Abstract
Purpose
The influence of human activities on the development and functioning of urban soils and their profile characteristics is still inadequately understood. Microbial communities can change due to anthropogenic disturbances and it is unclear how they exist along urban soil profiles. This study investigates the dynamic soil properties (DSPs) and the bacterial communities along the profiles of urban soils in New York City (NYC) with varying degree of human disturbances.
Materials and methods
Eleven pedons were investigated across NYC as well as one control soil in a nearby non-urban area. Six soils are formed in naturally deposited materials (ND) and five in human-altered and human-transported materials (HAHT). For each soil, the profile was described and each horizon was sampled to assess DSPs and the bacterial community composition and diversity.
Results and discussion
The development and the DSPs of NYC soils are influenced by the incorporation of HAHT materials and atmospheric deposits. The most abundant bacterial taxa observed in the NYC soils are also present in most natural and urban soils worldwide. The bacterial diversity was lower in some soils formed in ND materials, in which the contribution of low-abundance taxa was more restricted. Some differences in bacterial community composition separated the soils formed in ND materials and in dredged sediments from the soils formed in high artifact fill and serpentinite till. Changes in bacterial community composition between soil horizons were more noticeable in urban soils formed in ND materials than in those formed in HAHT materials which display less differentiated profiles and in the non-urban highly weathered soil.
Conclusions
The bacterial diversity is not linked to the degree of disturbance of the urban soils but the variations in community composition between pedons and along soil profiles could be the result of changes in soil development and properties related to human activities and should be consistently characterized in urban soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In the context of increasing urbanization at global level, sustainable use and management of urban soils have to be considered to maximize the ecosystem services provided by these soils (Morel et al. 2014). Within this aim, knowledge about the development and the functioning of urban soils is needed. Human activities are likely to impact the formation and functions of urban soils. This impact can be direct, such as human disturbances and soil management (e.g., excavation, mixing, incorporation of man-made materials, sealing, fertilization, irrigation), or indirect through changes of environmental conditions (e.g., atmospheric depositions, urban heat island effect). This results in high spatial heterogeneity of soils (“urban soil mosaic”) with varying degrees of soil disturbances (Pouyat et al. 2010) and an extensive range of soil properties in urban areas (Schleuss et al. 1998; De Kimpe and Morel 2000). Soil properties that change with natural and anthropogenic disturbances are referred to as dynamic soil properties (DSPs). Profiles of urban soils are often complex, resulting from the burying of native soils by inputs of anthropogenic materials and the turbation induced by human activities, and provide a record of the changing land uses of the area (Prokof’eva and Poputnikov, 2010).
Despite these human disturbances, urban soils may display a high degree of biological activity and have high species richness (Pouyat et al. 2010) due to the active role of microorganisms. Microbial communities are essential catalysts in many soil functions, including organic matter (OM) decomposition and nutrient cycling (Moore et al. 2005), and they are sensitive to both natural and anthropogenic disturbances. For instance, microbial abundance, community composition, functional diversity or activity have been shown to be influenced by urban land uses (Zhao et al. 2013), soil sealing (Piotrowska-Długosz and Charzyński 2015) or pollution (e.g., Subrahmanyam et al. 2016).
Recent advances in molecular genetics and sequencing technology permit detailed analyses of soil microbial community composition and diversity. Most studies on soil microbial communities have been performed on natural and agricultural soils although a handful of more recent studies have examined microbial communities in urban park soils (e.g., Xu et al. 2013; Ramirez et al. 2014). Since these studies have been limited to the surface soil horizons (top 5 or 10 cm) the significance of deeper horizons is unknown.
Deeper horizons of urban soils may be enriched in OM and can contain substantial microbial biomass and activity (Lorenz and Kandeler 2005), even if the microbial biomass generally decreases with soil depth (e.g., Braun et al. 2006). Several studies on natural and agricultural soils have shown that microbial community composition and diversity vary with depth and are influenced by soil horizon features (e.g., Hansel et al. 2008; Will et al. 2010). The variations in the community composition with depth within a soil profile can be as distinct as the differences in community composition of soils separated by long distances and found in different biomes (Eilers et al. 2012). As a soil develops and horizons differentiate, the changes in community composition between A and B horizons increase, suggesting there is selection of bacteria adapted to the specific soil properties in each horizon (Michel and Williams 2011). The investigation of the bacterial community composition along the profiles of urban soils may reveal the influence of human activities and provide a more detailed picture of the biogeochemical functions in these soils. This study explores various soils across New York City (NYC) to evaluate DSPs and microbial communities in an important urban center.
A city-wide soil survey of NYC at a 1:12,000 scale was completed by USDA-NRCS Soil Survey group and is available on the Web Soil Survey site (http://websoilsurvey.sc.egov.usda.gov/App/HomePage.htm). This survey shows that NYC is covered mainly by impervious surfaces, such as building and pavement (62.7 % of land area city-wide). In the open spaces, the soils formed in naturally deposited (ND) materials (8.6 % of land area city-wide) are generally surrounded by the soils formed in human-altered and human-transported (HAHT) materials (27.6 % of land area city-wide). Primary naturally deposited parent materials of NYC soils are glacial till (4.6 % of the land area), tidal marsh (1.9 %), and marine sands (0.5 %). Other parent materials are also present in small areas across the city, such as serpentinite till in Staten Island and eolian deposits in northern Manhattan. Primary human-transported parent materials are low artifact (<10 %) loamy fill (14.9 % of the land area), construction debris (5.9 %), dredged materials (3 %), domestic wastes (landfills; 1.6 %), and coal combustion ash (0.1 %). The legend of the soil survey has 236 map units composed of 37 soil series in ND materials, including 4 new series established in NYC and 29 soil series in HAHT materials, including 27 established in NYC, showing the high diversity of soils within the urban area (NYC Soil Survey Staff 2005).
1.1 Objectives
The objective of this work is to measure dynamic soil properties (DSPs) and examine microbial community composition along the continuum of urban soils across NYC. The soil sampling sites were selected among the high diversity of NYC soils according an increasing degree of human influence, from soils developed without direct impact of human activities to soils formed in man-made materials. The selection was also done with the objective to complete the acquisition of DSP data within the framework of the USDA soil survey (Shaw et al. 2016). These soils represent different parent materials (ND or HAHT materials), vegetative cover (managed or unmanaged), topography, water regime, and age. An additional soil formed in a neighboring non-urban area was also examined to compare with the urban soils.
2 Materials and methods
2.1 Site description
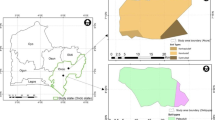
The description of the investigated sites is summarized in Table 1. Eleven pedons were investigated in parks across the five boroughs of NYC. In addition, one soil formed in glacial till (Rockaway series) but located in a non-urban area in New Jersey, 85 km to the northeast of NYC (Wawayanda State Park; Fig. 1), was sampled as example of soil with lower human influence. Among the soils collected in NYC, five soils did not display significant direct impact of human activities. They formed in ND materials consisting primarily of glacial till, sometimes covered by eolian or slopewash deposits (Haledon, Deerfield, Charlton, Siwanoy series) including serpentinite till (Todthill series). The six remaining urban soils were more impacted by human activities as they formed in HAHT materials. Two of these soils are developed on human-transported earthy materials or sediments, such as loamy fill (Flatbush series) or sandy dredged materials (Fortress series). These materials contained less than 10 % artifacts (low artifact fill). The four other soils are formed in pure or amended with soil man-made materials, such as coal ash (Rikers and Mosholu series) and construction debris (Laguardia and Secaucus series). These materials contained more than 10 % artifacts (high artifact fill). All the urban soils are more or less strongly subjected to indirect influence of human activities (e.g., atmospheric deposits). Most of these soils are under unmanaged vegetation cover, mainly woodland or grasses and shrubs, with only one soil found under turf (Secaucus series). In each category of soils (ND and HAHT soils), soils with varying drainage classes were selected, from somewhat excessively drained to poorly drained (Schoeneberger et al. 2012; Table 1). According to the categorization of soils of urban, industrial, traffic, mining, and military areas (SUITMAs; Morel et al. 2014), the soils formed in ND materials belong to the vegetated pseudo-natural SUITMAs whereas the soils formed from high artifact fill belong to the dumping sites SUITMAs.
Localization of the investigated pedons across New York City and in New Jersey. Six soils are formed in naturally deposited materials (ND) from the following soil series: Haledon, Charlton, Deerfield, Todthill, Siwanoy, and Rockaway. Six soils are formed in human-altered and human-transported materials (HAHT) from the following soil series: Flatbush, Fortress, Rikers, Mosholu, Laguardia, and Secaucus
2.2 Soil sample collection and preparation
Pedons from the Haledon, Deerfield, Todthill, and Charlton series were sampled in October 2013. Those from the Rikers, Mosholu, Laguardia, and Secaucus series were collected in June 2014. The pedons from the Siwanoy, Rockaway, and Fortress series were sampled in September 2014 and the one from Flatbush series in August of 2015.
For each soil, a pit was dug to a depth of at least 1 m, except if the bedrock or the water table was reached within the first meter. The profile was described following USDA-NRCS criteria (Schoeneberger et al. 2012). At each horizon, samples of approximately 10 g of soil were collected in 16-mL tubes for microbial analysis. A sample of 0.5–1 kg of soil was collected in each horizon for soil analyses, and then it was air-dried and sieved at 2 mm. The volumetric percentage of coarse natural and artifactual fragments was estimated in the laboratory when not assessed in the field.
2.3 Soil analyses
The fine soil fraction was analyzed for pH, measured in water at a ratio of 1:1 (v/v). The salt content was measured at a ratio of 1:2 (v/v) using a conductivity meter. Carbonate contents were analyzed using the volumetric method (Schleiber method) on ground air-dried soil samples, according to the French standard NF ISO 10693 (AFNOR 1995). The results were corrected by the residual water content and are expressed in gram per kilogram of oven-dried soil. Organic C content was assessed by loss on ignition. Two grams of oven-dried (105 °C) and sieved (<2 mm) soil samples were burned at 550 °C in a furnace for 20 min. Organic matter (OM) content was calculated as the percent of mass lost in this process. Total C, N, and S contents were determined by dry combustion at Auburn University Soil Testing Laboratory for ground samples dried at 50–60 °C and then corrected by residual water content. Total organic carbon (TOC), used to assess the C:N ratio, was calculated by subtracting inorganic carbon content (C bound to carbonates) from total C content. Elemental composition (K, Fe, Ca, Ti, Cr, Mn, Ni, Cu, Zn, Rb, Sr, Zr, Pb) was analyzed using a portable XRF analyzer on air-dried and 2-mm-sieved samples. The measurements were performed through a clear plastic storage bag three times for 90 s each after mixing the soil sample. Averages were calculated and then corrected by the residual water content.
Available micronutrients (B, Na, Mg, P, Ca, Fe, Mn, Cu, Zn) were determined using the modified Morgan extraction method (McIntosh 1969). Two grams of air-dried (<2 mm) soil were extracted with 10 mL of 1 M ammonium acetate adjusted to pH 4.8 in a shaker at 100 rpm for 20 min at room temperature. Suspension aliquots were centrifuged at 6,000 rpm for 10 min and the supernatants were diluted 100-fold with an aqueous solution containing 1 % HNO3 and 50 μg L−1 Ge solution as internal standard. Solutions were analyzed using ICP-MS. Method blanks were added in each series of analyses. Micronutrient concentrations were corrected for the soil residual water content.
The particle-size distribution was analyzed using the hydrometer method by following the simplified clay fraction procedure (Gee et al. 1986) on 40 g of air-dried and 2-mm-sieved soil samples. Organic matter content was removed for the samples containing more than 5 % of organic C content by adding hydrogen peroxide (concentration 30 %) until the effervescence ceased. The particle-size distribution of the samples collected in October 2013 and the bulk density of most samples were measured at the Kellogg Soil Survey Laboratory (USDA-NRCS).
2.4 DNA extraction and microbial data analysis
Soil samples (∼10 g) were collected from three to five areas along the clean wall of the pit within each identified horizon. For the Rockaway pedon, the organic horizon was too shallow to be sampled for soil analysis. Three samples were collected around the pit and used to isolate DNA to assess the bulk microbial community within the O horizon of this soil. Of all profiles of the study, the total genomic DNA was extracted from each sample (a 0.25 g soil subsample, according to the manufacturer’s protocol) using the MoBio PowerSoil DNA extraction kit (Carlsbad, CA, USA), and the DNA concentration and quality were measured using a NanoDrop 2000 spectrophotometer. The soil-extracted DNA was express-mailed to Molecular Research Lab (Shallowater, TX, USA) for Illumina MiSeq sequencing. The bacterial DNA in the sample was targeted through amplicon sequencing of the V4 or V1–3 regions of the 16S rRNA gene. There were some soil horizons from which we were unable to extract sufficient amounts of DNA for sequencing, and were not included in the analysis (these horizons are labeled in Fig. 4 as “not analyzed”). This may be the result of materials or chemicals in the soil horizon that interfered with the DNA extraction.
Samples from each horizon of all pedons (except for the soil from the Flatbush series; data still being processed) were analyzed (total of 76 samples). Raw sequence data had paired-end reads joined and then all sequences were filtered and trimmed using QIIME v1.8 (Caporaso et al. 2010). Operational taxonomic units (OTUs) were assigned by matching the reads to the Greengenes database (DeSantis et al. 2006) and alpha diversity was calculated. Data were summarized and graphed with R (R Core Team 2013). The bacterial community characteristic of a pedon was determined by identifying OTUs that were present in 50 % or more of the horizon samples and had more than 100 sequences per OTU. The Shannon–Weiner diversity index (H′; alpha diversity) was calculated and then converted to the “effective number of species” (ENS; in this study species is considered as equivalent to OTU) to represent diversity as a number of equally abundant species (or OTUs), which facilitates comparison across samples (Jost 2006; Chao et al. 2010). The predicted OTUs are an alpha diversity calculation (Chao 1) for the sample and the observed OTUs is the number of different OTUs that were actually detected in the sample.
3 Results
3.1 Description of the soil profiles and dynamic soil properties
Detailed descriptions of the soil profiles and the dynamic soil properties (DSPs) are included in the Electronic Supplementary Material. The photos of the soil profiles are shown in Fig. 2 and the ranges of the DSPs are summarized in Table 2.
Description of soil profiles and vertical distribution of metal concentrations (Pb, Cu, Zn) in soils of NYC. The pedons of 12 soil series were presented in order of increasing influence of human activities (localization in urban area, human-transported earthy or sediments as parent materials, pure or amended with soil man-made materials as parent materials). The dotted line represents the bottom of the pit when the pedons were investigated. Circumflex accent: presence of human-transported materials; apostrophe: indicator of the recurrence of identical horizon descriptor(s) in a profile; a: highly decomposed OM (used only with O); b: buried genetic horizon; e: moderately decomposed OM (used only with O); g: strong gley; h: illuvial OM accumulation; s: illuvial sesquioxide and OM accumulation; t: illuvial accumulation of silicate clay; u: presence of human-manufactured materials (artifacts); w: weak color or structure within B; x: fragipan characteristics
The soils formed in ND materials displayed an (O)-A-B-C profile type. The HAHT materials had an (O)-^A-^C profile type, except the soil formed in loamy fill (Flatbush) in which a ^Bw horizon has developed. Some profiles were complex and showed buried surface horizons (e.g., Ab horizon in the Deerfield pedon) or the buried native soils under the human-transported materials (e.g., Fortress and Flatbush). Organic (O) horizons have developed at the surface of some of the soils formed under woodland (Deerfield, Haledon, Rikers). In particular, the Deerfield pedon showed an accumulation of decomposed OM. The Mosholu pedon displayed a thick O horizon composed mainly of human-deposited wood chips. The Rockaway and Haledon pedons showed both argillic (Bt) and fragipan (Bx) horizons, while other soils formed in ND materials displayed Bw horizons. Redoximorphic features were observed in the soils with limited drainage (Haledon, Deerfield and Siwanoy series). Gley colors were present in the some horizons of the Deerfield pedon.
Overall, soils formed in coal ash (Rikers and Mosholu) and construction debris (Laguardia and Secaucus) as well as the serpentine soil (Todthill) contained a large proportion of coarse fragments, up to 75 % (v/v) in the C horizon of the Mosholu pedon. These coarse fragments were mainly artifacts in the soils formed in coal ash, which contained coal and slag, and in the soils formed in construction debris, in which bricks, concrete, glass, coal, slag, or bivalve shells were found. Proportions were generally higher in Bw and C horizons and varied markedly between the layers of dredged materials in the soil from the Fortress series.
As to particle-size class, pedons from the Rikers, Mosholu, and Deerfield series are sandy, Fortress is sandy over loamy; the Flatbush, Charlton, Haledon, and Rockaway pedons are coarse loamy and Siwanoy is coarse silty; Todthill, Laguardia, and Secacus are loamy skeletal. The texture varied gradually along the profile of most soils with increase in clay in Bt horizons (Haledon and Rockaway). But some marked changes were observed in the soil formed in sandy over loamy dredged materials (Fortress) or in the Deerfield pedon at the boundary with the buried surface silty A horizon.
The pH of most of the soils was acidic. The soils formed in ND materials, including the non-urban soil (Rockaway), had a pH between 3.5 and 5, except the serpentine soil (Todthill), which displayed a pH between 5.3 and 6.7. The soils formed in low artifact fill (Fortress and Flatbush) as well as soils formed in coal ash had a pH between 4.0 and 6.0. Only the soils formed in construction debris (Laguardia and Secaucus) reached slightly to moderately alkaline pH levels (7–8.2), due to the presence of carbonates, up to 83 mg kg−1, in some horizons.
The Haledon, Deerfield, and Charlton pedons, and, to a lesser extent, the Rikers and Flatbush pedons, showed a higher pH in the surface horizon. By contrast, surface horizons of the soils formed in construction debris (Laguardia and Secaucus) had a lower pH compared to the C horizons. This trend was also observed in the Mosholu and Todthill pedons. The pH varied markedly along the profile of the Fortress pedon, displaying a decrease in an organic dredged sediment layer (5^C horizon) and an increase in the buried native soil (6ABb horizon).
None of the analyzed soils was saline. However, higher salt and available Na concentrations were measured in deeper horizons of the Deerfield pedon (up to 1,000 mg L−1 of salts), and, to a lesser extent, the Siwanoy pedon. These two soils are adjacent to salt marshes, which can explain the increased salt concentrations in deeper horizons. Most of other soils displayed an increase in salt concentrations in surface horizons. Some increases in salt concentrations were observed in C horizons of the HAHT soils (Fortress, Rikers, and Secaucus).
The accumulation of OM and available micronutrients was higher in the surface horizons of the soils formed in ND materials under woodland, such as Charlton, Haledon, and Deerfield. The soils formed in coal ash (Rikers and Mosholu) displayed high concentrations of TOC with a high C:N ratio. In general, TOC concentrations decreased with depth but high TOC concentrations were observed in the C horizons of the HAHT soils (e.g., 8 % in the ^Cu3 horizon of the Laguardia soil or 16 % in the ^Cu1 horizon of the Mosholu soil). Likewise, available micronutrients were generally present in higher concentrations in surface horizons but some deeper horizons also displayed significant amounts, such as the buried surface horizons of the Deerfield and Rikers pedons or some C horizons from the HAHT soils. In particular, the soils formed in construction debris (Laguardia and Secaucus) had high concentrations in available Ca in some C horizons.
In terms of mean elemental composition along the soil profiles, the soils formed in high artifact fill (Rikers, Mosholu, Laguardia, Secaucus) were enriched in Ca (×8), S (×4), Cu (×3), Zn (×4), Sr (×3), Ti (×2), and Pb (×4) compared to the urban ND soils. They also displayed enrichment in Ni (×3) and Cr (×2) compared to the urban ND soils except the serpentine soil (Todthill), which was characterized by very high levels of Cr, Ni, Fe, Mn, and available Mg. Mean concentrations of Ti and Sr were higher in soils formed in coal ash, especially the Mosholu pedon, compared to the concentrations in the other soils. Average metal concentrations along the profiles of the soils formed in low artifact fill (Flatbush, Fortress) were in the same range of those of the urban ND soils and displayed an enrichment in Pb (×4–5) and in Cu (×5–7) compared to the non-urban soil (Rockaway).
Overall, the distribution of metals (Pb, Cu, Zn) along the soil profiles showed an enrichment in metals in the surface organic horizons in soils formed in ND materials (Fig. 2). Concentrations of Cu and Pb reached, respectively, 487 and 612 mg kg−1 in the Oa horizon of the soil of Deerfield series. Concentration of Zn reached 286 mg kg−1 in the Ab horizon in the same soil, showing that some buried surface horizons were also enriched in metals. In soils, the HAHT soils, surface horizons were often enriched in metals but some deeper horizons were as well, such as an organic clayey layer of dredged deposits (5^C horizon) in the Fortress soil or C horizons in soils formed in construction debris or coal ash. For example, Pb concentration reached 1049 mg kg−1 in the ^Cu3 horizon of the Laguardia soil and Cu concentration 743 mg kg−1 in the ^Cu4 horizon of the Rikers soil.
3.2 Bacterial community
3.2.1 Bacterial community composition
The comparison of the bacterial communities across all the soil samples was based on the characteristic set of bacterial taxa for each soil. Therefore, the influence of rare OTUs was minimized and comparisons between soils focused on the prominent representatives of each community, which remained highly diverse (Fig. 3).
Bacterial orders characteristic (“core”) of 11 analyzed pedons of NYC. Characteristic bacteria of each soil series comprised 40 % or more of the bacteria communities. Bacteria taxa are differentiated by color representing the Phyla assignment then gradations of the Phyla color to indicate Order. Rockaway is the non-urban soil with urban soils separated into groups of naturally deposited material (NDM) and human-associated and human-transported material (HAHTM)
There were 155 bacterial orders in 28 phyla that formed the characteristic set of bacteria present in the communities (Fig. 3). Across all pedons, the most abundant orders were (in decreasing abundance) Rhizobiales, Xanthomonadales Rhodospirillales, and Actinomycetales. Three additional identified orders were abundant though absent in one or two pedons: Ellin6513(AcidobacteriaDA052), Acidobacteriales, and Solibacterales. Nine phyla were present in all samples, regardless of the pedon and horizon type: Acidobacteria, Actinobacteria, AD3, Chloroflexi, Gemmatimonadetes, Nitrospirae, Planctomycetes, Proteobacteria, and Verrucomicrobia. However, within each pedon, these phyla were not necessarily among of the top five phyla in terms of abundance. Only Acidobacteria and Proteobacteria were among the five most abundant phyla in all soils. The soils from the Charlton, Haledon, Rockaway, Deerfield, and Siwanoy pedons, formed in ND materials, as well as the soil from the Fortress pedon, formed in dredged sediments, had the characteristic orders representing >60 % of the bacteria in the sample. In the serpentine soil (Todthill) and most soils formed in HAHT materials (Rikers, Laguardia, and Secaucus), the characteristic orders represented 50–60 % of the bacteria in the sample. In the soil from the Mosholu series formed in coal ash, the characteristic set of orders was least represented, with only 40 % of the total bacterial community, suggesting this soil has a heavier representation from low-abundance OTUs, which in aggregate, make up a significant fraction of the community.
The bacterial community composition varied differently along the depth of each profile. To illustrate these variations, the community was represented within each horizon by the summed percentage of the five most abundant bacterial orders for each pedon (rather than the predominant bacterial orders for the entire study; Fig. 4).
Soils formed in construction debris (Laguardia and Secaucus) as well as the non-urban soil (Rockaway) had less variation in community composition than the others. The non-urban soil had a small decrease in relative abundance of the five top orders in B and C horizons. In the soils formed in construction debris, there was a slight change in relative abundance of the top five orders between A and C horizons in the Laguardia pedon, while there was a noticeable increase of the abundance of some top orders with depth in the Secaucus pedon.
Among the other HAHT soils, some showed gradual shifts in the community composition with increasing depth. For instance, the soil formed in coal ash (Rikers) displayed an increase of the relative abundance of some orders with depth. Along the profile of the soil formed in dredged sediments (Fortress), the relative abundance of the five top orders in the deeper layers was low. Slight changes in the community structure seemed to differentiate surface horizons from the C horizons and from the OM-rich, clayey, and acidic sediment layer (5^C horizon) and the buried surface horizons.
The remaining soils formed in ND materials (Todthill, Charlton, Deerfield, and Haledon) showed relatively marked variations of bacterial communities depending on horizons. Changes in the relative abundance of the top five orders differentiated A, Bw, and BC or C horizons along the profiles of the soils from Todthill and Charlton pedons. The community composition varied markedly along the complex profile of the soil from the Deerfield pedon. Sharp shifts in community structure can be observed even between two consecutive horizons. The BC3 horizon had a high relative abundance of Rhizobiales compared to the other BC horizons. The proportion of the five most abundant orders present in the overlying horizons was very low in buried surface horizon (Ab) and underlying gley horizon (C’g1) compared to overlying horizons. The community composition of the soil of the Haledon series displayed pronounced changes along its profile. In particular, fragipan horizons had a unique community structure, dominated by Pseudomonadales order that was absent in the other horizons.
3.2.2 Bacterial community diversity
The bacterial communities of all soils were relatively similar in terms of the number of observed and predicted OTUs (Chao1 estimator). Since not all OTUs are equally abundant in our soils, the ENS and the observed number of OTUs will differ, but the diversity value (ENS) will be comparable across different soils. Differences were observed in the diversity of the soils (Fig. 5). Soils from the Charlton, Deerfield, and Haledon series had the lowest diversity, with an ENS below 10,000. However, this number was similar to their predicted and observed numbers of OTUs. Most other soils had an effective number of species twice as high as their observed and expected OTU values. The only non-urban soil (Rockaway) presented the highest number of expected and observed OTUs, as well as the highest ENS (Fig. 5).
Soil bacterial community diversity across pedons of NYC. Bacterial community diversity presented as the predicted number of OTUs (prediction of the potential number of OTUs from the alpha diversity metric Chao 1), the observed number of OTUs (number of OTUs detected in the sample) and the effective number of species (ENS) (conversion of the Shannon–Weiner index to a number of species or OTU). Error bars are one standard deviation from the mean. The number of soil samples processed (N) for each soil series is indicated below the bars, in some cases more soil samples than the number of horizons were processed and averaged into horizon samples
4 Discussion
4.1 Human activities influence on the development of NYC soils
Soil development in urban areas is influenced directly by human activities such as excavation, mixing, introduction of human-transported materials, and management of vegetation cover, or indirectly through atmospheric deposition and the introduction of invasive plants, insects, and animals. This study targeted a number of urban soils in NYC with an increasing degree of human influence. The soils formed in ND materials under unmanaged cover (woodland or grasses and shrubs: Charlton, Deerfield, Haledon, Siwanoy, and Todthill) have not had significant recent direct physical disturbances. In addition, the soil from a non-urban area (the Rockaway pedon) was investigated to highlight the effects of urbanization. The majority of soils in open space in NYC are formed in HAHT materials. Most of these materials are low artifact fill, such as earthy materials in the Flatbush soil or dredged sediments in the Fortress soil. The soils formed from artifactual materials, either pure or amended with soil, such as coal ash (Mosholu and Rikers) or construction debris (Laguardia and Secaucus) represent a higher level of human influence. Within the soils described here, only the soil from the Secaucus series is under turf and has undergone lawn management practices.
The main soil-forming processes observed in the soils described in this study are OM accumulation and, in some cases, development of structure as well as the translocation and accumulation of clay. Wetter soils (Haledon, Deerfield, and Siwanoy pedons) displayed signs of redoximorphic feature development. All the HAHT soils are weakly developed soils. The soil formed in loamy fill (Flatbush) as well as the soils formed in ND materials from the Deerfield, Charlton, Siwanoy, and Todthill series displayed the inception of soil development, characterized by the presence of Bw horizons. The more highly weathered soils (Haledon and Rockaway) displayed an argillic horizon (Bt), resulting from the translocation and accumulation of clay, as well as a fragipan horizon (Bx).
The influence of urbanization appears through the enrichment in metals (especially Pb and Cu) compared to the non-urban soil (Rockaway) and the New York State rural surface soils (New York State Department of Environmental Conservation 2005). The incorporation of man-made materials (e.g., coal ash, construction debris) contributes also to the enrichment in some other metals (e.g., Zn, Ni, Cr) in the soils, even if NYC soils can contain naturally high contents in Ni and Cr due to the presence of serpentine bedrock in Staten Island. The distribution of metals along the soil profiles shows higher metal concentrations (especially Pb, Zn, and Cu) in organic surface horizons. Most sites did not have direct sources of metals; therefore, the most likely source is atmospheric deposits from local traffic and industrial activities, as observed in NYC and other cities (e.g., Imperato et al. 2003; Pouyat et al. 2010). Garden topsoils of New York City have been shown to display relatively high Pb concentrations (600 ppm in average), which can be assigned to the past use of leaded gasoline, leaded paint, and refuse incineration (Cheng et al. 2015). This effect of atmospheric deposits is less detectable in the pedon from Pelham Park Bay (Siwanoy) further from the city center as well as in the soils formed in high artifact fill (Rikers, Laguardia, and Secaucus soils) since these materials can already contain elevated amounts of these metals. Buried organic surface horizons are also enriched in metals, which could be considered as evidence of past atmospheric deposition.
The influence of the vegetative cover management is difficult to investigate within the range of studied soils. By comparing soils formed in construction debris, one formed under weedy cover (Laguardia) and the other under turf (Secaucus), it appears that the organic horizons under turf are enriched in OM with a lower C:N. This is likely because of the OM input from the dense root system of the turf-grass, as well as the lawn management (e.g., irrigation, fertilization; Pouyat et al. 2010). The dataset does not allow us to observe an enrichment in nutrients such as P and K in disturbed soils under turf, as described in several cities (Pouyat et al. 2015).
Overall, profiles of soils formed in ND materials display more differentiated profiles (A-B-C type) than HAHT soils (mainly of ^A-^C type). The introduction of HT materials has modified the native soils, as observed in the Fortress and Flatbush pedons, which created new parent materials for soil formation. Consequently, these new young soils are characterized by organo-mineral (A) horizons developed over the HT materials (C horizons), as often reported in these soils (e.g., Schleuss et al. 1998; Howard et al. 2015). Only the Flatbush pedon showed the development of a Bw horizon in the human-deposited loamy fill. Depending on the mode of deposition of the HT materials, these parent materials can be stratified presenting sharp discontinuities between the different layers (e.g., dredged deposits in the Fortress pedon) or be continuous but with high vertical variability (e.g., construction debris in the Laguardia and Secaucus pedons). Therefore, the distribution of some DSPs (e.g., metals and organic C) along the profile of these soils can be uneven and dependent on the nature and variability of the parent materials. This can contribute to the presence of high organic C in deeper horizons, as observed in other urban soils (e.g., Lorenz and Kandeler 2005).
The introduction of HT materials modifies the DSPs compared to those of native soils of the region. Anthropogenic materials may contain large amounts of coarse fragments (gravels and cobbles), including artifacts derived from human activities. The presence of coarse fragments as well as textural boundaries, as observed along the profile formed in sandy over loamy dredged sediments (Fortress), may modify the water movement in the soil. Some artifacts influence the DSPs, such as metal concentrations (El Khalil et al. 2008) or water and nutrient storage (Nehls et al. 2013). The presence of anthropogenic organic compounds mixed with natural OM is also a characteristic of urban soils. Notably, the particulate form of C resulting from the incomplete combustion of organic materials (black carbon) contributes significantly to the total organic C in urban soils (Lorenz and Kandeler 2005; Nehls and Shaw 2010). This could explain the high C:N ratios measured in deeper horizons of the soils formed in coal combustion by-products (Mosholu and Rikers). While the native soils of the NYC area are acidic, the soils formed in construction debris (Laguardia and Secaucus) show a neutral to moderate alkaline pH, high available Ca concentrations, and the presence of carbonates are likely because of the presence of calcareous artifacts like concrete or shells. An alkaline pH is commonly reported in highly disturbed urban soils and associated with the presence of materials used in infrastructure and building (Pouyat et al. 2015).
4.2 Bacterial communities in NYC soils
4.2.1 Comparison of NYC soil bacterial communities with other soil communities
The most abundant bacterial taxa observed in this study of NYC soils are common in natural and urban soils across the globe. Particularly, the phyla Acidobacteria, Actinobacteria, and Proteobacteria, were prominent in NYC soils and, are observed in relative high abundance in most soils from different biomes across North and South America or Europe (Janssen 2006; Lauber et al. 2009; Fierer et al. 2012). These phyla are also dominant in urban soils in China (Xu et al. 2013) and in NYC soils along with Bacteroidetes (Central Park, Ramirez et al. 2014; urban parks and median streets, Reese et al. 2015). Other abundant phyla detected in this study, such as Chloroflexi, Verrumicrobia, Bacteroidetes, Gemmatimonadetes, Planctomycetes, or Firmicutes, are also commonly encountered in soils across the globe with varying abundance (Janssen 2006). The most noticeable observation among the soils we analyzed is the prevalence of the candidate phylum AD3 in soils formed in ND materials, which is especially abundant in Charlton and Deerfield soils. The only other instances in which it has been reported as abundant are in surface soil samples from Mitchell Peninsula, Antarctica (Ji et al. 2015), and from a red soil in China (Ren et al. 2015). The soil conditions driving the presence of these bacterial taxa remain to be defined.
The high diversity of the bacterial communities we have found in the soils included in this study makes it difficult to determine a set of commonly shared taxa, or “core” (Li et al. 2013), a situation that is not unprecedented in microbiome studies. Even the intensely studied human microbiome has shown to be difficult to characterize by a core bacterial community shared by all individuals (Grice and Segre 2012; Huse et al. 2012; Li et al. 2013). The comparison of soil bacterial community composition studies is also complicated by the difference of methodology in soil sampling and data analyses. Most studies focused on soil surface horizons whereas it was shown that some phyla are more abundant in surface horizons (e.g., Bacteroidetes, Planctomycetes) while others are more abundant in deeper horizons (e.g., Chloroflexi, Gemmatimonadetes, Nitrospirae, Acidobacteria; Will et al. 2010; Michel and Williams 2011; Eilers et al. 2012). These trends were also observed in this study.
4.2.2 Bacterial community diversity across the soils and along the profiles
The bacterial community composition and diversity vary across the soils and along the profile of each soil. Despite this variability, some trends in community structure allow the soils to be distinguishable based on the nature of the parent materials and some DSPs. The non-urban soil (Rockaway) has the greatest diversity of all soils in this study. Among urban soils, diversity is lower in the Haledon, Deerfield, and Charlton pedons formed in ND materials compared to the HAHT soils, the serpentine till soil (Totdthill) and the upper horizons of the Siwanoy pedon. Provided that HAHT soils have been recently disturbed, it might be expected that diversity would be greater since the conditions to shed transient species from the bacterial community and enrich for specialists have not yet developed (Roxburgh et al. 2004). However, the large difference in diversity between the soils formed in glacial till with a fragipan in the non-urban area (Rockaway) as the urban area (Haledon) suggests that factors other than the nature of the parent materials are driving the soil bacterial diversity. For instance, the difference in drainage in these two soils could influence the bacterial diversity, by constraining the water content and the oxygen availability and creating anoxic zones.
The characteristic set of bacteria for each soil represents the majority of the community for most of the soils formed in ND materials and the soil formed in dredged sediments (low artifact fill) over the buried native soil (Fortress). By contrast, this characteristic set constitutes a reduced component of bacterial community of the HAHT soils (high artifact fill) and the serpentine soil (Todthill). This suggests that these soils have generally more low-abundance taxa relative to the soils formed in ND materials (except serpentinite till). Serpentine soils have elevated metal levels (especially Ni and Cr), and a high Mg to Ca ratio that is very unfavorable or even prohibitive to most plants, and could constrain bacterial community composition, as in soils formed in high artifact fill.
Some specific differences in community composition further distinguish the soils formed in ND materials (except serpentinite till) and in dredged sediments from the soils formed in high artifact fill and serpentinite till. In the soils formed in ND materials and in dredged sediments, the predominant bacterial orders are Acidobacteriales, Ellin6513(AcidobacteriaDA052), Rhizobiales, Xanthomonadales, and Pseudomonadales. Furthermore, Pseudomonadales is only present in these soils. In soils formed in high artifact fill and serpentinite till, the predominant bacterial orders are also Rhizobiales and Xanthomonadales in addition to RB41 (Chloracidobacteria), Syntrophobacterales, Nitrospirales, and Acidimicrobiales. No bacterial order was found exclusively in the soils formed in high artifact fill and serpentinite till. Some changes in community composition seem to be linked to soil pH. Acidobacteria are known to be abundant in acidic soils (Griffiths et al. 2011; Fierer et al. 2012; Ramirez et al. 2014) and the soils in this study further support this trend: Acidobacteriales and Ellin6513 orders are absent in alkaline soils formed in construction debris (Laguardia and Secaucus) while in high abundance in acidic Haledon pedon.
The bacterial community composition varies along the soil profiles and these variations corresponded mostly with different types of horizons (O, A, B, and C), delimitated based on field description and DSPs analysis. However, these changes in community structure are more or less pronounced depending on the soil. Some soils displayed a relatively constant community structure all along the profile with only slight changes, such as the soils formed in construction debris (Laguardia and Secaucus) or the non-urban soil from the Rockaway series. In some soils, the shifts in community composition are gradual with depth (e.g., in the soil formed in coal ash, Rikers). By contrast, some soils (e.g., Deerfield and Haledon) show marked changes in community composition between consecutive horizons. Some horizons with particular characteristics may have harbored specific communities (e.g., the fragipan horizons in the soil from Haledon soil), indicating that bacteria could have undergone selection to adapt to the properties in the horizon leading to a differentiation of community composition between horizons as the soil developed, as proposed by Michel and Williams (2011). This could also explain the lower bacterial diversity and the lower proportion of rare bacteria observed in these differentiated soils. The intensity of changes in community structure with depth is likely related to the degree of differentiation in horizons and the variability of DSPs along the profile. The soils formed in HAHT materials are still relatively little differentiated (^A-^C profile) and show relatively slight changes in community composition along their profiles. However, initial strong variability in HAHT materials may induce significant shifts in total bacterial community structure, as it has been observed in the different layers of constructed Technosols made from compost, paper by-products and treated industrial soil (Hafeez et al. 2012). The development of horizons with time could create more distinct habitats for microorganisms with depth, as observed in the soils formed in ND materials. The relative constant community composition of the highly weathered soil from the Rockaway series remains to be explained but could be partly due to the relative homogeneization of most DSPs along the soil profile due to the high degree of weathering.
The evolution of the composition of bacterial communities with depth during the soil development could be a useful parameter to monitor within the aim of better understanding the functioning and the ecosystem services provided by urban soils. Compared to the categorization of SUITMAs (Morel et al. 2014), this study shows that some vegetated dumping sites SUITMAs were likely to support higher microbial diversity than vegetated pseudo-natural SUITMAs. However, the role of these microorganisms in the soil functioning requires further investigations. Changes in global microbial community structure along the soil profile may contribute to changes in the expression and/or rate of soil processes, as it has been suggested for the changes in N-cycling processes rates with depth in constructed Technosols (Hafeez et al. 2012).
5 Conclusions
The present study is unprecedented due to the number of urban soil types and the depth at which the bacterial community was examined. It focused on the description of soil profile characteristics and associated bacterial communities in soils of NYC, with varying degrees of human disturbances and management. The development and the DSPs of these soils are influenced by human activities, such as atmospheric deposits or the introduction of human-transported materials. The general bacterial communities present in these soils, even in those strongly impacted by human activities, are not different from the communities found in other soils. The bacterial diversity varied across the investigated soils and was lower in some soils formed in naturally deposited materials displaying marked variations of the community structure along their profiles. The changes in bacterial community composition depending on the soil horizons were less pronounced in less differentiated soils, such as the soils formed in human-transported materials or in weathered soils with relatively stable distribution of soil properties along their profiles. This suggests that potential links exist between human activities, soil development, dynamic soil properties, and bacterial community composition in NYC soils. However, further data analyses are required to better understand which soil properties are driving the microbial diversity in these soils. The influence of human activities on soil development and functioning in relation to the microbial diversity needs to be investigated across a wider range of urban soils within the aim of a sustainable management of these soils.
References
AFNOR (1995) Soil quality—determination of carbonate content—volumetric method, NF ISO 10693. Association Française de Normalisation ed, Paris
Braun B, Böckelmann U, Grohmann E, Szewzyk U (2006) Polyphasic characterization of the bacterial community in an urban soil profile with in situ and culture-dependent methods. Appl Soil Ecol 31:267–279
Caporaso JG, Kuczynski J, Stombaugh J, et al. (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Meth 7:335–336
Chao A, Chiu C-H, Jost L (2010) Phylogenetic diversity measures based on hill numbers. Phil Trans R Soc B 365:3599–3609
Cheng Z, Paltseva A, Li I, et al. (2015) Trace metal contamination in New York City garden soils. Soil Sci 180:167–174
De Kimpe CR, Morel JL (2000) Urban soil management: a growing concern. Soil Sci 165:31–40
DeSantis TZ, Hugenholtz P, Larsen N, et al. (2006) Greengenes, a chimera-checked 16S rRNA Gene database and workbench compatible with ARB. Appl Environ Microbiol 72(7):5069–5072
Eilers KG, Debenport S, Anderson S, Fierer N (2012) Digging deeper to find unique microbial communities: the strong effect of depth on the structure of bacterial and archaeal communities in soil. Soil Biol Biochem 50:58–65
El Khalil H, Schwartz C, Elhamiani O, Kubiniok J, Morel JL, Boularbah A (2008) Contribution of technic materials to the mobile fraction of metals in urban soils in Marrakech (Morocco. J Soils Sediments 8:17–22
Fierer N, Leff JW, Adams BJ, et al. (2012) Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. P Natl Acad Sci 109:21390–21395
Gee GW, Bauder JW, Klute A (1986) Particle-size analysis. In: Klute A (ed) Methods of soil analysis—part 1. Physical and mineralogical methods, pp 383–411
Grice EA, Segre JA (2012) The human microbiome: our second genome. Annu Rev Genomics Human Genet 13:151–170
Griffiths RI, Thomson BC, James P, Bell T, Bailey MJ, Whiteley AS (2011) The bacterial biogeography of British soils. Environ Microbiol 13:1642–1654
Hafeez F, Spor A, Breuil M-C, et al. (2012) Distribution of bacteria and nitrogen-cycling microbial communities along constructed Technosol depth-profiles. J Hazard Mater 231–232:88–97
Hansel CM, Fendorf S, Jardine PM, Francis CA (2008) Changes in bacterial and archaeal community structure and functional diversity along a geochemically variable soil profile. Appl Environ Microbiol 74:1620–1633
Howard JL, Ryzewski K, Dubay BR, Killion TW (2015) Artifact preservation and post-depositional site-formation processes in an urban setting: a geoarchaeological study of a nineteenth century neighborhood in Detroit, Michigan, USA. J Archaeol Sci 53:178–189
Huse SM, Ye Y, Zhou Y, Fodor AA (2012) A core human microbiome as viewed through 16S rRNA sequence clusters. PLoS One 7:e34242
Imperato M, Adamo P, Naimo D, Arienzo M, Stanzione D, Violante P (2003) Spatial distribution of heavy metals in urban soils of Naples city (Italy. Environ Pollut 124:247–256
Janssen PH (2006) Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Appl Environ Microbiol 72:1719–1728
Ji M, van Dorst J, Bissett A, Brown MV, Palmer AS, Snape I, Siciliano SD, Ferrari BC (2015) Microbial diversity at Mitchell Peninsula, Eastern Antarctica: a potential biodiversity “hotspot. Polar Biol 39:237–249
Jost L (2006) Entropy and diversity. Oikos 113:363–375
Lauber CL, Hamady M, Knight R, Fierer N (2009) Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl Environ Microbiol 75:5111–5120
Li K, Bihan M, Methé BA (2013) Analyses of the stability and core taxonomic memberships of the human microbiome. PLoS One 8:e63139
Lorenz K, Kandeler E (2005) Biochemical characterization of urban soil profiles from Stuttgart, Germany. Soil Biol Biochem 37:1373–1385
McIntosh JL (1969) Bray and Morgan soil test extractants modified for testing acid soils from different parent materials. Agron J 61:259–265
Michel HM, Williams MA (2011) Soil habitat and horizon properties impact bacterial diversity and composition. Soil Sci Soc Am J 75:1440–1448
Moore JC, McCann K, de Ruiter PC (2005) Modeling trophic pathways, nutrient cycling, and dynamic stability in soils. Pedobiologia 49:499–510
Morel JL, Chenu C, Lorenz K (2014) Ecosystem services provided by soils of urban, industrial, traffic, mining, and military areas (SUITMAs. J Soils Sediments 15:1659–1666
Nehls T, Shaw RK (2010) Black carbon in soils: relevance, analysis, distribution. Soil Surv Horiz 51:79–84
Nehls T, Rokia S, Mekiffer B, Schwartz C, Wessolek G (2013) Contribution of bricks to urban soil properties. J Soils Sediments 13:575–584
New York City Soil Survey Staff (2005) New York City reconnaissance soil survey. United States Department of Agriculture. Natural Resources Conservation Service, Staten Island, NY
New York State Department of Environmental Conservation (2005) Concentrations of selected analytes in rural New York state surface soils: a summary report on the statewide rural surface soil survey. Available at http://www.dec.ny.gov/docs/remediation_hudson_pdf/appendixde.pdf. Accessed July 17, 2016
Piotrowska-Długosz A, Charzyński P (2015) The impact of the soil sealing degree on microbial biomass, enzymatic activity, and physicochemical properties in the Ekranic Technosols of Toruń (Poland). J Soils Sediments 15:47–59
Pouyat RV, Szlavecz K, Yesilonis ID, Groffman PM, Schwarz K (2010) Chemical, physical, and biological characteristics of urban soils. In: Aitkenhead-Peterson J, Volder A (eds) Urban ecosystem ecology, agronomy monograph 55. ASA-CSSA-SSSA, Madison, WI, pp. 119–152
Pouyat RV, Yesilonis ID, Dombos M, et al. (2015) A global comparison of surface soil characteristics across five cities: a test of the urban ecosystem convergence hypothesis. Soil Sci 180:136–145
Prokof’eva TV, Poputnikov VO (2010) Anthropogenic transformation of soils in the Pokrovskoe-Streshnevo park (Moscow) and adjacent residential areas. Eurasian Soil Sci 43:701–711
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna http://www.R-project.org
Ramirez KS, Leff JW, Barberán A, et al. (2014) Biogeographic patterns in below-ground diversity in New York City’s Central Park are similar to those observed globally. Proc R Soc B 281:20141988
Reese AT, Savage A, Youngsteadt E et al (2015) Urban stress is associated with variation in microbial species composition—but not richness—in Manhattan. ISME J 1–10
Ren G, Ren W, Teng Y, Li Z (2015) Evident bacterial community changes but only slight degradation when polluted with pyrene in a red soil. Front Microbiol 6:22
Roxburgh SH, Shea K, Wilson JB (2004) The intermediate disturbance hypothesis: patch dynamics and mechanisms of species coexistence. Ecology 85:359–371
Schleuss U, Wu Q, Blume H-P (1998) Variability of soils in urban and periurban areas in northern Germany. Catena 33:255–270
Schoeneberger PJ, Wysocky DA, Benham EC, Soil Survey Staff (2012) Field book for describing and sampling soils, version 3.0. Natural Resources Conservation Service, National Soil Survey Center, Lincoln, NE
Shaw RK, Hernandez L, Levin M, Muñiz E (2016) Promoting soil science in the urban environment—partnerships in New York City, NY, USA. J Soils Sediments 1–6. doi: 10.1007/s11368-016-1456-8
Subrahmanyam G, Shen J-P, Liu Y-R, et al. (2016) Effect of long-term industrial waste effluent pollution on soil enzyme activities and bacterial community composition. Environ Monit Assess 188:1–13
Will C, Thürmer A, Wollherr A, et al. (2010) Horizon-specific bacterial community composition of German grassland soils, as revealed by pyrosequencing-based analysis of 16S rRNA genes. Appl Environ Microbiol 76:6751–6759
Xu H-J, Li S, Su J-Q, et al. (2013) Does urbanization shape bacterial community composition in urban park soils? A case study in 16 representative Chinese cities based on the pyrosequencing method. FEMS Microbiol Ecol 87:182–192
Zhao D, Li F, Yang Q, et al. (2013) The influence of different types of urban land use on soil microbial biomass and functional diversity in Beijing, China. Soil Use Manage 29:230–239
Acknowledgments
The authors would like to thank the USDA-NRCS soil scientists (Lisa Krall, Edwin Muñiz, Fred Schoenagel, Marissa Theve, Olga Vargas, and Ron Taylor) for their help in describing and sampling the soils. They also wish to thank Zulema García-Blanco for mapping the sampling sites and all the undergraduate and graduate students of Brooklyn College and the students of Midwood High School for their help to collect and analyze the soil samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by USDA-NRCS (Contract Number: 68-7482-13-524).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Stefan Norra
Electronic supplementary material
ESM 1
(DOC 768 kb)
Rights and permissions
About this article
Cite this article
Huot, H., Joyner, J., Córdoba, A. et al. Characterizing urban soils in New York City: profile properties and bacterial communities. J Soils Sediments 17, 393–407 (2017). https://doi.org/10.1007/s11368-016-1552-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-016-1552-9