Abstract
Purpose
Positive priming effect following the incorporation of biochar may partially reduce the biochar-induced soil carbon (C) sequestration. We hypothesize that repeated biochar incorporations induce further priming effect in the soil that was already amended with biochar.
Materials and methods
Two biochars prepared from Miscanthus giganteus at 350 and 700 °C, at a rate of 50 g C kg−1 soil, were added into the soil previously received each biochar separately with a pre-incubation of 1.5 years.
Results and discussion
The second addition of fresh biochar caused 108 and 26.9 μg CO2-C g−1 soil of soil C losses, which is the same order but less amount (about 72 and 56%) of C losses as the first biochar addition. This might be due to the depleted soil C after 1.5 years of incubation. Also, it might be attributed to the altered soil microbial community with lower proportions (%) of gram-positive bacteria, and higher proportions of fungi, actinobacteria and gram-negative bacterial phospholipid fatty acids (PLFAs) with repeated biochar addition.
Conclusions
Our research provided insights into the second addition of biochar-induced priming effects and responsible PLFAs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
It is widely known that soil organic matter mineralisation can be either accelerated or decelerated by substrate addition, which is refers to as positive or negative priming effect, respectively (Kuzyakov et al. 2000). Biochar addition increases mineralisation of soil organic matter (SOM), i.e. positive priming effect, which raised a very controversial issue and debate (Lehmann and Sohi 2008; Wardle et al. 2008a; Wardle et al. 2008b). Clearly, any increase in C sequestration due to biochar incorporation would be diminished due to positive priming effect on SOM caused by biochar amendment, or by increasing biochar mineralisation following the introduction of a substrate. In contrast, negative priming effect following biochar addition has been also measured (Woolf et al. 2010; Lu et al. 2014).
The inconsistent results on biochar priming effect may be attributed to the differences in the biochars and soils (Maestrini et al. 2015; Awad et al. 2017). Generally, positive priming effect was observed in the early stage and might be mainly attributed to the labile organic C in biochar (Luo et al. 2011; Jien et al. 2015). The magnitude of biochar-induced priming is also determined by the content of easily mineralizable SOM. Whitman et al. (2014) found that biochar addition caused less absolute primed CO2 in 6-month pre-incubated soils compared to 1-day pre-incubated soils, and they explained that the decreased biochar-induced priming effects was due to the 60% reduction of water-extractable C during the 6-month pre-incubation. Biochar-caused priming effects also largely depend on soil properties like pH (Luo et al. 2011), aggregate composition (Liang et al. 2010), microbial biomass (Luo et al. 2013; Thiessen et al. 2013; Watzinger et al. 2014), activity (Steinbeiss et al. 2009), community (Watzinger et al. 2014) (Lehmann et al. 2011) and enzyme activity (Jones et al. 2011). Environment factors, like temperature (Fang et al. 2014), nitrogen (N) fertilizer (Lu and Zhang 2015), forest burning (Nocentini et al. 2010) and presence of plant root (Weng et al. 2015), can also affect the direction and magnitude of biochar-induced priming effect. It was widely reported that biochar has certain effects on soil physio-chemical properties, like water-filled pore spaces, habitat structure, soil aeration, moisture, pH and nutrient availability (Chan et al. 2007; Sohi et al. 2010; Verheijen et al. 2010). As soil properties might be changed with biochar addition, the SOC in biochar-amended and unamended soils may respond differently to added substrates; thus, the direction or magnitude of priming effects caused by second addition of biochar might be different to the first addition as soil properties are different.
Hamer et al. (2005) found that repeated inputs of substrates caused higher priming effects, indicating that the available soil organic C (SOC) pool was not depleted, and priming effects were not limited by availability of SOC. Considering that biochar-caused priming effect is similar to that of other organic inputs, despite its low decomposability, it remains largely unknown whether the biochar previously added into soil would physically protect SOC from microbial decomposition or intrigue higher soil priming effects due to previously added biochar-induced changes to soil properties, like higher pH and microbial activity. This calls for more studies that contribute to our understanding about the impact of continuous input of biochar on soil organic matter decomposition (Maestrini et al. 2015).
The impacts of substrate-induced priming effects as related to microbial community structure have been extensively investigated. In contrast to litter, investigations into the effects of biochar on soil microbial community structure are mainly based on traditional biochemical and C isotopic assays (Watzinger et al. 2014) and few studies investigated on the relationship between soil C priming and microbial community changes. High-throughput sequencing provides robust and comprehensive insights into the phylogenetic diversity and taxonomic composition of microbial communities and biochar-induced priming effects (Whitman et al. 2014). A greater understanding of microbial functions on priming effects in biochar-amended soils needs to be elucidated by linking with microbial community. Thus, we hypothesize that because microbial community after first addition of biochar is different from soil that has never received biochar, the second biochar addition may stimulate different priming effects with different microbial compositions. The objectives were to quantify the primed CO2-C in the biochar-containing soil following repeatedly biochar addition, and find the possible mechanisms, mainly microbial community, for biochar-induced priming effect.
2 Materials and methods
2.1 Soils and biochars
The used biochars were made from Miscanthus giganteus straw. The straw was dried at 105 °C for 24 h, milled to <1 mm and pyrolyzed in a Carbolite CWF 1200 furnace with a sealable retort (Carbolite, Hope, UK), at 350 or 700 °C for 30 min (BC350 and BC700, respectively), while being flushed with argon.
An extremely acidic soil (pH 3.7) was sampled (0–23 cm) with a Dutch auger from the Hoosfield acid strip at Rothamsted Research, UK. Soil had 0.87% of C content and 0.1% of N content. Biochar had a pH value of 7.8 (BC350) and 10.8 (BC700), water-extractable C of 4430 mg C g−1 soil (BC350) and 323 (BC700). More details about the soil and biochars were given by Luo et al. (2011).
2.2 Experiment design
Two portions of the soil were thoroughly mixed with BC350 and BC700 at 50 g C kg−1 soil (d.w. basis), and then incubated for 431 days following moisture adjustment to 50% of water-holding capacity (WHC). The soils were added water every week to keep the moisture by weighing the incubation box, thus avoiding any driedout soil samples during the long period of incubation. They were named as SBC350 and SBC700. Another portion of the soil without adding biochar (SCK) was incubated for 431 days as described above.
After 431 days of incubation, both soils (SBC350 and SBC700) were again separately amended with two biochars of BC350 and BC700 at 50 g C kg−1 soil, named as SR-BC350 and SR-BC700 respectively, and M. giganteus straw (<1 mm, SM-BC350 and SM-BC700), to investigate the priming effects caused by repeated biochar addition and compare the differences between raw material and the corresponding biochar. Nitrogen at 170 μg NH4NO3-N g−1 soil was added to avoid any N limitation, and then incubated for a further 28 days following thorough mixing and adjusting to 50% WHC. SCK received no addition of either biochar or M. giganteus straw during 28 days of incubation was set as control (SR-CK). Also, SR-CK went through the mixing procedure to avoid any disturbing effects different from other treatments.
Each moist soil sample (40 g d.w. basis) was incubated in a 100-ml glass jar inside a 1-l brown glass jar, with 20 ml 1 M NaOH in a vial and 10 ml water in the base of the brown jar. Three jars with only water and NaOH as blanks were also included as above. All the jars were sealed with a rubber bung and incubated in a randomized block design at 25 °C for the 28 days of incubation. The NaOH vials were changed after 1, 3, 7, 14 and 28 days for determination of evolved CO2 and 13C–CO2 (‰). Both biomass C and PLFAs were determined for the soils at the end of incubation. The mineralisation of SOC (C3) was distinguished from biochar (C4) based on the changes of stable isotopic composition (δ13C) over 1, 3, 7, 14 and 28 days of incubation.
2.3 Chemical analysis
2.3.1 Soil CO2 emission
The evolved CO2 during soil incubation was determined according to Tinsley et al. (1951). In brief, 5 ml of solution was mixed with 10 ml deionized water and then titrated with standardized 0.5 M HCl using a TIM840 autotitrator (Radiometer Analytical, Villeurbanne Cedex, France).
2.3.2 13C in soil CO2 emission
To determine the δ13C (‰) of the trapped CO2-C, 8-ml aliquots were added to 8 ml 1.5 M BaCl2 in vials (Aoyama et al. 2000). The resulting BaCO3 precipitate was filtered with a glass fibre filter (90 mm, Whatman GF/A, UK), and then carefully rinsed with deionized water to make sure all BaCO3 precipitate was collected. The precipitate was dried overnight at 80 °C and then scraped off the filter. 13C of the precipitate was analysed with an elemental analyser-isotope ratio mass spectrometer (Sercon Ltd., Crewe, UK).
2.3.3 Soil microbial biomass C
Soil microbial biomass C was determined by fumigation extraction (Vance et al. 1987; Wu et al. 1990). The K2SO4 (0.5 M)-extractable organic C was determined using an organic carbon autoanalyser (Shimadzu, Analytical Sciences, Kyoto, Japan). Soil microbial biomass C (Bc) was calculated from Bc = 2.22 Ec, where Ec = [(organic C extracted from fumigated soil) minus (organic C extracted from non-fumigated soil)].
2.3.4 Phospholipid fatty acid
A modified Bligh Dyer extraction procedure was applied for the extraction of PLFA (Dungait et al. 2013). In brief, Bligh Dyer solvent (KH2PO4-buffered H2O, pH 7.2:MeOH/CHCl3 in 4:10:5 v/v/v) was used to liberate a total lipid extract (TLE) from freeze-dried soils. Silicic fractionation was used to isolate the phospholipid fraction from the TLE using the solvent system: CHCl3 (neutral lipids), (CH3)2CO (glycolipids) and MeOH (polar lipids). The polar lipids were saponified (0.5 M methanolic NaOH; 1 h, 80 °C) and acidified (1 M HCl), and the liberated PLFAs were methylated with acidified dry MeOH (1 h; 80 °C). PLFAs were analysed as their methyl esters using gas chromatography-mass spectrometry (GC-MS; Rothamsted Research North Wyke, Okehampton, UK).
PLFA molecular structures are described using standard nomenclature. The first number refers to the total number of C atoms, and the number after the colon refers to the number of double bonds. A number following a ‘v’ is the location of the first double bond relative to the aliphatic end of the molecule. Notations ‘Me’, ‘OH’ and ‘cy’ are, respectively, methyl, hydroxy and cyclopropane groups and notations ‘i’ and ‘a’, respectively, are iso- and anteiso-branched fatty acids. PLFA biomarkers were grouped as Gramme-positive bacterial (i15:0, a15:0, i16:0, i17:0, a17:0, 7Me-17:0), Gramme-negative bacterial (16:1v5, 16:1v7, 17:1v8, 7,9cy-17:0, 18:1v7, 7,8cy-19:0, 19:1) and fungal (18:2v6, 18:1v9) (Nottingham et al. 2009).
2.4 Calculation
The mineralisation of SOC was calculated based on the changes of stable isotopic composition (δ13C) over time. The standard equation for determining δ13C (‰) is derived from
where R sample is the mass ratio of 13C to 12C of the sample and RVPDB is the mass ratio of 13C to 12C of the international Pee Dee belemnite (PDB) standard (Craig 1953).
According to Amelung et al. (2008), the labelled 13C (%) was then estimated from
where CO2-13C (%) is the proportion of evolved CO2 from SOC (C3), δ treatment is the δ13C (‰) of evolved CO2 in treatment, δC3 is the δ13C (‰) of original soil C without biochar addition and δC4 is the δ13C (‰) from C4 materials (Miscanthus biochar). Thus, the CO2-C produced during the incubation was calculated from
where CO2-Ctreatment is the non-isotopically labelled CO2-C evolved from biochar-amended soil. The primed soil CO2-C with the addition of biochar was calculated from
where CO2-Ccontrol is total CO2-C evolved from control soil without second addition of biochar.
2.5 Statistics
Data meeting assumptions of normality and equality of variances were analysed by ANOVA. Data not meeting the assumptions were logarithmically transformed. All analyses were performed using GenStat software (13th edition). A one-way analysis of variance was undertaken to determine the significance (p < 0.05) of differences between the treatments of priming effects with BC350 and BC700. LSD was chosen for the comparisons between treatments. All treatments were prepared in triplicates.
3 Results
3.1 CO2 evolution
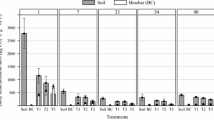
Biochar-unamended soil (SR-CK) had a very low CO2 evolution, less than 2.5 μg C g−1 soil within 28 days of incubation. Both biochar-containing soils of SBC350 and SBC700 had very low CO2 evolution, 32 and 40 μg C g−1 soil, respectively, within 28 days of incubation. The second biochar addition to the corresponding biochar-amended soils caused much more CO2 evolution, 411 and 93 μg C g−1 soil in SR-BC350 and SR-BC700, respectively (Fig. 1). Most CO2 emissions were evolved within the initial 2 weeks.
3.2 δ13C delta value of CO2 and priming effect
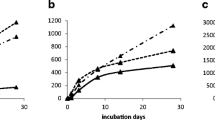
The tested soil cropped with wheat (C3) for many years had a δ13C value of −26.6‰, while the C4 plant of Miscanthus and the derived biochar had much higher δ13C of −11.8 and −12.0‰, respectively, showing that pyrolysis induces very small isotope fractionation (Fig. 2a). The δ13C value of the evolved CO2 ranged from −22.0 to −16.4‰, reaching to maximum at day 3 in SR-BC350, but at day 8 in SR-BC700, and then decreased slowly in both soils during further incubation. In general, the evolved CO2 from SR-BC350 had higher δ13C value (−18.3 to −16.4‰) than those from SR-BC700 (−22.0 to −19.5‰).
Change of δ13C value (a) in evolved (top) and primed CO2 (b) in the soils during the incubation. The bottom dashed line (at the top figure) in a shows δ13C values of C3 control soil (SR-CK,). The upper solid and dotted lines are δ13C values of biochar. SR-BC350 signifies repeated BC350 addition into SBC350 soil, SR-BC700 repeated BC700 addition into SBC700 soil
The primed CO2 that evolved from SR-BC350 and SR-BC700 during 28 days of incubation was 108.0 and 26.9 μg CO2-C g−1 soil, respectively (Fig. 2b), about 72 and 56% of primed CO2 caused by the first addition of biochar (SBC350 and SBC700) (Electronic Supplementary Material, Table S1). The soil of SR-BC350 had much higher priming rate, 3.86 μg CO2-C day−1 g−1 soil on average, than SR-BC700, 0.96 μg CO2-C day−1 g−1 soil. Both soils had a much stronger priming effect in the initial 3 days, 14.8 and 2.16 μg CO2-C day−1 g−1 soil, respectively. Instead of turning feedstock into biochar, Miscanthus caused a much larger priming effect, giving primed soil C losses of 243 μg CO2-C g−1 soil (SM-BC350) and 413 μg CO2-C g−1 soil (SM-BC700), respectively, at the end of incubation (Electronic Supplementary Material, Fig. S1).
3.3 Microbial biomass C and PLFA
Both biochar-containing soils of SBC350 and SBC700 had larger microbial biomass C than biochar-free soil (SCK) with only 22 μg C g−1. The second biochar addition to the previously biochar-amended soils increased biomass C from 84 to 147 μg C g−1 in SR-BC350 and from 48 to 89 μg C g−1 in SR-BC350, respectively (Fig. 3). The total mass of PLFA biomarkers was similar to microbial biomass C, higher in repeated biochar addition soils of SR-BC350 and SR-BC700 (p < 0.05, Fig. 4), compared to the control soil. PLFA followed the order Gramme-positive bacterial > negative bacteria > fungi > actinobacteria (Fig. 4). The most abundant PLFAs were branched saturated fatty acids i15:0, a15:0, i16:0, i17:0, and a17:0 (Gramme-positive bacteria), 18:1w9 (Gramme-negative bacteria) and 18:2w6,9 (fungi). Both soils of repeated biochar addition had lower proportions of Gramme-positive bacteria, 53 and 49% in SR-BC350 and SR-BC700, respectively, compared to 61% in the control soil of SR-CK, while it had a higher proportion of fungi, actinobacteria and Gramme-negative bacterial PLFAs (Fig. 5).
4 Discussion
4.1 Repeated biochar addition-induced priming effects
Our previous study showed biochar-induced priming effects (Luo et al. 2011). We conducted a 28-day-incubation study based on a pre-incubation of 1.5 years, to investigate repeated biochar-induced priming effects. This is mainly because the influence of a single substrate C addition on priming effect is not representative of litterfall and root exudation in many terrestrial ecosystems (Qiao et al. 2014). Instead, repeated or pulses of substrate input may reflect reality, as dead roots, leaf, shoot residues and root exudates are regarded as continuous C inputs with fast and slow decomposition rates (Zhu et al. 2014). Currently, a few studies have examined the effect of continuous input on priming effect (De Nobili et al. 2001; Hamer and Marschner 2005; Kuzyakov 2010). Hamer et al. (2005) observed larger priming effect induced by subsequent substrate additions than the first one in most cases, suggesting that the pool of SOC available for priming performance was not depleted. Duong et al. (2009) found that frequent residue additions increased SOC mineralisation, while Blagodatskaya et al. (2007) argued that this response was lower than that with the second addition. Major et al. (2010) measured less primed CO2 from the soil with the repeated biochar addition at the second year (6% of priming effect) compared with the first year (40%). Similarly, our study showed that the primed CO2 volumes from the second addition of either BC350 or BC700 were slightly lower than those at the first addition (Electronic Supplementary Material, Table S1). This could be explained by the depleted soil C after 431 days of incubation without any fresh C input. Whitman et al. (2014) used two soils with different organic C contents adjusted by pre-incubations of 1 day (high easily mineralizable SOC) and 6 months (low easily mineralizable SOC), and found much higher absolute priming effect in the high organic C soil. How biochar-containing soil with depleted SOC content level (exclude biochar C) responds to continuous (more than twice) biochar addition still remains largely unknown and awaits further research.
Priming effects caused by repeated biochar addition observed in this study cannot be distinguished or attributed to the aged or fresh biochar application. It would be interesting to know the contribution of first addition of biochar to total priming effects after second addition of biochar. Approaches using two stable isotopes (13C and12C) here were limited and can be only partition two sources (biochar C and SOC). More complex methodological approaches, like 14C labelling combined with 13C natural abundance (Luo et al. 2017), which are able to discern three C sources (aged biochar, fresh biochar, native SOC) are needed to understand the contribution of biochar at different addition times and its interactions between the three C sources.
Incorporation of biochar will clearly increase total SOC. The budget of C in biochar-amended soil should consider both the amount of mineralized C from SOM and added C through biochar (Kuzyakov 2010). The conclusion to the SOC content could be justified only if the input and output of C calculation to the biochar soil system in the long term is given (Woolf et al. 2010). If priming effects with single biochar addition can be largely negligible compared to biochar C sequestrated into soil C pool, the primed CO2 emission caused by continuous biochar addition in the biochar-amended soil system needs to be carefully watched. Anyhow, from this study, biochar amendment induced less CO2 evolution from soil compared to fresh organic matter addition (Miscanthus) to biochar-containing soil (Electronic Supplementary Material, Fig. S1). This may indicate that turning raw feedstock into biochar still is an approach to diminish SOC loss if priming effects are ubiquitous following substrate addition (Blagodatskaya and Kuzyakov 2008). Also, we found larger Miscanthus-induced priming effects in BC700 incorporated soil compared to BC350, indicating that pyrolysis temperature can be adjusted to reduce any substrate-induced priming effects in biochar-enriched soil.
4.2 Mechanisms
The mechanisms for biochar-intrigued priming effect are still unclear. Previous studies have shown that labile C within biochar and organic C sorption on the added char sphere may be responsible for the priming effect (Steinbeiss et al. 2009; Jones et al. 2011; Luo et al. 2011; Maestrini et al. 2015; Whitman et al. 2014). The labile C compounds within biochar may intrigue microbes and induce much higher priming effect during the initial several days of incubation following biochar addition (Luo et al. 2011), making no difference from priming effect induced by any substrates containing available organic C (Blagodatskaya and Kuzyakov 2008). In addition, biochar-caused priming effect may also attribute to the improvement of soil physical and chemical properties by biochar addition. Biochar amendment usually results in low bulk density, but high values of soil porosity (Verheijen et al. 2010), middle aggregates (Atkinson et al. 2010), pH (Yuan et al. 2011), CEC (Cheng et al. 2006; Jien and Wang 2013) and nutrients (Chan 2009). The changes of soil physical structure may be a benefit to microbial colonisation, thus escaping from preying and even exposing organic matter to microbial attack (Luo et al. 2013). The improvements of soil physio-chemical properties can enhance soil microbial activity (Steinbeiss et al. 2009) and favour certain groups of microorganisms, like mycorrhizal fungi (Paterson et al. 2008) and Gramme-negative bacteria (Cretoiu et al. 2014). Similarly, in our study, soils with the second addition of biochar had enhanced fungi, actinobacteria and Gramme-negative bacterial PLFAs (Fig. 4). Fungi and actinobacteria are usually regarded as the k-strategists responsible for the decomposition of a more complex soil organic matter, causing real priming effect in the relatively long term (Fontaine et al. 2003; Kuzyakov 2010). In the previous incubation study (early stage in pre-incubation of 431 days), the fast initiation of priming effects following biochar indicated r-strategist might be stimulated by available C in biochar in the early stage (Luo et al. 2011), which could more rapidly degrade and assimilate the available biochar C fraction, and thus enhance microbial biomass turnover and cause apparent priming effects (Blagodatskaya and Kuzyakov 2008). We exclude ‘apparent’ priming effects caused by increased biomass C turnover as the microbial biomasses were less than the primed CO2 in all treatments (Figs. 2b and 3).
After the labile biochar C vanished, k-strategists might be largely activated in the late stage of 431 days of incubation. Another addition of biochar might continuously stimulate k-strategists (including fungi, actinobacteria and Gramme-negative bacterial PLFAs) after further 28 days of incubation (Fig. 4). However, research is needed to determine the specific microbial groups at phylum taxon level and identify their contributions to SOC mineralisation in the char sphere, using advanced techniques e.g. DNA-SIP, coupled with high-throughput sequencing.
5 Conclusions
Our research observed the second addition of fresh biochar-induced priming effects, which gave the primed CO2 of 108.0 μg CO2-C g−1 soil and 26.9 μg CO2-C g−1 evolved from BC350-containing soil (SR-BC350) and BC700-containing soil (SR-BC700) during 28 days of incubation. Priming effects caused by the first addition of biochar had the same order but less amounts of soil C losses (about 72 and 56%). This might be because of the depleted soil C after 431 days of incubation without any fresh C input. Also, it may be attributed to an altered soil microbial community with lower proportions of Gramme-positive bacteria, and higher proportion of fungi, actinobacteria and Gramme-negative bacterial phospholipid fatty acids (PLFAs). Our research provided insights into the second addition of biochar-induced priming effects on biochar-enriched soil (received first addition of biochar). How biochar-containing soil responds to continuous (more than twice) biochar addition or other substrates (root exudates) still remains largely unknown and awaits further research.
References
Amelung W, Brodowski S, Sandhage-Hofmann A, Bol R (2008) Combining biomarker with stable isotope analyses for assessing the transformation and turnover of soil organic matter. In: Donald LS (ed) Adv Agron, vol Volume 100. Academic Press, Burlington, pp 155–250
Aoyama M, Angers DA, N’Dayegamiye A, Bissonnette N (2000) Metabolism of 13C-labeled glucose in aggregates from soils with manure application. Soil Biol Biochem 32:295–300
Atkinson CJ, Fitzgerald JD, Hipps NA (2010) Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 337:1–18
Awad YM, Lee SS, Ok YS, Kuzyakov Y (2017) Effects of biochar and polyacrylamide on decomposition of soil organic matter and 14C-labeled alfalfa residues. J. Soils Sediments 17:611–620
Blagodatskaya E, Blagodatsky S, Anderson T, Kuzyakov Y (2007) Priming effects in Chernozem induced by glucose and N in relation to microbial growth strategies. Appl Soil Ecol 37:95–105
Blagodatskaya Е, Kuzyakov Y (2008) Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol Fertil Soils 45:115–131
Chan KY, Van Zwieten L, Meszaros I, Downie A, Joseph S (2007) Agronomic values of greenwaste biochar as a soil amendment. Aust J Soil Res 45:629–634
Chan KY, Xu, Z. (2009) Biochar: nutrient properties and their enhancement. In: Lehmann J, Joseph, S (ed) Biochar for environmental management: science and technology. Earthscan, London, pp 67–84
Cheng CH, Lehmann J, Thies JE, Burton SD, Engelhard M (2006) Oxidation of black carbon by biotic and abiotic processes. Org Geochem 37:1477–1488
Craig H (1953) The geochemistry of the stable carbon isotopes. Geochim Cosmochim Acta 3:53–92
Cretoiu MS, Kielak AM, Schluter A, van Elsas JD (2014) Bacterial communities in chitin-amended soil as revealed by 16S rRNA gene based pyrosequencing. Soil Biol Biochem 76:5–11
De Nobili M, Contin M, Mondini C, Brookes PC (2001) Soil microbial biomass is triggered into activity by trace amounts of substrate. Soil Biol Biochem 33:1163–1170
Dungait JAJ, Kemmitt SJ, Michallon L, Guo S, Wen Q, Brookes PC, Evershed RP (2013) The variable response of soil microorganisms to trace concentrations of low molecular weight organic substrates of increasing complexity. Soil Biol Biochem 64:57–64
Duong TTT, Baumann K, Marschner P (2009) Frequent addition of wheat straw residues to soil enhances carbon mineralization rate. Soil Biol Biochem 41:1475–1482
Fang Y, Singh BP, Singh B (2014) Temperature sensitivity of biochar and native carbon mineralisation in biochar-amended soils. Agric Ecosyst Environ 191:158–167
Fontaine S, Mariotti A, Abbadie L (2003) The priming effect of organic matter: a question of microbial competition? Soil Biol Biochem 35:837–843
Hamer U, Marschner B (2005) Priming effects in soils after combined and repeated substrate additions. Geoderma 128:38–51
Jien S-H, Wang C-S (2013) Effects of biochar on soil properties and erosion potential in a highly weathered soil. Catena 110:225–233
Jien SH, Wang CC, Lee CH, Lee TY (2015) Stabilization of organic matter by biochar application in compost-amended soils with contrasting pH values and textures. Sustainability (Switzerland) 7:13317–13333
Jones DL, Murphy DV, Khalid M, Ahmad W, Edwards-Jones G, DeLuca TH (2011) Short-term biochar-induced increase in soil CO2 release is both biotically and abiotically mediated. Soil Biol Biochem 43:1723–1731
Kuzyakov Y (2010) Priming effects: interactions between living & dead organic matter. Soil Biol Biochem 42:1363–1371
Kuzyakov Y, Friedel JK, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–1498
Lehmann J, Rillig M, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota—a review. Soil Biol Biochem 43:1812–1835
Lehmann J, Sohi SP (2008) Comment on “fire-derived charcoal causes loss of forest humus”. Science 321:1295
Liang BQ et al (2010) Black carbon affects the cycling of non-black carbon in soil. Org Geochem 41:206–213
Lu W, Ding W, Zhang J, Li Y, Luo J, Bolan N, Xie Z (2014) Biochar suppressed the decomposition of organic carbon in a cultivated sandy loam soil: a negative priming effect. Soil Biol Biochem 76:12–21
Lu W, Zhang H (2015) Response of biochar induced carbon mineralization priming effects to additional nitrogen in a sandy loam soil. Appl Soil Ecol 96:165–171
Luo Y, Durenkamp M, De Nobili M, Lin Q, Brookes PC (2011) Short term soil priming effects and the mineralisation of biochar following its incorporation to soils of different pH. Soil Biol Biochem 43:2304–2314
Luo Y, Durenkamp M, De Nobili M, Lin Q, Devonshire BJ, Brookes PC (2013) Microbial biomass growth, following incorporation of biochars produced at 350 °C or 700 °C, in a silty-clay loam soil of high and low pH. Soil Biol Biochem 57:513–523
Luo Y, Zang H, Yu Z, Chen Z, Gunina A, Kuzyakov Y, Xu J, Zhang K, Brookes PC (2017) Priming effects in biochar enriched soils using a three-source-partitioning approach: 14C labelling and 13C natural abundance. Soil Biol Biochem 106:28–35
Maestrini B, Nannipieri P, Abiven S (2015) A meta-analysis on pyrogenic organic matter induced priming effect. GCB Bioenergy 7:577–590
LuoMajor J, Lehmann J, Rondon M, Goodale C (2010) Fate of soil-applied black carbon: downward migration, leaching and soil respiration. Glob Chang Biol 16(4):1366–1379
Nocentini C, Guenet B, Di Mattia E, Certini G, Bardoux G, Rumpel C (2010) Charcoal mineralisation potential of microbial inocula from burned and unburned forest soil with and without substrate addition. Soil Biol Biochem 42:1472–1478
Nottingham AT, Griffiths H, Chamberlain PM, Stott AW, Tanner EVJ (2009) Soil priming by sugar and leaf-litter substrates: A link to microbial groups. Appl Soil Ecol 42(3):183–190
Paterson E, Osler G, Dawson LA, Gebbing T, Sim A, Ord B (2008) Labile and recalcitrant plant fractions are utilised by distinct microbial communities in soil: independent of the presence of roots and mycorrhizal fungi. Soil Biol Biochem 40:1103–1113
Qiao N, Schaefer D, Blagodatskaya E, Zou X, Xu X, Kuzyakov Y (2014) Labile carbon retention compensates for CO2 released by priming in forest soils. Glob Chang Biol 20:1943–1954
Sohi SP, Krull E, Lopez-Capel E, Bol R (2010) A review of biochar and its use and function in soil. Adv Agron 105:47–82
Steinbeiss S, Gleixner G, Antonietti M (2009) Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol Biochem 41:1301–1310
Thiessen S, Gleixner G, Wutzler T, Reichstein M (2013) Both priming and temperature sensitivity of soil organic matter decomposition depend on microbial biomass—an incubation study. Soil Biol Biochem 57:739–748
Tinsley J, Taylor TG, Moore JH (1951) The determination of carbon dioxide derived from carbonates in agricultural and biological materials. Analyst 76:300–310
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Verheijen FGA, Jeffery S, Bastos AC, van der Velde MID (2010) Biochar application to soils: a critical scientific review of effects on soil properties, processes and functions. Office for the Official Publications of the European Communities, Luxembourg
Wardle DA, Nilsson MC, Zackrisson O (2008a) Fire-derived charcoal causes loss of forest humus. Science 320:629
Wardle DA, Nilsson MC, Zackrisson O (2008b) Response to comment on “fire-derived charcoal causes loss of forest humus”. Science 321
Watzinger A et al (2014) Soil microbial communities responded to biochar application in temperate soils and slowly metabolized13C-labelled biochar as revealed by13C PLFA analyses: results from a short-term incubation and pot experiment. Eur J Soil Sci 65:40–51
Weng Z, Van Zwieten L, Singh BP, Kimber S, Morris S, Cowie A, Macdonald LM (2015) Plant-biochar interactions drive the negative priming of soil organic carbon in an annual ryegrass field system. Soil Biol Biochem 90:111–121
Whitman T, Zhu Z, Lehmann J (2014) Carbon mineralizability determines interactive effects on mineralization of pyrogenic organic matter and soil organic carbon. Environ Sci Technol 48:13727–13734
Woolf D, Amonette JE, Street-Perrott FA, Lehmann J, Joseph S (2010) Sustainable biochar to mitigate global climate change. Nat Commun 1:1–9
Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC (1990) Measurement of soil microbial biomass C by fumigation-extraction: an automated procedure. Soil Biol Biochem 22:1167–1169
Yuan J-H, Xu R-K, Zhang H (2011) The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour Technol 102:3488–3497
Zhu B, Gutknecht JLM, Herman DJ, Keck DC, Firestone MK, Cheng W (2014) Rhizosphere priming effects on soil carbon and nitrogen mineralization. Soil Biol Biochem 76:183–192
Acknowledgements
We thank J. Zhou for chemical analyses, A. Duffey for δ13C (‰) analyses and R. P. White for statistical analyses. This work was supported by the National Scientific Foundation Council (No. 41301250, 41671233).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Yong Sik Ok
Electronic supplementary material
ESM 1
(DOCX 113 kb)
Rights and permissions
About this article
Cite this article
Luo, Y., Lin, Q., Durenkamp, M. et al. Does repeated biochar incorporation induce further soil priming effect?. J Soils Sediments 18, 128–135 (2018). https://doi.org/10.1007/s11368-017-1705-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-017-1705-5