) Determining the mass attenuation coefficient, effective atomic number, and electron density of raw wood and binderless particleboards of Rhizophora spp. by using Monte Carlo simulation. Results Phys 5:228–234
Medhat ME (2011) Studies on effective atomic numbers and electron densities in different solid state track detectors in the energy range 1keV–100GeV. Ann Nucl Energy 38:1252–1263
Medhat ME, Pires LF, Arthur RCJ (2014) Analysis of photon interaction parameters as function of soil composition. J Radioanal Nucl Chem 300:1105–1112
Mora ML, Schnettler B, Demanet R (1999) Effect of liming and gypsum on soil chemistry, yield, and mineral composition of ryegrass grown in an acidic Andisol. Commun Soil Sci Plant Anal 30:1251–1266
Mudahar GS, Sahota HS (1988) Effective atomic number studies in different soils for total photon interaction in the energy region 10–5000 keV. Int J Radiat Appl Instrumentation Part A Appl Radiat Isot 39:1251–1254
Önder P, Turşucu A, Demir D, Gürol A (2012) Studies on mass attenuation coefficient, effective atomic number and electron density of some thermoluminescent dosimetric compounds. Nucl Instrum Methods Phys Res Sect B Beam Interact Mater Atoms 292:1–10
Pavan MA, Bloch MF, Zempulski HC et al (1992) Manual de análise química do solo e controle de qualidade. Instituto Agronômico do Paraná (IAPAR), Londrina, BR
Pires LF, Medhat ME (2016) Different methods of mass attenuation coefficient evaluation: influences in the measurement of some soil physical properties. Appl Radiat Isot 111:66–74
Pires LF, Pereira ABP (2014) Gamma-ray attenuation to evaluate soil porosity: an analysis of methods. ScientificWorldJournal 2014:723041
Pires LF, Bacchi OOS, Reichardt K (2005) Soil water retention curve determined by gamma-ray beam attenuation. Soil Tillage Res 82:89–97
Pires LF, Rosa JA, Pereira AB et al (2009) Gamma-ray attenuation method as an efficient tool to investigate soil bulk density spatial variability. Ann Nucl Energy 36:1734–1739
Pires LF, Prandel LV, Saab SC (2014) The effect of wetting and drying cycles on soil chemical composition and their impact on bulk density evaluation: an analysis by using XCOM data and gamma-ray computed tomography. Geoderma 213:512–520
Pires LF, Brinatti AM, Prandel LV, da Costa Saab S (2016) Mineralogical composition of hardsetting soils and its effect on the radiation attenuation characteristics. J Soils Sediments 16:1059–1068
Rangacharyulu C (2014) Physics of nuclear radiations: concepts, techniques and applications. CRC Press, Boca Raton
Reginato RJ (1974) Gamma radiation measurements of bulk density changes in a soil Pedon following Irrigation1. Soil Sci Soc Am J 38:24
Rheinheimer DS, Santos EJS, Kaminski J et al (2000) Alterações de atributos do solo pela calagem superficial e incorporada a partir de pastagem natural. Rev Bras Ciência do Solo 24:797–805
Soil Survey Staff (2013) Simplified guide to soil taxonomy. USDA-Natural Resources Conservation Service, National Soil Survey Center, Lincoln
Un A, Sahin Y (2012) Determination of mass attenuation coefficients, effective atomic numbers, effective electron numbers and kermas for earth and Martian soils. Nucl Instrum Methods Phys Res Sect B Beam Interact Mater Atoms 288:42–47
van Raij B, Andrade JC de A, Cantarella H, Quaggio JA (2001) Análise química para avaliação da fertilidade de solos tropicais. Instituto Agronômico de Campinas, Campinas
Acknowledgements
We would like to thank Dr. José A. Baptista from IAPAR and the farmers for making the experimental area available.
Funding
The authors acknowledge CNPq (“Conselho Nacional de Desenvolvimento Científico e Tecnológico”) for the research grant for Dr. L.F. Pires (303726/2015-6) and CAPES (“Coordenação de Aperfeiçoamento de Pessoal de Nível Superior”) for the PhD grant for MS. T.R. Ferreira and MS. A.C. Auler.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Arnaud Temme
Rights and permissions
About this article
Cite this article
Ferreira, T.R., Pires, L.F., Brinatti, A.M. et al. Surface liming effects on soil radiation attenuation properties. J Soils Sediments 18, 1641–1653 (2018). https://doi.org/10.1007/s11368-017-1866-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-017-1866-2