Abstract
Purpose
Plantation is an important strategy for forest restoration and carbon (C) storage. Plantations with different tree species could significantly affect soil properties, including soil pH, soil nutrient content, soil microbial activities, and soil dissolved organic C. Changes in these abiotic and biotic factors could regulate mineralization of soil organic C (SOC). However, it remains unclear to what extent these factors affect the mineralization of SOC under different tree species plantations.
Materials and methods
Soil was collected at 0–10 cm depth from plantations with Pinus elliottii Engelm. var. elliottii, Araucaria cunninghamii, and Agathis australis, respectively, in southeast Queensland, Australia. Soil samples were assayed for soil organic C; organic N and mineralization of SOC; soil particle size; total C, N, and P; and pH. In addition, a 42-day laboratory incubation with substrate additions was done to examine the influence of different substrates and their combinations on bio-available organic C.
Results and discussion
Our results suggested that SOC mineralization was mainly determined by soil pH and soil C content among plantations with different tree species, whereas SOC mineralization was not correlated with soil N and P contents. These results were further confirmed by the substrate addition experiments. SOC mineralization of soils from slash pine showed greater response to C (glucose) addition than soils from other two plantations, which suggested significant differences in SOC mineralization among plantations with different tree species. However, neither N addition nor P addition had significant effects on SOC mineralization.
Conclusions
Our results indicated that plantations with different tree species substantially affect the mineralization and stability of soil organic C pool mainly by soil pH and soil C content.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The stock of soil organic carbon (SOC), which is approximately 1500 Pg in the upper 100 cm and accounts for two thirds of the global terrestrial carbon pool (Batjes 1996), plays a critical role in the carbon cycle between soil and atmosphere (Heimann and Reichstein 2008; Schmidt et al. 2011). Given the large stock of SOC in soils, even subtle changes in the mineralization of SOC can trigger a severe feedback to atmospheric CO2 concentrations (Jenkinson et al. 1991; Álvaro-Fuentes et al. 2012). It is believed that the mineralization of SOC is sensitive to management practice such as plantations of tree species. Although plantation of tree species has widely existed worldwide, especially in tropical and subtropical area, how the mineralization of SOC responds to the plantations with different tree species is largely unknown. Therefore, understanding the effect of plantations with different tree species on the mineralization of SOC is important to evaluate the ecological effects of plantations and optimize the designs of tree plantations.
Three dominant hypotheses have been proposed to explain the mechanisms underlying the effect of plantations with different tree species on SOC mineralization (Table 1). The first hypothesis is that plantations with different tree species can affect the mineralization of SOC through changing soil pH (Finzi et al. 1998; Berthrong et al. 2009). This is particularly important for plantations with coniferous species as they can produce acidic leaf litters and root exudation of H+, which in turn lower soil pH (Johnson et al. 1991). The second hypothesis is that plantation of tree species can affect the mineralization of SOC by directly changing soil microbial activities, especially by altering microbial biomass carbon (MBC) and dissolved organic carbon (DOC) in soil (Grayston et al. 1997; Jenkins 2002; Ehrenfeld et al. 2005). The third hypothesis is that plantation of tree species will affect soil nutrient availability (i.e., nitrogen, phosphorus and soil organic matter) via substrate availability, which in turn had a great influence on the mineralization of SOC (Elliott 1986). Although all these three hypotheses may simultaneously affect the mineralization of SOC, given the fact that different tree species differed in the ability to change soil pH, nitrogen (N), and phosphorus (P) availability (Ste-Marie and Paré 1999; Burton et al. 2007) and plant–microbe associations also differed among species (Chen and Xu 2008; Vivanco and Austin 2008), it is possible that these mechanisms contributed unequally to the mineralization of SOC in plantations with different tree species. However, studies on the relative contribution of these three mechanisms to changes in the mineralization of SOC in plantations with different tree species were limited.
The present study was designed to investigate the impacts of 73-year-old plantations with slash, hoop, and kauri pines on the rate of mineralization of SOC in subtropical Australia. The plantations with the three species had similar climatic condition and management actives but varied in soil pH, total C, C/N ratio, DOC, and dissolved inorganic nitrogen (DIN) due to the different abilities between plant and soil interaction (Lu et al. 2012), which provided an ideal chance to study the influence of plant-soil interaction on the mineralization of SOC. Particularly, we expected to answer the following questions: (1) how do soil pH, substrates availability, and microbial activity affect the mineralization of SOC in the three plantations of pine species? (2) What is the relative contribution of soil pH, substrates availability and microbial activity to the mineralization of SOC in the three plantations of pine species?
2 Materials and methods
2.1 Site description and soil sampling
The site is located at Cooloola, Tin Can Bay, southeast Queensland, Australia (25° 56′ 49″ S, 153° 5′ 27″ E). The altitude at the site is 43 m above sea level. The soil is classified as gleyic acrisol (FAO Soil Classification), developed from quartz-rich sandstones. Annual rainfall varies from 741 to 2106 mm, with an average of 1287 mm. About 40% of annual rainfall (501 mm) is distributed in summer (Dec–Feb), while about 15% (192 mm) falls in winter (Jun–Aug). Winter temperatures range from 7 to 23 °C and summer temperatures from 18 to 30 °C.
The tree species, Pinus elliottii Engelm. var. elliottii, Araucaria cunninghamii, and Agathis australis, were established in 1921 on the original banana farm, with the plot of 1.087, 0.308, and 0.428 ha, respectively. No fertilizer has been applied on these plots since tree species had been planted. After brush tending in 1940, 1948, and merchantable thinning in 1963, the final stocking densities of P. elliottii var. elliottii, A. cunninghamii, and Agathis robusta were 140, 120, and 120 trees ha−1, respectively.
Soil samples were taken in the November in 2008 when the plantations of these three tree species were 73 years old. Within each plantation, four 10 × 20 m subplots were randomly placed for soil sampling. After removing litter and fermentation layers, a total of ten soil cores at the 0–10 cm depths were randomly collected within each subplot using a 7.5 cm diameter auger and mixed as a composite sample in situ. Then, soil samples were placed in a sterile plastic bag, sealed and transported to the laboratory with ice for further analysis. After removing fine roots and large debris and sieving through 2 mm mesh, the collected soil samples were then divided into two subsamples. One subsample was stored at 4 °C for analysis of soil DOC, dissolved organic nitrogen (DON), DIN, MBC, and microbial biomass nitrogen (MBN). One subsample was air dried and stored at room temperature to analyze of soil particle size; total C, N, and P; and pH.
2.2 Measurement of biologically available soil organic C
Biologically available soil organic C (bio-available organic C) was determined using the incubation method as described by Chen et al. (2004). In brief, 25 g of air-dried soil was adjusted to 60% of the field capacity and aerobically incubated at 22 °C in a 1-L sealed glass jar. Then, CO2 evolved from soil was trapped in 0.1 M NaOH and measured by 0.1 M HCl titration after 1, 3, 7, 14, 21, 28, 35, and 42 days, respectively. The bio-available organic C was estimated by calculating the cumulative production of CO2 from soils during the 42-day incubation.
To examine the influence of different substrates and their combinations on bio-available organic C, we amended the soil samples of each plantation with glycine (Acros Organics, NJ, USA), NH4NO3 (Fisher Scientific Ltd., Leicestershire, UK) and NaPO4 (monobasic, monohydrate; Acros Organics), and each pairwise combination of these four substrates (six combinations) as well as a distilled water control. The substrates were added in solution with enough water to raise soil to 60% of WHC. Particularly, the organic compounds were added at a rate of 15 mg substrate C per g soil C (Table 2). To ensure that equal amounts of N were added the inorganic forms, NH4NO3 was added at the rate of 8.97 mg N per g soil C (Table 2). NaPO4 was added at the rate of 0.625 mg P per g soil C (Table 2). Then, the rate of CO2 production was monitored for 42 days for each soil samples after substrate addition. Because the total number of soil samples to be incubated (132) exceeded the number of chambers in the respirometer, the experiment was run twice with two replicates for each soil types in each run. When the first run was conducted, the soil samples for the second run were stored at 4 °C. Results from two-way analysis of variance (ANOVA) shown that, in all substrate additions, there was no significant bio-available organic C variation in between two runs (P > 0.282).
2.3 Measurement of other soil properties
Soil particle size and bulk density were measured using the standard methods described by Rayment and Higginson (1992). Soil pH was determined in 1:5 (v/v) soil/water extracts using a combination glass electrode and moisture by drying at 105 °C for 48 h. Soil total C and N were determined using an isotope ratio mass spectrometer with a Eurovector Elemental Analyzer (Isoprime-EuroEA 3000, Milan, Italy) (Lu et al. 2012). Soil microbial biomass C and N were determined by chloroform fumigation–extraction based on the method developed by Brookes et al. (1985).
2.4 Statistical analysis
One-way ANOVA, followed by Fisher’s least significant difference (LSD) test, was employed to examine the effect of different tree species on MBC, MBN, dissolved organic C and N, bio-available C, and other soil properties. The normality of all data was checked before ANOVA. Pearson’s correlation coefficient was used to assess the relationship between soil pH and other soil parameters. These chemical and biochemical parameters were also subject to the principal component analysis (PCA) to determine if tree species had distinct impacts on the soils. All statistical analyses were performed using SPSS 17.0 software (SPSS Inc., USA).
3 Results
3.1 Soil properties
Surface litter biomass in slash pine (13.2 t ha−1) was greater than that in hoop (12.3 t ha−1) and kauri (11.2 t ha−1) pines. Without addition of substrate, soil pH in the slash pine plantation was lower than that in hoop and kauri pine plantations (all P < 0.05) (Table 3). In addition, total C, soil moisture, and C/N ratio in slash pine plantation were greater than that in hoop and kauri pines plantations (Table 3), whereas no significant differences were found in total N and total P concentrations among these three pine plantations (Table 3). Among these three pine plantations, slash–pine plantation had the lowest DOC concentrations (88.67 ± 13.06 mg kg−1), and hoop–pine plantation had the highest DOC concentrations (149.84 ± 13.83 mg kg−1) (Table 3). The average concentration of MBC ranged from 62.5 to 147.63 mg kg−1, and the concentrations of MBC in slash and kauri pine plantations were greater than that in hoop pine plantation (P < 0.01).
Soil pH in all plantations was higher under the substrate addition treatment than that under no substrate addition (Lu et al. 2012). Soil pH under slash pine (pH 4.5) was lower compared with those under hoop (pH 6.0) and kauri (pH 6.2) pines (Table 3).
3.2 KCl-extractable organic C and N
DON concentrations ranged from 8.37 to 10.98 mg N kg−1, accounting for 1.55–2.11% of total N (Table 3), but the differences among the three pine plantations were not significant. DOC concentrations ranged from 88.67 to 149.84 mg kg−1, with greater concentrations in hoop and kauri pines, although the differences among tree species were not significant (Table 3). In addition, the differences of DOC/DON ratio among these three plantations were not significant (Table 3).
3.3 Soil microbial biomass C and N
The average concentration of MBC was 62.5–147.63 mg kg−1 (Table 3). Concentrations of MBC were greater in soils under slash and kauri pine than hoop pine (P < 0.01). The average concentration of MBN was 8.49–24.59 mg kg−1. A similar trend (to MBC) was observed in soil MBN across different plantations (Table 3), but the MBN was not significantly different among all plantations.
3.4 Soil total CO2 production
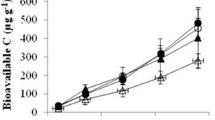
Soil total CO2 production in soils was measured as the cumulative CO2 evolved during the 42-day incubation and shown in Figs. 1 and 2. In all three soils, NH4NO3, Na2HPO4, and NH4NO3 together with Na2HPO4 addition had no significant effect on total CO2 production (Fig. 1). When the results from all three soils were considered, the addition of both glucose and one or two substrates together significantly increased total CO2 production relative to the controls, with an increase of total CO2 production by 11, 24, and 2% in the slash, hoop, and kauri pine soils, respectively. However, these trends of all soils were not statistically significant.
Total soil CO2 production from plantations with different tree species under different substrate addition treatments during the 42-day incubation. Microbial biomass carbon (MBC) was determined for the soils sampled in the 42nd day. Error bars represented mean ± SE (n = 3). For a given treatment, bars with the same letters were not significantly different in Duncan’s multiple range tests reported from one-way ANOVA (P > 0.05). CK control (dH2O), C glucose, N NH4NO3, P Na2HPO4, CN glucose + NH4NO3, CP glucose + Na2HPO4, NP NH4NO3 + Na2HPO4, CNP glucose + NH4NO3 + Na2HPO4
Changes in respiration rate over the 42-day incubation period in each of the three soils for each substrate addition treatment (mean ± SE, n = 3). Significant differences between soils from different tree species are indicated as follows: ns P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001
Total CO2 production increased significantly when NH4NO3 and Na2HPO4 were added (Fig. 1), with glucose addition resulting in significantly more CO2 production than no glucose addition (P < 0.001). The addition of glucose increased CO2 production beyond that observed for either substrate separately in all soils (P < 0.001). The addition of the glucose had little effect on CO2 production compared with adding both glucose and one or two inorganic substrates on their own.
3.5 Relationships between soil properties and soil total CO2 production
Soil total CO2 production was significantly positively correlated with soil DOC, moisture, and pH (Fig. 3b, e, h), whereas significantly negatively with soil total carbon concentration and soil moisture (Fig. 3a, R 2 = 0.47, P = 0.024; Fig. 3h, R 2 = 0.5388, P = 0.0244). There was no correlation between soil total CO2 production and total P, total N, DON, DIN, MBC, and MBN (Fig. 3c, d, f, g, i, j).
Relationships between total CO2 production during the 42-day incubation and soil properties, including total phosphorus (TP), total carbon (TC), total nitrogen(TN), dissolved organic carbon (DOC), dissolved organic nitrogen (DON), dissolved inorganic nitrogen (DIN), moisture, pH, microbial biomass carbon (MBC), and microbial biomass nitrogen (MBN) under three 73-year-old pine plantations
4 Discussion
4.1 The effects of plantations with coniferous species on soil properties
Our results suggested that 73-year-old plantations with different tree species had significant effects on soil properties. Soils from different plantations mainly differed in soil pH and labile organic matter (i.e., DOC; Table 1) (Lu et al. 2012). Soil pH under slash pine (pH 4.5) was lower compared with those under hoop (pH 6.0) and kauri (pH 6.2) pines (Table 1). Soils under coniferous species were usually acidified because of the production of acidic leaf litters (Jongkind et al. 2007; Menyailo et al. 2002). Greater amount of acidic litters under slash pine might explain why soil under slash pine has lower pH compared with soils under hoop and kauri pines (Lu et al. 2012).
Soil C and N contents can be largely different under plantations with different tree species because of root–soil interactions and the production of litters with different quantity and quality, which could significantly alter soil environment and microbial communities (Menyailo et al. 2002; Chen and Xu 2008; Vivanco and Austin 2008; Witt and Setälä 2010). In our study, soil pH was negatively correlated with soil total C content but positively correlated with soil DOC (Fig. 3). Lower soil pH under slash pine favored the accumulation of soil C and thus led to lower amount of DOC compared with hoop and kauri pines. Our results were consistent with previous laboratory studies that DOC positively correlated with soil pH (Curtin and Smillie 1986; You et al. 1999). Soil acidification could decrease DOC through various mechanisms, i.e., decreased organic matter solubility (Erich and Trusty 1997; Tombácz et al. 1999), decreased microbial activity (Guggenberger et al. 1994), or increased Al3+ toxicity (Castro and Logan 1991).
In our study, soil microbial respiration under slash pine was much lower than that under hoop and kauri pines, but soil microbial biomass C and N under slash pine were greater than hoop pine and lower than kauri pines (Table 3). Soil pH is an important factor controlling microbial composition and activities (Kemmitt et al. 2006). In our study, microbial respiration rate was positively correlated with soil pH (Fig. 3), but there was no significant correlation between soil pH and MBC or MBN (Table 3). Therefore, rather than affecting MBC or MBN, lower soil pH under slash pine could decrease microbial activity compared with hoop and kauri pines and thus limit microbial decomposition and lead to greater C accumulation in the soils under slash pine.
Soil nutrients could affect tree growth, but trees could in turn affect soil nutrient content and cycling (i.e., nutrient absorption, litter production, mineralization). In our study, soils under slash pine had lower total P concentration, C/N ratio, lower DON, and DIN than soils under other two species (Table 3). But there was no significant difference in total N concentration among soils under these three plantations (Table 3). Slash pine had lower amounts of N, P, and K in the litter fall than other conifers (Gholz et al. 1985; Maggs 1985). It has been reported that hoop pine litter C was predominately in a recalcitrant form and associated N might not be rapidly released during decomposition (Bubb et al. 1998), while kauri pine tends to accumulate relatively recalcitrant N in the forest floor materials (Silvester 2000).
4.2 Correlations between soil properties and soil carbon mineralization
Soil carbon pool is an important factor of soil carbon cycle, which will affect the soil original SOC mineralization dynamic and microbial community (Stewart et al. 2009; Wang et al. 2014). Previous studies have shown that soils with high C content could have high SOC mineralization rate. Our results suggested that soil carbon content was one of the critical factors determining the SOC mineralization (Stewart et al. 2008; Kuzyakov 2011). As a biological process, soil carbon mineralization in laboratory was mainly determined by substrate availability and microbial composition and activities. In our study, soil carbon mineralization only significantly correlated with DOC, moisture, and soil pH, with no significant correlations with soil N and P concentrations or DON, MBC, and MBN (Fig. 3). Since soil pH could affect DOC and microbial activities, our results indicated that substrate availability and microbial activities were two key factors in determining soil carbon mineralization.
These results supported hypotheses 1 and 2 that tree species could affect soil carbon mineralization via affecting soil pH and DOC availability. Our results did not support hypotheses 3, because although tree species could change soil microbial community, such changes could not lead to modifications of soil carbon mineralization.
Meanwhile, effects of soil pH on soil carbon mineralization depends on substrate availability. Soil pH explained a greater proportion of variance in mineralization under no C addition treatments than under C addition treatments (Fig. 4a, b), indicating that the magnitude of effects of soil pH on soil carbon mineralization decreased with substrate availability. Furthermore, effects of C addition on CO2 production were not affected by soil pH (Fig. 4c ), indicating that soil pH affect CO2 production mainly through changes in substrate availability rather than microbial activity. Decreased soil pH could decrease bacterial growth but increase fungi growth, so total CO2 production was less affected by soil pH than microbial composition because of the functional redundancy in carbon mineralization (Rousk et al. 2009).
4.3 Effects of substrate addition on soil carbon mineralization
In our study, neither NH4NO3 nor Na2HPO4 addition had significant effects on total CO2 production (Fig. 1; Table 4), indicating that soil microbial respiration was limited neither by N nor by P availability. One possible explanation is that N addition had dual effects on soil organic carbon decomposition. While there are positive effects on decomposition, higher N availability could promote the formation of more recalcitrant compounds during lignin decomposition and thus reduce the overall rate of lignin decomposition (Berg 2000). In previous studies, N additions could increase (Cheshire and Chapman 1996; Fierer et al. 2003), decrease (Söderström et al. 1983; FOG 1988; Cheshire and Chapman 1996; Fierer et al. 2003; Bradford et al. 2008; Ouyang et al. 2008), or have no effects (Sjöberg and Persson 1998; Yoshitake et al. 2007) on soil carbon mineralization. However, P addition generally stimulated soil respiration rates (Amador and Jones 1993; Cheshire and Chapman 1996; Fierer et al. 2003; Bradford et al. 2008; Ouyang et al. 2008), with few negative results observed. Meanwhile, some studies found significant interactions of N and P additions on soil organic carbon mineralization (Hartley et al. 2010). Amador and Jones (1993) and Cheshire and Chapman (1996) found that N additions decrease respiration rates only when the natural P availability was low but increase or have no effects when P availability was high. Our results and those studies suggested that N and P additions do not consistently increase soil respiration rates, even in ecosystems characterized by low N and P availabilities.
As the C source of soil microbial respiration, glucose addition directly increased soil respiration rates, but the C effects depended on the sampling days. CO2 production greatly increased under C addition at the third day and then quickly decreased, with no significant changes at the 42nd day. Glucose addition could also indirectly increase CO2 production through priming effects. Although our study did not directly determine the priming effects, soil microbial biomass C was increased by C addition even at the 42nd day. In our experiment, the amount of glucose-C added was approximately equal to the size of the microbial biomass-C pool. Such amount of glucose addition could initially increase microbial biomass followed by a subsequent decline (Schneckenberger et al. 2008) and therefore increased microbial biomass turnover, potentially contributing to the positive priming effect (Hartley et al. 2010). Therefore, our results supported that the priming effects may be caused by an acceleration of microbial biomass turnover in the medium term (Blagodatskaya et al. 2007; Blagodatskaya and Kuzyakov 2008).
In our study, effects of C addition on CO2 production was differed among soils from different tree species. Soils under kauri pine have a greater response to C addition than soils under slash and hoop pines. However, given that soils under kauri pine was very similar to soils under hoop pine in most soil properties (Table 3), the underlying mechanisms of plant species affecting C addition effects were unclear. For example, although soil pH could significantly affect CO2 production, soil pH had no significant correlation with C addition effects on CO2 production (Fig. 4). Further studies could focus on the mechanisms why C addition effects differed among soils under different tree species.
5 Conclusions
Our results clearly demonstrated that soils under slash pine had lower pH, DOC, and greater total C content than under hoop and kauri pines. But soil N concentration (i.e., total N, DON, DIN) and total P concentration were similar among soils under different plantations. Soils under hoop pine had the lowest MBC and MBN. CO2 production was negatively correlated with soil pH and total C content, but positively correlated with DOC. However, CO2 production did not correlate with soil N or P content, MBC, and MBN. These results supported the hypotheses that different tree species could affect soil carbon mineralization through changing soil pH and DOC but did not support the pathways of changing soil nutrient availability and soil microbial community. These conclusions were further supported by a substrate and nutrient addition experiments, which CO2 production was significantly increased by glucose addition but did not respond to N or P addition. Meanwhile, neither N nor P addition could affect the differences in soil carbon mineralization under different trees, but the differences would be decreased under glucose addition. Although slash pines could decrease soil pH and soil fertility, plantation with slash pine is a better strategy in maintaining long-term soil carbon stock through decreasing SOC mineralization.
References
Álvaro-Fuentes J, Easter M, Paustian K (2012) Climate change effects on organic carbon storage in agricultural soils of northeastern Spain. Agric Ecosyst Environ 155:87–94
Amador J, Jones RD (1993) Nutrient limitations on microbial respiration in peat soils with different total phosphorus content. Soil Biol Biochem 25:793–801
Batjes NH (1996) Total carbon and nitrogen in the soils of the world. Eur J Soil Sci 47:151–163
Berg B (2000) Litter decomposition and organic matter turnover in northern forest soils. Forest Ecol Manag 133:13–22
Berthrong ST, Jobbágy EG, Jackson RB (2009) A global meta-analysis of soil exchangeable cations, pH, carbon, and nitrogen with afforestation. Ecol Appl 19:2228–2241
Blagodatskaya Е, Kuzyakov Y (2008) Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol Fert Soils 45:115–131
Blagodatskaya EV, Blagodatsky SA, Anderson TH, Kuzyakov Y (2007) Priming effects in Chernozem induced by glucose and N in relation to microbial growth strategies. Appl Soil Ecol 37:95–105
Bradford MA, Davies CA, Frey SD, Maddox TR, Melillo JM, Mohan JE, Reynolds JF, Treseder KK, Wallenstein MD (2008) Thermal adaptation of soil microbial respiration to elevated temperature. Ecol Lett 11:1316–1327
Brookes PC, Landman A, Pruden G, Jenkinson D (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Bubb K, Xu Z, Simpson J, Saffigna P (1998) Some nutrient dynamics associated with litterfall and litter decomposition in hoop pine plantations of southeast Queensland, Australia. Forest Ecol Manag 110:343–352
Burton J, Chen C, Xu Z, Ghadiri H (2007) Gross nitrogen transformations in adjacent native and plantation forests of subtropical Australia. Soil Biol Biochem 39:426–433
Castro C, Logan T (1991) Liming effects on the stability and erodibility of some Brazilian Oxisols. Soil Sci Soc Am J 55:1407–1413
Chen CR, Xu ZH (2008) Analysis and behavior of soluble organic nitrogen in forest soils. J Soils Sediments 8:363–378
Chen C, Xu Z, Mathers N (2004) Soil carbon pools in adjacent natural and plantation forests of subtropical Australia. Soil Sci Soc Am J 68:282–291
Cheshire M, Chapman S (1996) Influence of the N and P status of plant material and of added N and P on the mineralization of C from 14C-labelled ryegrass in soil. Biol Fert Soils 21:166–170
Curtin D, Smillie G (1986) Effects of liming on soil chemical characteristics and grass growth in laboratory and long-term field-amended soils. Plant Soil 95:23–31
Ehrenfeld JG, Ravit B, Elgersma K (2005) Feedback in the plant-soil system. Annu Rev Environ Resour 30:75–115
Elliott E (1986) Aggregate structure and carbon, nitrogen, and phosphorus in native and cultivated soils. Soil Sci Soc Am J 50:627–633
Erich MS, Trusty GM (1997) Chemical characterization of dissolved organic matter released by limed and unlimed forest soil horizons. Can J Soil Sci 77:405–413
Fierer N, Schimel JP, Holden PA (2003) Variations in microbial community composition through two soil depth profiles. Soil Biol Biochem 35:167–176
Finzi AC, Canham CD, Van Breemen N (1998) Canopy tree-soil interactions within temperate forests: species effects on pH and cations. Ecol Appl 8:447–454
Fog K (1988) The effect of added nitrogen on the rate of decomposition of organic matter. Biol Rev 63:433–462
Gholz H, Fisher R, Prichett W (1985) Nutrient dynamics in slash pine plantation ecosystems. Ecology 66:647–659
Grayston S, Vaughan D, Jones D (1997) Rhizosphere carbon flow in trees, in comparison with annual plants: the importance of root exudation and its impact on microbial activity and nutrient availability. Appl Soil Ecol 5:29–56
Guggenberger G, Zech W, Schulten HR (1994) Formation and mobilization pathways of dissolved organic matter: evidence from chemical structural studies of organic matter fractions in acid forest floor solutions. Org Geochem 21:51–66
Hartley IP, Hopkins DW, Sommerkorn M, Wookey PA (2010) The response of organic matter mineralisation to nutrient and substrate additions in sub-arctic soils. Soil Biol Biochem 42:92–100
Heimann M, Reichstein M (2008) Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 451:289–292
Jenkins AB (2002) Organic carbon and fertility of forest soils on the Allegheny plateau of West Virginia. West Virginia University, Doctoral dissertation
Jenkinson D, Adams D, Wild A (1991) Model estimates of CO2 emissions from soil in response to global warming. Nature 351:304–306
Johnson DW, Miegroet HV, Lindberg SE, Todd DE, Harrison RB (1991) Nutrient cycling in red spruce forests of the Great Smoky Mountains. Can J For Res 21:769–787
Jongkind AG, Velthorst E, Buurman P (2007) Soil chemical properties under kauri (Agathis australis) in the Waitakere Ranges, New Zealand. Geoderma 141:320–331
Kemmitt SJ, Wright D, Goulding KW, Jones DL (2006) pH regulation of carbon and nitrogen dynamics in two agricultural soils. Soil Biol Biochem 38:898–911
Kuzyakov Y (2011) How to link soil C pools with CO2 fluxes. Biogeosciences 8:1523–1537
Lu S, Chen C, Zhou X, Xu Z, Bacon G, Rui Y, Guo X (2012) Responses of soil dissolved organic matter to long-term plantations of three coniferous tree species. Geoderma 170:136–143
Maggs J (1985) Litter fall and retranslocation of nutrients in a refertilized and prescribed burned Pinus elliottii plantation. Forest Ecol Manag 12:253–268
Menyailo OV, Hungate BA, Zech W (2002) Tree species mediated soil chemical changes in a Siberian artificial afforestation experiment. Plant Soil 242:171–182
Ouyang XJ, Zhou GY, Huang ZL, Liu JX, Zhang DQ, Li J (2008) Effect of simulated acid rain on potential carbon and nitrogen mineralization in forest soils. Pedosphere 18:503–514
Rayment G, Higginson FR (1992) Australian laboratory handbook of soil and water chemical methods. Inkata Press Pty Ltd, Melbourne, p 330
Rousk J, Brookes PC, Bååth E (2009) Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl Environ Microbiol 75:1589–1596
Schmidt MW, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA, Kleber M, Kögel-Knabner I, Lehmann J, Manning DA (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56
Schneckenberger K, Demin D, Stahr K, Kuzyakov Y (2008) Microbial utilization and mineralization of [14C] glucose added in six orders of concentration to soil. Soil Biol Biochem 40:1981–1988
Silvester WB (2000) The biology of kauri (Agathis australis) in New Zealand 11. Nitrogen cycling in four kauri forest remnants. New Zeal J Bot 38:205–220
Sjöberg RM, Persson T (1998) Turnover of carbon and nitrogen in coniferous forest soils of different N-status and under different 15NH4-N application rate. Environ Pollut 102:385–393
Söderström B, Bååth E, Lundgren B (1983) Decrease in soil microbial activity and biomasses owing to nitrogen amendments. Can J Microbiol 29:1500–1506
Ste-Marie C, Paré D (1999) Soil, pH and N availability effects on net nitrification in the forest floors of a range of boreal forest stands. Soil Biol Biochem 31:1579–1589
Stewart CE, Paustian K, Conant RT, Plante AF, Six J (2008) Soil carbon saturation: evaluation and corroboration by long-term incubations. Soil Biol Biochem 40:1741–1750
Stewart CE, Paustian K, Conant RT, Plante AF, Six J (2009) Soil carbon saturation: implications for measurable carbon pool dynamics in long-term incubations. Soil Biol Biochem 41:357–366
Tombácz E, Rice JA, Ghabbour EA, Davies G (1999) Changes of colloidal state in aqueous systems of humic acids. Understanding humic substances. Adv Methods Prop Appl, pp 69–78
Vivanco L, Austin AT (2008) Tree species identity alters forest litter decomposition through long-term plant and soil interactions in Patagonia, Argentina. J Ecol 96:727–736
Wang QK, Wang SL, He TX, Liu L, Wu JB (2014) Response of organic carbon mineralization and microbial community to leaf litter and nutrient additions in subtropical forest soils. Soil Biol Biochem 71:13–20
Witt C, Setälä H (2010) Do plant species of different resource qualities form dissimilar energy channels below-ground? Appl Soil Ecol 44:270–278
Yoshitake S, Uchida M, Koizumi H, Nakatsubo T (2007) Carbon and nitrogen limitation of soil microbial respiration in a High Arctic successional glacier foreland near Ny-Ålesund, Svalbard. Polar Res 26:22–30
You SJ, Yin Y, Allen HE (1999) Partitioning of organic matter in soils: effects of pH and water/soil ratio. Sci Total Environ 227:155–160
Acknowledgements
We acknowledge the funding support from the National Natural Science Foundation of China (31360136, 31560168, 31660072), the Jiangxi Province Natural Science Foundation of China (20151BAB204007, 20161BAB204175), the Opening Foundation of Key Laboratory of Poyang Lake Wetland and Watershed Research (Jiangxi Normal University), and the Ministry of Education (PK2014009).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hailong Wang
Rights and permissions
About this article
Cite this article
Lu, S., Zhang, Y., Chen, C. et al. Plant–soil interaction affects the mineralization of soil organic carbon: evidence from 73-year-old plantations with three coniferous tree species in subtropical Australia. J Soils Sediments 17, 985–995 (2017). https://doi.org/10.1007/s11368-016-1602-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-016-1602-3