Abstract
Purpose
Guadalentin River (SE Spain) has been affected by tannery industries, where their effluents, containing Cr, were spilled until 2003. The untreated tannery effluent is characterized by the presence of inorganic and organic substances including a basic chromium (III) sulfate salt. Chromium contents in sediments represent permanent environmental and human health risks. The main objectives of this research were to evaluate the contamination, the spatial distribution, and the speciation of chromium in the sediments.
Materials and methods
In order to determine the degree of Cr pollution and to evaluate the influence of sediment properties in the behavior of total Cr, Cr(VI), and Cr(III) concentrations, a sediment sampling was carried out in a stretch of 1500 m of the dry riverbed, from 0 to 100 cm deep. Total, soluble, and exchangeable Cr(III+IV) and Cr(III) were measured using graphite furnace atomic absorption spectrometry (GFAAS), and Cr(VI) was selectively extracted by EPA 3060A method. A physicochemical characterization of the riverbed sediments was done with the aim of evaluating the influence of some sediment properties related to the contents of total Cr and its speciation.
Results and discussion
Chromium total concentration was high in the riverbed (11–11099 mg kg−1) up to 100 cm deep, exceeding in almost all the study stretch, the background level, and the generic reference values of Cr for Murcia Province. The highest degrees of sediment pollution (over 10,000 mg Cr kg−1) are located 20–50 cm deep, at the first 600 m east of the city center, and in the last 300 m of the studied area, which reveals that the Cr contents in sediments are relatively higher near the discharge point of the tannery facilities. Chromium(III) is the predominant oxidation state with 95.87 % (mean value) of total Cr in the sediments.
Conclusions
The results (maximum values 11099 Cr mg kg−1 and 79 Cr(VI) mg kg−1) indicated Cr leaching from the surface until 100 cm deep. Chromium(VI) represents 4.13 % of total Cr, so Cr(III) was the predominant oxidation state. The riverbed sediment pollution by Cr (total) and Cr(VI) was caused by an anthropogenic activity (tannery industry).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Tannery is one of the major sources of Cr pollution in sediments (Callender 2003; Pawlikowski et al. 2006; Pertsemli and Voutsa 2007; Dong et al. 2013). The untreated tannery effluent is characterized by the presence of organic substances, Cr salts, chloride ions, sulfides, sulfates, and nitrogen (Bajza and Vrcek 2001), being the basic chromium sulfate (salt) used for tanning the most important source of Cr(III) (Thorstensen 1984).
According to Taylor and Diefendorf (1990), the use of Cr in tanning procedures and the subsequent spill to soil/sediments or waterbodies accelerate the mobility and transport rates of Cr in natural environment. In fact, the amount of Cr by far exceeds the rates of natural cycle processes triggering serious problems in those countries involved in the tanning industry.
In the surroundings of the Guadalentin River (SE Spain), a high tannery industry activity has contributed to the contamination of the riverbed sediments due to the use of high wastewater quantities which were spilled into the river without previous treatment until 2003.
Guadalentin River does not present a permanent flow rate, since there is a dam upstream to control the flow and storage of the water for agricultural use during dry season; only when the reservoir is full are the doors of the dam open, and the flow rate can reach 0.20 m3 s−1 maximum. This water flow can cause a major extension of contamination by Cr in the river due to the surface runoff and the downstream contaminated sediment movement. The contamination by Cr of these areas may constitute a major concern for the (bio)accumulation of this metal in sediments and plants.
Chromium is found mainly in two oxidation states in the environment: trivalent Cr(III) and hexavalent Cr(VI). Hexavalent chromium is mobile and highly toxic for humans (Bini et al. 2008), and it is considered a skin irritant and an A class carcinogen by inhalation, whereas Cr(III) can be immobilized in soils/sediments. According to Fendorf (1995), Cr(III) has a strong affinity for negatively charged ions and colloids in soils/sediments and gives sparingly soluble compounds such as Cr(OH)3. Moreover, Cr(III) has low toxicity (James et al. 1997) and it is considered to be an essential trace element in human metabolism (Lilly et al. 2015).
The behavior of Cr in sediments depends on its chemical form. Hydrogen chromate ion (HCrO4 −) predominates at pH <6.5, while chromate ion (CrO4 2−) predominates at pH 6.5 and dichromate ion (Cr2O7 2−) predominates at higher concentrations (>10 mM) and at pH from 2 to 6. Dichromate ions pose a greater health hazard than chromate ions. Because of the anionic nature of Cr(VI), its adsorption in sediments is limited due to the predominance of negatively charged exchange sites in sediments, while Cr(III) is readily the chemical form adsorbed (McLean and Bledsoe 1992). Griffin and Shimp (1978) found that Cr(III) was the least mobile of the studied metals in sediments at pH 5. In contrast, Cr(III) complexed with soluble organic ligands remaining in the sediment solution (James and Bartlett 1983).
According to several authors, bioavailability and toxicity of metals can depend considerably on speciation (Graham et al. 2009; Vink 2009); therefore, the speciation of Cr is particularly important due to the toxicity of Cr(VI) and the biodependence of Cr(III) in plants, soils, and sediments (Wang et al. 1997; Mandiwana 2008; Elci et al. 2010). Moreover, the chemical speciation of Cr is an important facet of the chemistry of this pollutant with respect to both environmental and plant uptake of metals (Otabbong 1989).
The Cr contents in sediments represent a permanent environmental risk because Cr could be re-dissolved in water, suspended, or transported downstream in the river. Several agricultural areas are in the surrounding media in the Guadalentin River resulting in a severe spread of contamination.
On the other hand, there are no enough studies or information about soluble and exchangeable Cr in riverbed sediments and multivariate statistical analyses relating to sediment characterization and Cr contents, so this research contributes to the increase in the knowledge of Cr behavior in sediments under a Mediterranean semiarid climatic condition.
The main objective of this study was to evaluate the influence of sediment properties in the behavior of total Cr, Cr(VI), and Cr(III) in a selected stretch of the Guadalentin riverbed sediments. In addition, the degree of contamination by total Cr in the river sediments and the identification of hot pots of Cr pollution and the spatial distribution of Cr(VI) and Cr(III) were also investigated.
The degree of Cr accumulation, speciation (mobility), and the characterization of river sediments are presented as the most important parameters to define the best alternatives/options to minimize the pollution in the Guadalentin riverbed.
2 Materials and methods
2.1 Site location and sampling
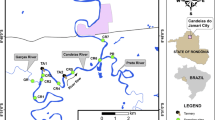
Guadalentin River is the major tributary of the Segura River (one of the largest Mediterranean basins in Spain). A representative stretch has been selected immediately downstream from the city center of Lorca (Region of Murcia, SE Spain). The total length was 1500 and 80 m of breadth. The area of the studied zone was 12 ha (Fig. 1).
Surface sediment samples have been collected according to a regular sampling grid of 2000 m2 with a sampling point each 45 m and a total of 60 sampling points. This grid was designed to cover all the area of the river stretch based on the aerial orthophoto (Fig. 1b). These samples were collected from 0 to 20 cm depth. In addition, other set of sediment samples were collected at three different depths (0–20, 20–50, 50–100 cm) in 30 sampling points.
2.2 Laboratory analyses
The collected samples were air-dried for 7 days, passed through a 2-mm sieve, and homogenized, and then they were stored in plastic bags at 4 °C prior to laboratory analyses. A part of each sample was kept without drying for alkaline digestion and Cr(VI) determination.
The sediment samples were analyzed for physical and chemical properties: pH and electrical conductivity (EC) were measured in a 1:1 and 1:5 deionized water/sediment ratio solution, respectively, according to Peech (1965). The equivalent calcium carbonate was determined by the volumetric method of Bernard’s calcimeter. Total organic carbon (TOC) was determined by dichromate method (Soil Survey Staff 2004), and total nitrogen (TN) was measured by Kjeldalh method (Duchaufour 1970). Cation exchange capacity (CEC) was determined following Roig et al. (1980). The clay, silt, and sand contents were performed by the Bouyoucos hydrometer method (Bouyoucos 1936); previously, the organic matter in the samples was removed by the addition of hydrogen peroxide. Available P was determined by the method of Burriel-Hernando (Díez 1982). Exchangeable cations such as K, Ca, and Mg were measured from CEC extracts with atomic absorption spectrometer (AAnalyst 800 PerkinElmer, USA). Nitrate, chloride, and sulfate were measured from 1:5 sediment/water extracts by ion chromatography (Metrohm 861 Advanced Compact IC, Switzerland).
A split of each sample was ground using an agate mortar (Retsch RM 100) for 15 min. One gram of ground sample was digested using 10 mL HNO3 and 10 mL H3ClO4 at 210 °C for 1.5 h in a heating block equipped with a temperature sensor and a time control panel to ensure digestion. To minimize the loss by evaporation or volatilization, a recovery system for gases was used in the procedure. After cooling, the samples were passed through a 0.2-μm filter for quantitative analysis, and 0.1 M HCl was added to volume in a 100-mL volumetric flask (Risser and Baker 1990). The total amounts of Cr were determined by atomic absorption spectrophotometer (AAnalyst 800, PerkinElmer USA). Exchangeable Cr was extracted using CaCl2 0.01 M (Pueyo et al. 2004), while soluble Cr was extracted using deionized water; after shaking, centrifugation, and filtering, exchangeable and soluble Cr were measured by a graphite furnace atomic absorption spectrometer (GFAAS PerkinElmer, USA) (Ernst 1996). Hexavalent Cr in sediments was determined by a selective extraction of Cr(VI) colorimetric method (US EPA 3060A (1996) and 7196 methods (1992)) following the modifications from several researchers (Romero et al. 2006; Severiche and Gonzalez 2013). Trivalent chromium was obtained by subtracting the total Cr and the Cr(VI) contents.
2.3 Quality assurance and quality control
Certified reference material (BAM-U110) from the Federal Institute for Materials Research and Testing and blanks were used as quality control samples during the analyses. Reference material was processed one time every ten sediment samples. We obtained recoveries of 93–106 % for Cr. Each sediment sample was prepared in duplicate for total Cr and hexavalent chromium determination. The relative percent difference of duplicate results was within 10 %.
2.4 Multivariate statistical analysis
Multivariate analysis methods provide techniques for classifying the interrelationship of sediment characteristics and metals. Principal component analysis (PCA) and cluster analysis (CA) are the most common multivariate statistical methods used in environmental studies (Mendiguchía et al. 2004; Tariq et al. 2006; Gutiérrez-Galindo et al. 2007; Peris et al. 2008; Acosta et al. 2009).
Descriptive statistics, Spearman correlation coefficient analysis, PCA, and CA were performed using the commercial statistics software package SPSS version 22.0 for Windows. Prior to statistical analysis, the data set distribution was evaluated using the Kolmogorov–Smirnov test; when the distribution was not normal, the data were log-transformed (Romic and Romic 2002) before statistical treatment. Spearman correlation coefficient (r) was used to measure the relationship between two quantitative variables. Multivariable statistical analyses such as PCA and CA were used to study the correlations among total Cr, Cr(VI), Cr(III), and sediment properties.
Principal component analysis is widely used to reduce data and to extract a smaller number of independent factors (principal components) and for analyzing relationships among observed variables (Tokalıŏglu and Kartal 2006). It starts with the correlation matrix describing the dispersion of the original variables and extracting the eigenvalues and eigenvectors (Xinwei et al. 2010). Principal component analysis can reduce the number of correlated variables to a smaller set of orthogonal factors (varimax rotation), making it easier to interpret a given multidimensional system by displaying the correlations among the original variables (Chandrasekaran et al. 2015).
Cluster analysis classifies a set of observations into two or more mutually exclusive unknown groups based on a combination of internal variables. Cluster analysis is often coupled with PCA to check results and to group individual parameters and variables (Xinwei et al. 2010).
2.5 Geospatial distribution
Geospatial methods are the most cost-effective tools in understanding the location of contaminants which also provide us with a comprehensive picture about their spatial distribution over a larger area (Nobi et al. 2010). In the present study, distribution patterns of Cr, Cr(III), Cr(VI), CaCl2-extractable Cr, and water-soluble Cr have been mapped using ArcView 3.1. The inverse distance weighted method was adopted for the interpolation of the data (Celine et al. 2006; Acosta et al. 2011)
3 Results and discussion
3.1 Physicochemical characterization of riverbed sediment
The physicochemical properties of the studied riverbed sediments are shown in Table 1. The riverbed sediments were alkaline, and the pH values ranged between 7.5 and 8.6 due to the presence of carbonates. The calcium carbonate contents range between 300 and 630 mg kg−1, with a mean value around 460 mg kg−1 at all the studied depths. The EC represents medium salinity content, with saline areas over 2 mS cm−1, especially at 20–100 cm deep. The low values of CEC in the sediments (lower than 10 cmol+ kg−1) indicate a low capacity for retaining nutrients adsorbed on colloids (clay and organic matter mainly) and for retaining Cr.
Clay content ranges around 200 mg kg−1 for all samples, with a higher clay content (210 mg kg−1) at 0–20 cm deep, the sand content being the most abundant fraction in the riverbed sediments (mean values of 600 mg kg−1). The sediments could be classified as sandy clay loam (USDA, 2015).
The TOC content is about 10 mg kg−1 in the most part of the riverbed with a mean value of 13 mg kg−1. A minimum drop in the TOC contents can be observed with the depth. The high C/N ratio (mean value 20.1) as a consequence of a very low amount of TOC in the sediments indicated a high mineralization of organic matter with a very poor quality (Brady and Weil 2008). The TN with contents below 3.0 mg kg−1 and a mean value of 2.3 mg kg−1 pointed out low values in the studied riverbed. Average available P concentration in the sediments was around 5 mg kg−1.
Mean values for the nitrate concentration in the sediments were 322, 434, and 273 mg kg−1 for each sampling depth. These values are in accordance with those in agricultural sediments (<500 mg kg−1) (Gómez-Garrido 2014) except some specific areas where an excessive nitrate content has been found (over 3000 mg kg−1). This may be explained due to several factors as a high C/N (>15) (Andrades 1996) connected to not enough phosphate content (no detected by ion chromatography in the study area) according to Acosta (2008).
Chloride and sulfate contents were very heterogeneous with values ranging from 4 to 4234 and from 90 to 8082 mg kg−1, respectively, indicating very high sulfate values mainly at 50–100 cm deep, as it could be expected since tanning agents (basic chromium sulfates) were used in the tanning processes according to Pawlikowski et al. (2006).
3.2 Spatial distribution of chromium in the sediments and identification of environmental risk areas
Figures 2 and 3 depict the geospatial distribution of total Cr, Cr(VI), Cr(III), and extractable and soluble Cr fractions in the riverbed sediments, from 0 to 20, 20 to 50, and 50 to 100 cm deep.
Total Cr concentration was high in the riverbed sediments (11–11099 mg kg−1) up to 100 cm deep, exceeding in almost all the study stretch, the background level, and generic reference values of Cr for Murcia Province (44.6 and 66.0 mg kg−1, respectively) (Martínez and Pérez 2007) and the US Environmental Protection Agency Sediment Screening Levels (SSL) for ingestion or inhalation of total Cr (390 mg kg−1). This observation indicates the sediment pollution by Cr until 100 cm deep. In addition, Cr contents reported in the sediments from Guadalentin River were higher than those determined by several authors (Köleli 2004; Dong et al. 2013; Lilly et al. 2015).
The highest degrees of sediment pollution (over 10,000 Cr mg kg−1) were located at 20–50 cm deep at the first 600 m east of the city center, and in the last 300 m of the studied area. Figure 2 reveals that the Cr contents in sediments were relatively higher near the discharge (point) of the tannery facility. This is consistent with the tannery industry location where the untreated tannery effluent was discharged for decades into the riverbed. This is one of the most prominent sources of Cr as it was described in other surveys (Pawlikowski et al. 2006; Pertsemli and Voutsa 2007).
The results about speciation of Cr (Fig. 3) showed higher concentrations of Cr(III) than Cr(VI) in all the sediments from the riverbed. Hexavalent Cr represented only a little fraction of the total Cr, with a mean value of 4.13 %. Therefore, Cr(III) was the predominant oxidation state with 95.87 % (mean value) of total Cr in the sediments. This finding is in accordance with Thorstensen (1984) who reported the presence of Cr(III) due to the use of a basic Cr sulfate salt containing Cr(III) for tanning industry and those reported by Dahl et al. (2013) where Cr was presented predominantly in the Cr(III) state.
Moreover, a reduction of Cr(VI) to Cr(III) could be produced in the sediments due to a slightly alkaline pH. Several researchers indicated that pH is one of the main factors affecting the rate and the extent of Cr(VI) reduction (Bartlett and Kimble 1976; Eary and Rai 1991). However, the presence of manganese oxides will be able to oxidize Cr(III) to Cr(VI) and increase the environmental risks (Fendorf and Zasoski 1992; Bartlett and James 1993)
The highest Cr(VI) concentrations were found in the same areas where high total Cr contents were observed. The maximum Cr(VI) value (78.92 mg kg−1) was located from 20 to 50 cm deep which could be explained by the leaching process, since Cr(VI) compounds (e.g., chromate) are quite mobile in the environment (Dhal et al. 2013).
The concentrations of exchangeable Cr and water-soluble Cr were low, indicating a very low Cr mobility in the studied sediments, likely due to the predominance of Cr(III) which is relatively immobile. The same pattern could be observed for the total Cr where the highest values were measured in the first 600 m in the riverbed and at 20–50 cm deep. The concentration of exchangeable fraction of Cr was very similar to the concentration of water-soluble Cr in the sediments. This fact points out that the bioavailability of Cr is very low due to the main presence of the less soluble substances containing Cr(III).
The highest Cr contamination was located in a small area in the northwest part of the studied riverbed at 100 cm deep. As it can be observed in the spatial distribution maps (Figs. 2 and 3), leaching of Cr has happened from the surface (spillage point) until 100 cm deep with the maximum concentration at 20–50 cm deep.
3.3 Behavior of chromium and their relation with sediment properties
The results of the PCA of Cr contents and physicochemical properties of sediment are shown in Table 2. Four principal components (PCs) with eigenvalues greater than unit were extracted. Principal component analysis leads to a reduction of initial dimension of the dataset to four factors which explains 67.7 % of the data variation. Therefore, these four factors play a significant role in explaining contamination by Cr in the riverbed. The communalities shown by the variables ranged from 28 % for EC to 93 % for total Cr; thus, all the elements were consequently well represented considering these four factors.
In detail, principal component 1 (PC1), which has high loadings of EC (0.41), CEC (0.60), Ca (0.80), Mg (0.76), Cl− (0.76), NO3 − (0.66), SO4 2− (0.76), and silt (0.75), accounts for 22.72 % of variance, being the most important component. PC1 could be better explained as salinity suggesting that the presence of salts is related to EC in the studied area as was supported based on the Spearman correlation matrix (Table 3). Modest and strong positive Spearman correlations, according to Fowler et al. (2006), have been found for EC with Ca (r =0.56, p < 0.01), Mg (r = 0.66, p < 0.01), Cl− (r = 0.71, p < 0.01), and SO4 2− (r = 0.78, p < 0.01).
Figure 4 displays the dendogram from the CA performed on the analyzed parameters with centroid linkage hierarchical clustering. The results showed that the studied parameters can be classified into three major clusters. Cluster 2 includes three sub-clusters that showed several associations of parameters with similar behavior in the study area: (1) clay and K; (2) Mg and Cl− (r = 0.79, p < 0.01) and C and SO4 2− (r = 0.69, p < 0.01) related to silt content; and (3) TN, NO3 −, CEC, OC, and EC. This cluster (number 2 and subclusters 2 and 3) is in accordance with the observation from PC1 (PCA) and Spearman correlation (related to anions and cations and its direct relationship with salinity).
PC2 has high loadings of total Cr (0.93), soluble Cr (0.94), exchangeable Cr (0.94), Cr(VI) (0.70), and Cr(III) (0.93). It accounts for 22.33 % of variance and is the most important component related to Cr content in the riverbed sediments. This is in concordance with results from CA and Spearman correlations—cluster number 1 (Fig. 4) that includes these parameters with strong relationship between total Cr–Cr(III) and soluble Cr–exchangeable Cr; the highest positive correlation coefficients (Table 3) have been found between Cr and soluble Cr (r = 0.89, p < 0.01), exchangeable Cr (r = 0.81, p < 0.01), Cr(VI) (r = 0.62, p < 0.01), and Cr(III) (r = 0.99, p < 0.01). Both CA and Spearman correlations indicate a high correlation and dependence among total Cr, soluble and exchangeable fractions, and speciation. Soluble Cr is highly related to Cr(III) (r = 0.89, p < 0.01), though Cr(III) present in sediments is relatively immobile because of its strong affinity for negatively charged ions and colloids, giving sparingly soluble compounds such as Cr(OH)3, dominating in the pH range 4–8 (Fendorf 1995). Nevertheless, an increment of content of organic matter could produce Cr(III)-soluble organic complexes (Stein and Schwedt 1994; Walsh 1996) and therefore enhance the environmental risk.
PC2 and the high correlation coefficients indicate that the measured concentrations of total Cr, soluble and exchangeable Cr, Cr(VI), and Cr(III) in the riverbed are not dependent of any sediment properties, and thus, the contamination by Cr in the Guadalentin riverbed has an anthropogenic origin mainly due to tannery effluents.
Several weak relationships (Fowler et al. 2006) between total Cr and TN or carbonates and soluble and exchangeable Cr with pH have been found (Table 3), although with low coefficient correlations. pH is generally known to be the main factor governing concentration of soluble metals (Ross 1994; Brallier et al. 1996; Acosta et al. 2011). However, low correlations were found between pH-soluble Cr (r = 0.362, p < 0.01) and pH-exchangeable Cr (r = −0.334, p < 0.01) in the studied sediment. According to Loayza (2008), the concentration of water-soluble Cr(VI) increases at pH >8, a value close to the mean values in the studied sediments.
PC3 was dependent upon OC, CO3 2−, TN, K, and pH and accounted for 11.87 % of the total variance (Table 2) but with low or medium correlation coefficients as it can be observed in Spearman correlation (Table 3). The presence of OC and TN in PC3 indicates that a certain amount of N in the sediments is associated to organic matter (Urbano 2001).
The values of pH and CO3 2− have a correlation coefficient of r = 0.49 (p < 0.01). This observation indicates that basic pH values in the sediments are hugely influenced by CO3 − contents. This relation is supported by CA (Fig. 4) in cluster 3.
Negative correlations between CO3 2− and water-soluble and exchangeable Cr in the sediments have been displayed in Table 3. This could be due to the high CO3 2− concentrations, which promote Cr precipitation and, consequently, its immobilization in the sediments. Additionally, the mechanism of increased sequestration is most likely a localized pH effect at the carbonate surface which promotes the formation of Cr(OH)3 species. Bioaccessibility of Cr(III) decreases as the carbonate content increases (r = −0.53, p < 0.01). According to Steward et al. (2013), Cr(III) sequestration is enhanced in sediments with high levels of carbonates which promotes the formation of solid-phase Cr(III) hydroxides that are sparingly soluble, even under acidic conditions. These hydroxides (i.e., Cr(OH)3) precipitate and are not easily bioaccesible.
PC4 only explained 10.73 % of the total variance and loaded positively on available P, clay, and sand. However, the correlation among them is very weak (r < 0.5) (Table 3), and therefore, further investigation is needed to elucidate the possible relation among these soil constituents.
4 Conclusions
High concentrations of Cr and Cr(VI) were found in the studied riverbed sediments, 11099 and 79 mg kg−1, respectively. These amounts exceeded in almost all the study stretch, the background level (44.6 mg kg−1), and reference values of Cr for Murcia Province (66 mg kg−1). Cr contamination until 100 cm deep was also observed. Based on Cr contents, the highest degree of sediment pollution was located at 20–50 cm deep.
Geospatial distribution indicated that the highest degree of pollution by Cr was located at the first 600 m from the city center, which is immediately east of the river, and in the last 300 m of the studied area. This revealed that the Cr content in the sediments was higher near the discharge point of the tannery industry which could be the main prominent source of Cr in the Guadalentin River.
Hexavalent chromium represented 4.13 % of the total Cr which indicated that Cr(III) was at the predominant oxidation state (95.8 %). This was expected since Cr(III) salt is used for tanning industry.
Pollution by Cr and Cr(VI) in Guadalentin River (SE of Spain) was related mainly to an anthropogenic origin (tannery industry), and the geochemical speciation is not associated to any sediment properties, indicating that the behavior of this element in the riverbed sediments depends only on the amount of total Cr in the sediments.
References
Acosta JA (2008) Caracterización y contenido en metales pesados de los suelos de la ciudad de Murcia y alrededores. Universidad Politécnica de Cartagena. PhD Thesis
Acosta JA, Faz A, Martinez-Martinez S (2009) Identification of heavy metal sources by multivariable analysis in a typical Mediterranean city (SE Spain). Environ Monit Assess 169:519–530
Acosta JA, Faz A, Martínez-Martínez S, Zornoza R, Carmona DM, Kabas S (2011) Multivariate statistical and GIS-based approach to evaluate heavy metals behavior in mine sites for future reclamation. J Geochem Explor 109:8–17
Andrades M (1996) Prácticas de edafología y climatología. Universidad de la Rioja Eds, Spain
Bajza Z, Vrcek IV (2001) Water quality analysis of mixtures obtained from tannery waste effluents. Ecotox Environ Safe 50:15–18
Bartlett RJ, James BR (1993) Redox chemistry of soils. In: Sparks DL (ed) Advances in agronomy. Academic, New York, pp 151–208
Bartlett RJ, Kimble JM (1976) Behavior of chromium in sediments II. Hexavalent forms. J Environ Qual 5(4):383–386
Bini C, Maleci L, Romanin A (2008) The chromium issue in sediments of the leather tannery district in Italy. J Geochem Explor 96:194–202
Bouyoucos GC (1936) Directions for making mechanical analysis of sediments by the hydrometer method. Sediment Sci 4:225–228
Brady N, Weil R (2008) The nature and properties of sediments, 14th edn. Pearson Prentice Hall, New Jersey, USA
Brallier S, Harrison RB, Henry CL, Dongsen X (1996) Liming effects on availability of Cd, Cu, Ni and Zn in sediment amended with sewage sludge 16 years previously. Water Air Soil Poll 86:195–206
Callender E (2003) Heavy metals in the environment historical trends. In: Holland HD, Turekian KK (eds) Treatise on geochemistry. Elsevier Publishers, Amsterdam, pp 67–105
Celine SL, Xiangdong L, Wenzhong S, Sharon C, Iain T (2006) Metal contamination in urban, suburban, and country park sediments of Hong Kong: a study based on GIS and multivariate statistics. Sci Total Environ 356:45–61
Chandrasekaran A, Ravisankar R, Harikrishnan N, Satapathy KK, Prasad MVR, Kanagasabapathy KV (2015) Multivariate statistical analysis of heavy metal concentration in sediments of Yelagiri Hills, Tamilnadu, India—spectroscopical approach. Spectrochim Acta A 137:589–600
Dhal B, Thatoi H, Das N, Pandey B (2013) Chemical and microbial remediation of hexavalent chromium from contaminated sediment and mining/metallurgical solid waste: a review. J Hazard Mater 250–251:272–291
Díez JA (1982) Consideraciones sobre la utilización de la técnica extractiva de Burriel-Hernando para la evaluación de fósforo asimilable en suelos. Anales de Edafología y Agrobiología 41:1345–1353
Dong C, Chen CW, Chen CF (2013) Distribution and contamination status of chromium in surface sediments of northern Kaohsiung Harbor, Taiwan. J Environ Sci 25(7):1450–1457
Duchaufour P (1970) Precis de Pedologie. Masson, Paris
Eary LE, Rai D (1991) Chromate reduction by subsurface sediments under acidic conditions. Sediment Sci Soc Am J 55:676–683
Elci L, Divrikli U, Akdogan A, Hol A, Cetin A, Soylak M (2010) Selective extraction of chromium(VI) using a leaching procedure with sodium carbonate from some plant leaves, sediment and sediment samples. J Hazard Mater 173(1–3):778–782
Ernst WHO (1996) Bioavailability of heavy metals and decontamination of sediments by plants. Appl Geochem 11:163–167
Fendorf SE (1995) Surface reactions of chromium in sediments and waters. Geoderma 67(1–2):55–71
Fendorf SE, Zasoski RJ (1992) Chromium (III): oxidation by –MnO2. I. Characterization. Environ Sci Technol 26(1):79–85
Fowler J, Cohen L, Jarvis P (2006) Practical statistics for field biology. Wiley, Chichester
Gómez-Garrido M (2014) Efectos ambientales de la valorización agronómica de purines de ganado porcino: dinámica del nitrógeno en el sistema suelo-agua-planta. PhD Tesis, Universidad Politécnica de Cartagena
Graham AM, Wadhawan AR, Bouwer EJ (2009) Chromium occurrence and speciation in Baltimore Harbour sediments and porewater, Baltimore, Maryland, USA. Environ Toxicol Chem 28:471–480
Griffin RA, Shimp NF (1978) Attenuation of pollutants in municipal landfill leachate by clay minerals. EPA-600/2-78-157
Gutiérrez-Galindo EA, Muñoz-Barbosa A, Walter L, Macías-Zamora JV, Segovia-Zavala JA (2007) Sources and factors influencing the spatial distribution of heavy metals in a coastal lagoon adjacent to the San Quintín volcanic field, Baja California, Mexico. Mar Pollut Bull 54:1962–1989
James BR, Bartlett RJ (1983) Behavior of chromium in sediments: V. Fate of organically complexed Cr(II) added to sediment. J Environ Qual 12:169–172
James BR, Petura JC, Vitale RJ, Mussoline GR (1997) Oxidation–reduction chemistry of chromium: relevance to the regulation and remediation of chromate-contaminated sediments. J Sediment Contam VI 6:569–580
Köleli N (2004) Speciation of chromium in 12 agricultural sediments form Turkey. Chemosphere 57:1473–1478
Lilly M, Moraetis D, Nikolaidis P, Karatzasa G, Kalogerakis N (2015) Characterization and mobility of geogenic chromium in sediments and riverbed sediments of Asopos basin. J Hazard Mater 281:12–19
Loayza J (2008) Metales pesados en los cultivos II. Boletín electrónico informativo sobre productos químicos y residuos 4(38):1–4. Universidad Nacional Mayor de San Marcos, Lima (Perú)
Mandiwana KL (2008) Rapid leaching of Cr(VI) in sediment with Na3PO4 in the determination of hexavalent chromium by electrothermal atomic absorption spectrometry. Talanta 74:736–740
Martínez MJ, Pérez C (2007) Niveles de fondo y niveles genéricos de referencia de metales pesados en suelos de la Región de Murcia. Consejería de Desarrollo Sostenible y Ordenación del Territorio, Murcia
McLean JE, Bledsoe BE (1992) Behaviour of metals in sediments. Ground Water Issue.EPA/540/S-92/018
Mendiguchía C, Moreno C, Galindo R, García-Vargas M (2004) Using chemometric tools to assess anthropogenic effects in river water. A case study: Guadalquivir River (Spain). Anal Chim Acta 515:143–149
Nobi E, Dilipan E, Thangarodjou T, Sivakumar K, Kannan L (2010) Geochemical and geo-statistical assessment of heavy metal concentration in the sediments of different coastal ecosystems of Andaman Islands, India. Estuar Coast Shelf S 87:253–264
Otabbong E (1989) Chemistry of Cr in some Swedish sediments. 1. Chromium speciation in sediment extracts: a comparison of different methods. Acta Agr Scand B-S P 39:119–129
Pawlikowski M, Szalinska E, Wardas M, Dominik J (2006) Cr originating from tanneries in river sediments: a preliminary investigation from the Upper Dunajec River (Poland). Pol J Environ Etud 15:885–894
Peech M (1965) Hidrogen-ion activity. In: Black CA (ed) Methods of sediment analysis, 2nd edn. Madison, American Society of Agronomy, pp 914–916
Peris M, Recatalá L, Micó C, Sánchez R, Sánchez J (2008) Increasing the knowledge of heavy metal contents and sources in agricultural sediments of the European Mediterranean region. Water Air Soil Poll 37:192–25
Pertsemli E, Voutsa D (2007) Distribution of heavy metals in Lake Doirani and Kerkini, Northern Greece. J Hazard Mater 148(3):529–537
Pueyo M, López-Sanchez JF, Rauret G (2004) Assessment of CaCl2, NaNO3 and NH4NO3 extraction procedures for the study of Cd, Cu, Pb and Zn extractability in contaminated sediments. Anal Chim Acta 504:217–226
Risser JA, Baker DE (1990) Testing sediments for toxic metals. In: Westerman RL (ed) Sediment testing and plant analysis, 3rd edn. Sediment Science Society of America Publisher, 3, Madison, Wisconsin, pp 275–298
Roig A, Romero M, Lax A, Fernández FG (1980) Estudio comparativo de métodos de determinación de capacidad de cambio catiónica en suelos calizos. Anales de Edafología y Agrobiología 39:2021–2032
Romero C, Pellerano R, Acevedo H, Vázquez F (2006) Estandarización condiciones preliminares para la determinación de cromo en muestras medioambientales. Comunicaciones científicas y tecnológicas. Resumen E-039. Universidad Nacional el Nordeste (Argentina)
Romic M, Romic D (2002) Heavy metals distribution in agricultural top sediments in urban area. Environ Geol 43:795–805
Ross SM (1994) Retention, transformation and mobility of toxic metals in sediments. In: Ross SM (ed) Toxic metals in sediment–plant systems. John Wiley and Sons Ltd, Chichester, UK, pp 63–152
Severiche C, Gonzalez HV (2013) Assesment of an analytical method for determining hexavalent chromium in water using spectrophotometry.Revista. Facultad de Ingenierías USBMed 4(1):22–26
Soil Survey Staff (2004) Sediment survey laboratory methods manual.Version No. 4.0.USDA NRCS. Sediment Survey Investigations Report No. 42. U.S. Govt. Print. Office, Washington, DC
Stein K, Schwedt G (1994) Chromium speciation in the wastewater from a tannery. Fresen J Anal Chem 350:38–41
Steward MA, Jardine PM, Barnett MO, Mehlhom TL, Hyder LK, Mckay LD (2013) Influence of sediment geochemical and physical properties on the sorption and bioaccessibility of chromium(III). J Environ Qual 32:129–136
Tariq SR, Shah MH, Shaheen N (2006) Multivariate analysis of trace metal levels in tannery effluents in relation to sediment and water: a case study from Peshawar, Pakistan. J Environ Manage 79:20–29
Taylor M, Diefendorf G (1990) Enzymatic treatment of chrome shavings. J Am Chem Soc 85(9):261–282
Thorstensen TC (1984) Practical leather technology. Robert E Krieger Publishing Company, Malabar, Florida
Tokalıŏglu S, Kartal S (2006) Multivariate analysis of the data and speciation of heavy metals in street dust samples from the Organized Industrial District in Kayseri (Turkey). Atmos Environ 40:2797–2805
Urbano P (2001) Tratado de fitotecnia general. Ed. Mundi Prensa, Madrid
US EPA Method 3060A (1996) Alkaline digestión for hexavalent chromium.
US EPA Method 7196A (1992) Chromium, hexavalent (colorimetric)
USDA (2015) Web Sediment Survey www.nrcs.usda.gov. Accessed may 2015
Vink JPM (2009) The origin of speciation: trace metal kinetics over natural water/sediment interfaces and the consequences for bioaccumulation. Environ Pollut 157:519–527
Walsh AR, O'Halloran J (1996) Chromium speciation in tannery effluent-I. An assessment of techniques and role of organic Cr(III) complexes. Water Res 30:2393–2400
Wang J, Ashley K, Kennedy ER, Neumeister C (1997) Determination of hexavalent chromium in industrial hygiene samples using ultrasonic extraction and flow injection analysis. Analyst 122:1307–1312
Xinwei L, Lijun W, Li LY, Kai L, Huang L, Kang D (2010) Multivariate statistical analysis of heavy metals in street dust of Baoji, NW China. J Hazard Mater 173(1):744–749
Acknowledgements
This work was funded by the EU-LIFE+ project RiverPhy (LIFE11 ENV/ES/000506) “Rehabilitation of a heavy metal contaminated riverbed by phytoextraction technique.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Maria Manuela Abreu
Rights and permissions
About this article
Cite this article
Rosales, R.M., Faz, A., Gómez-Garrido, M. et al. Geochemical speciation of chromium related to sediments properties in the riverbed contaminated by tannery effluents. J Soils Sediments 17, 1437–1448 (2017). https://doi.org/10.1007/s11368-016-1412-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-016-1412-7