Abstract
Purpose
Salt marsh plants are colonising wastes from a steel plant deposited on the Coina River Banks posing a potential contamination risk to the Tagus estuary ecosystem. The objectives of this study were to assess the uptake, accumulation and translocation of hazardous elements/nutrients in three spontaneous halophytic species, to evaluate the capacity of Tamarix africana to stabilise a contaminated salt marsh soil, and to evaluate the ecotoxicity of the pore water and elutriates from phytostabilised soils.
Materials and methods
The work comprises the following: fieldwork collection of soil samples from Coina River (an affluent of Tagus River) bank landfill, estuarine water and spontaneous plants (Aster tripolium, Halimione portulacoides and Sarcocornia sp.), and greenhouse studies (microcosm assay) with T. africana growing in one landfill salt marsh soil, for 97 days, and watered with estuarine water. Soils were analysed for pH, EC, Corganic, NPK, iron and manganese oxides. Soils total (acid digestion) elemental concentrations were determined by ICP/INAA. Estuarine waters, plants roots and shoots (acid digestion), soils available fraction (diluted organic acids extraction-RHIZO or pore water), and salts collected from the T. africana leaves surface were analysed for metals/metalloids (ICP-MS). Ecotoxicity assays were performed in T. africana soil elutriates and pore waters using Artemia franciscana and Brachionus plicatillis.
Results and discussion
Soils were contaminated, containing high total concentrations of arsenic, cadmium, chromium, copper, lead and zinc. However, their concentrations in the available fraction were <4 % of the total. The estuarine waters were contaminated with cadmium, but negligible ecotoxicological effect was observed. The spontaneous plants had significant uptake of the above elements, being mostly stored in the roots. Elemental concentrations in the shoots were within the normal range for plants. These species are not hazardous elements accumulators. Tamarix africana was well adapted to the contaminated saline soils, stored the contaminants in the roots, and had small concentrations of hazardous elements in the shoots. Excretion of hazardous elements by the salt glands was also observed. Elutriates from soils with and without plant did not show ecotoxicity.
Conclusions
The salt marsh species play an important role in the stabilisation of the soils in natural conditions. Tamarix africana showed potential for phytostabilisation of saline-contaminated soils. The low translocation of the elements from roots to shoots and/or active excretion of the elements by the salt glands was a tolerance mechanism in T. africana.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Estuaries are dynamic ecosystems with the highest specific and functional diversity and production in the world (Bianchi 2007). Being privileged sites for human settlements as well as agricultural and industrial activities, estuaries can also be a sink for wastes of the multiple human actions. According to the waste type and land disposal, severe consequences on water, sediment and/or biodiversity can occur, including the accumulation of potentially hazardous elements in the environment and, in some cases, their entry into the food chain representing an ecological risk and a threat to human health (Serafim et al.2013).
The River Tagus estuary (Portugal) received for decades, directly in the water or in the riverbanks, untreated hazardous wastes and sewage from several industries (e.g. chemical, petrochemical, steel, cement production and shipyards), agriculture and livestock contributing to high total concentrations of several potentially hazardous elements (e.g. cadmium, copper, lead and zinc), compounds and microorganisms in the salt marsh sediments (Mil-Homens et al. 2009; Duarte et al. 2010). However, the total concentrations of potentially hazardous elements in the soils and/or sediments do not reflect their bioavailability and, consequently, their potential environmental risk. Hazardous elements in salt marsh sediments are, generally, in an unavailable form, but erosion processes, tide and flooding events can be responsible for the sediment remobilisation and transport leading to the spread of contaminants.
Some plants, namely halophytes, colonise naturally contaminated salt marsh sediments, contributing to the improvement of the physical and chemical properties of these materials. Reduction of the stream velocity, water erosion and element availability are some advantages of the salt marsh plants development (Caçador et al. 1996; Almeida et al. 2011). The changing of the sediment-root environment by the increase of the organic matter and release of root exudates can contribute to the precipitation and/or chelation of elements in the sediments. Nevertheless, element availability can be reduced in the rhizosphere as a result of their accumulation in the root tissues and/or adsorption onto the root surfaces. Taking into account the active role of the spontaneous salt marsh plants in the attenuation of the contamination in sediments (Weis and Weis 2004; Reboreda and Caçador 2007a; Duarte et al. 2010; Andrades-Moreno et al. 2013), the implementation of a phytostabilisation process with different tolerant and autochthones plant species can be a promising strategy for sediments and/or salt marsh soil remediation. This in situ technique is considered adequate for the reclamation of contaminated heavy textured soils/sediments, especially if large areas of materials have to be treated, when other techniques are impracticable and most of all unprofitable (Vassilev et al. 2004). In general, the purpose of this technique is to reduce the spread and transfer of hazardous elements into the neighbouring environment and food chain as well as to stabilise the medium. The revegetation of the bare and contaminated soils/sediments must be done with plants tolerant to high salinity environments that are able to store the hazardous elements, mainly, in their roots, with restricted elements translocation to the aerial part.
Some salt marsh plants are annual species and have relatively low biomass, and/or environmental release of the elements accumulated inside the tissues after plant death. These facts lead to a controversy on the role played by salt marsh plants in the wetland remediation (Weis and Weis 2004; Caçador et al. 2009). In this context, the use of perennial plant species with high biomass production, and dense and deep root systems should be studied, especially their tolerance to estuarine environments and contaminated sediments/soils. Depending on their behaviour, those perennial species could be used in association with salt marsh species to develop more effective vegetation cover, to prevent water erosion and to immobilise contaminants from affected areas.
The chemical analysis of contaminated soils/sediments is not sufficient to fully evaluate the potential ecotoxicological risk of the contaminants in the environment. Those analyses do not provide information on the effects of the contaminants in the leaving organisms. To estimate the environmental risk of any contaminants, chemical methods need to be complemented with biological procedures (García-Lorenzo et al. 2014). Biotests are an example of biomonitoring tools commonly used. They are rapid and cost-effective techniques that provide a more direct measure of environmentally relevant toxicity of contaminated sites (Allan et al. 2006; Baran and Tarnawski 2013). Aquatic bioassays with elutriates have already been used to evaluate the toxicity of contaminated soils or sediments (Loureiro et al. 2005).
The objectives of this study were the following: (i) to assess the uptake, accumulation and translocation of potentially hazardous elements and nutrients in three representative halophytic species that grow spontaneously in contaminated soils of a River Coina salt marsh; (ii) to evaluate the capacity of Salix salviifolia, Flueggea tinctoria and Tamarix africana for the stabilisation of a contaminated salt marsh soil sparsely colonised by other halophytes; and (iii) to evaluate the ecotoxicity of the pore water and elutriates from the salt marsh soil subjected to the phytostabilisation experiments.
2 Materials and methods
2.1 Sampling site description
The former Portuguese steel industry “Siderurgia Nacional” was located in the industrial park of Paio Pires Village, in the left bank of the River Coina, an affluent of the River Tagus estuary. This industry produced steel between 1961 and 2001 using raw materials that included iron ore, limestone, mineral coal and pyrite ashes (Rollo 2005). According to the Portuguese Environmental Agency (APA 2008), over the 40 years of operation, 1.4 Tg of wastes were produced and deposited in warehouses and in a lagoon, creating a landfill. Around 21 Mg of those wastes are still in the industrial park. The deposited wastes contained iron, zinc and manganese sludge and dust, in its elemental and oxidised forms, aluminium oxide, silicon dioxide, organic carbon, asbestos, naphtha sludge, sulphur and calcium oxide (APA 2008).
The landfill is connected to the River Coina that is subject to tidal influence, which shaped it into a salt marsh and a lagoon. Nowadays, the salt marsh is colonised by several halophytic species, namely Atriplex halimus L., Aster tripolium subsp. pannonicus (Jacq.) Soó, Cotula coronopifolia L., Phagnalon saxatile (L.) Cass., Halimione portulacoides (L.) Aellen and Sarcocornia sp.
2.2 Field study
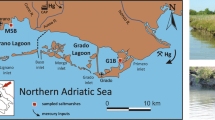
Three sampling areas with ≈1 m2 each were selected inside the landfill (Fig. 1) to include representative soils where the dominant salt marsh plants species were growing spontaneously. The sampling occurred in April 2013 during the low tide. In each sampling area, composite samples of soils (SPLS1, SPLS2 and SPLS3, ≈2 kg of homogenate) were collected, with a stainless steel shovel, in the surrounding of the radicular systems (0–20 cm) from the collected plants. In each sampling area were collected roots and shoots of three halophytic species: Aster tripolium ssp. pannonicus (Jacq.) Soó, Halimione portulacoides (L.) Aellen and Sarcocornia sp. Roots and shoots were immediately separated in the field, to prevent the translocation of the elements after collection. Plant samples represented composite samples of a homogenate of all individuals (>10 plants) of each species growing in each sampling area.
In February 2013 and during the low tide, a composite sample of soil (SPLS4) was also collected (≈150 kg, <20 cm depth), using a stainless steel shovel, inside the landfill in an area sparsely colonised by other halophytes, close to the exit point of the water from the lagoon (Fig. 1). Two water samples (250 L each) from the lagoon (estuarine water) were collected in February (LW1) and April (LW2) 2013 in the intersection point of the lagoon water with the River Coina (Fig. 1). The soil was homogenised and kept moist, in closed plastic bags, until its use for chemical characterisation and in the microcosm assays. The lagoon water was kept in the laboratory at room temperature.
2.2.1 Microcosm assay: experimental set-up and monitoring
A microcosm assay was set up with two experimental sets (CG1-positive control group without plants and TG1-test group with plants, n = 4 each) for each riparian plant species (Salix salviifolia Brot., Flueggea tinctoria (L.) G.L. Webster, and Tamarix africana Poiret), in pots filled with 16 kg of soil (SPLS4) each and watered daily with lagoon water. In order to evaluate the potential of each species for the phytostabilisation of contaminated salt marsh soils, positive control pots (CG1) remained bare. Plants resulting from vegetative propagation of cuttings harvested in uncontaminated sites from Portugal (S. salviifolia: Zêzere watershed; F. tinctoria and T. africana: Constança, Tagus watershed) and planted in commercial peat were used in the assay (cuttings 15–30 cm long) (Fig. 2). In order to adapt the plants to the saline stress, plants were irrigated during eight days with the saline water from lagoon before transplantation. These species were chosen because they are riparian species, autochthones from Portugal, and their root systems are extensive and deep, which can be an advantage in the stabilisation of sediments/soils and, consequently, erosion control.
Twenty plants of each species were carefully removed from the peat, washed in tap water and then with deionised water, and transplanted to the pots of test group (TG1, five plants per pot). The soils in the pots were kept moist, maintaining the pots in a tray with 2.5–4.5 cm high of the lagoon water, in order to simulate field moist conditions of a medium salt marsh where those species can grow. A second negative control group with T. africana plants (CG2, n = 2) was carried out in cell seed tray (160 cm3) filled with peat and watered daily with deionised water. In the CG2 were used a composite sample of five plants per replicate. All pots were kept in a greenhouse for 97 days (between February and May). Additionally, an independent test group (TG2, n = 2) was set up similarly to TG1 in order to collect the salts formed at the surface of the T. africana leaves. In this experiment, five plants per pot were transplanted to the soil and watered daily with the lagoon water during 97 days.
2.3 Sample characterisation
2.3.1 Water, sediment and peat
The water samples were filtered (0.45 μm pore size cellulose nitrate membrane) and characterised for pH, electrical conductivity (EC), concentrations of chloride by potentiometry, total sulphate by gravimetry as barium sulphate and hydrogen carbonate by acid–base titration. Total concentrations of aluminium, arsenic, cadmium, calcium, copper, chromium, iron, lead, magnesium, manganese, nickel, potassium, sodium and zinc were determined by inductively coupled plasma mass spectrometry (ICP-MS) after acidification with HNO3 to pH < 2 (Activation Laboratories 2014a).
The collected moist soils (SPLS1 to SPLS4) were homogenised and divided in subsamples. For samples SPLS1, SPLS2 and SPLS3 were considered two subsamples: One subsample (≈100 g) was kept in sterilised plastic containers and frozen at −20 °C, while the remaining sample was air dried and sieved (<2 mm). Sample SPLS4 was divided in three subsamples as follows: One subsample (≈100 g) was kept in sterilised plastic containers and frozen at −20 °C similarly to the other soil samples; a second subsample (≈500 g) was air-dried and sieved (<2 mm); the remaining soil was kept moist until to be used in the microcosm assays. Samples of the commercial peat used for rooting of the cuttings and for CG2 experiments were dried and ground.
Soil dried subsamples (fraction < 2 mm) were characterised for pH and EC in water suspension (1:2.5 m/V), organic carbon by dry combustion (Ströhlein method) (Póvoas and Barral 1992), extractable phosphorous and potassium (Égner et al. 1960), and mineral nitrogen (molecular absorption spectrophotometry in segmented flow autoanalyser preceded by Micro Kjeldahl acid digestion for ammonia nitrogen). In the soils and peat, the total concentrations of the same elements than in the lagoon water were determined by inductively coupled plasma atomic emission spectroscopy (ICP-EAS) and instrumental neutron activation analysis (INAA) after four-acid digestion (perchloric acid + nitric acid + hydrochloric acid + hydrofluoric acid), in a certified laboratory (Activation Laboratories 2014b).
The frozen samples of soils were used to determine the iron from total iron oxides (De Endredy 1963, extraction with Tamm reagent (0.1 mol/L oxalic acid + 0.175 mol/L ammonium oxalate at pH 3.25) under UV radiation for 4 h) and non-crystalline iron oxides (Schwertmann 1964, extraction with Tamm reagent in obscurity for 4 h), and manganese from manganese oxides (Chao 1972; extraction with 0.1 mol/L hydroxylamine hydrochloride solution at pH 2.0 for 30 min).
The chemical elements of the soils (frozen samples) and moist peat in the available fraction were extracted using an aqueous solution composed of a diluted aqueous mixture of organic acids (acetic acid + lactic acid + citric acid + malic acid + formic acid at 10 mmol/L for 16 h) that simulate the rhizosphere conditions (RHIZO solution; Feng et al. 2005). The concentrations of aluminium, arsenic, cadmium, calcium, copper, chromium, iron, lead, magnesium, manganese, nickel, potassium, sodium and zinc in the available fraction were analysed by ICP-MS (Activation Laboratories 2014b).
Pore water was collected by centrifugation from moist SPLS4 soil before microcosm experiments and in the end of the microcosm assay (97 days after plant transplantation). The pore water from each pot was collected and filtered (<0.45-μm cellulose acetate membranes), and the concentrations of the same elements analysed in the RHIZO solution were determined by ICP-MS (Activation Laboratories 2014b).
2.3.2 Plants
The plant samples (roots and shoots) collected in the salt marsh and those from the T. africana experiments, except TG2, were washed in abundant tap water followed by deionised water. Additionally, the roots were sonicated in deionised water, in an ultrasound-assisted bath until the physical removal of the soil particles and peat residues. Roots and shoots were dried, at 40 °C until reaching constant weight, homogenised and finely ground. The multielemental total concentrations in the shoots and roots were analysed by ICP-MS, after ashing (475 °C) and nitric acid digestion (Activation Laboratories 2014c).
The T. africana shoots from the TG2 were washed with 0.1 % (V/V) HNO3 for 2 min (Hagemeyer and Waisel 1988) in order to dissolve the salts formed at the surface of the leaves. The resulting solution was analysed by ICP-MS (Activation Laboratories 2014b).
2.4 Ecotoxicity evaluation
The toxicity to aquatic organisms was evaluated in pore water and elutriates from soil used in the microcosm assay. Elutriates were obtained by continuous agitation of soil samples and LW2 lagoon water (1:10 m/V) during 16 h in a rotatory agitator. Elutriates pH and EC were measured, and then the elutriate samples were centrifuged and stored at 4 °C in the dark until their use. A lagoon water sample LW2 was also tested for its ecotoxicity. Acute aquatic assays were conducted with two aquatic organisms: the brine shrimp Artemia franciscana and the rotifer Brachionus plicatillis, both main standard organisms used in aquatic risk assessment studies (MicroBioTests 2003, 2012). The bioassays were performed by determining the mortality of A. franciscana larvae exposed for 24 h and mortality of B. plicatillis juveniles exposed for 48 h according to the bioassay procedure (MicroBioTests 2003, 2012). Test dilutions with a gradient concentration range of 100, 50, 25, 12.5 and 6.25 % (V/V) were conducted with standard artificial seawater of normal salinity (35 g/kg seawater) in order to mimic the lagoon water. Results were expressed in mortality percentage for both bioassays.
2.5 Data analysis
Statistical analysis was performed with the statistical programme SPSS v18.0 for Windows. Differences among elements concentrations in the roots and shoots of plants from the landfill were analysed by a one-way ANOVA and post hoc Tukey HSD test (p < 0.05). Differences between elements concentrations in roots and shoots of T. africana growing in the assays were analysed non-parametrically using Kruskal–Wallis ANOVA by ranks test. Bivariate Pearson correlations were used to correlate plant and soil characteristics (r > 0.90). The quality control of the analysis was performed by blanks, analytical replicate samples, standard reference materials and laboratory standards at the international accredited laboratory Activation Laboratories (ISO/IEC 17025).
The soil-to-plant transfer coefficient, translocation coefficient and biological absorption coefficient were calculated in order to evaluate the plant behaviour. Soil-to-plant transfer coefficient (TransferC = [total element in the shoots] / [total element in the soil]) characterises the accumulation of a specific chemical element in the shoots (Abreu et al. 2014). The translocation coefficient (TranslC = [total element in the shoots] / [total element in the roots]) evaluates the plant capacity to translocate an element from the roots to the shoots (Abreu et al. 2014). The biological absorption coefficient (BAC = [total element in the roots] / [element in the available fraction of the soil]) evaluates the uptake capacity of an element by the plant. According to the Perelman classification of the BAC, this coefficient can be divided into five groups, which indicate the intensity of the element absorption by the plant roots (intensive 100 > BAC > 10; strong 10 > BAC > 1; intermediate 1 > BAC > 0.1; weak 0.1 > BAC > 0.01; very weak 0.01 > BAC > 0.001) (Perelman 1966).
3 Results and discussion
3.1 Salt marsh soil characteristics
The chemical characteristics of the soils collected in the River Coina salt marsh are given in Tables 1 and 2. The soils are neutral, strongly saline and with high concentrations of extractable phosphorus and potassium, while nitrogen concentration was low (except in SPLS1, Table 1) (INIA-LQARS 2000) as was also reported by several authors for sediments from Tagus estuary (Caçador et al. 2009; Duarte et al. 2010). The concentrations of extractable potassium can be related to the estuarine waters, but phosphorus can be explained by the deposition of steel wastes in the landfill, which contained, among other minerals, phosphates as apatite and lazulite (Santos 1965).
The organic matter concentrations of the studied soils (255 to 457 g/kg) were higher than the values reported by several authors for sediments collected in different areas of the Tagus estuary (Caçador et al. 2000; Reboreda and Caçador 2007a, b; Duarte et al. 2010). The life cycle of the majority of the plant species collected in the lagoon can have contributed to the increase of the organic matter in the soils by decomposition of dead plant tissues (Caçador et al. 2009; Duarte et al. 2010). Moreover, the past deposition of sludge naphtha in the landfill (APA 2008) and possibly some coal can also justify the high concentrations of organic carbon in the studied soils.
The high concentrations of iron in iron oxides in the soils (Table 1), mostly in the non-crystalline fraction (>65 % of the total), reflect the composition of the iron ore used in the steel production and, consequently, its wastes (APA 2008). As expected, iron oxide concentrations were much more abundant than the manganese oxides.
Regarding the multielemental characterisation (Table 2), all soil samples presented much higher total concentrations than those reported in the literature for samples collected in other areas of the Tagus estuary (Caçador et al. 2000, 2009; Reboreda and Caçador 2007a, b; Vinagre et al. 2008; Santos-Echeandía et al. 2010). According to the Sediment Quality Guidelines for the Protection of Aquatic Life (CCME 2001), the studied samples have total concentrations of arsenic, cadmium, copper, chromium, lead and zinc above the interim marine sediment quality guidelines (ISQG) and probable effect level (PEL) (except for cadmium in SPLS1 and copper in all samples) representing a potential contamination source.
In spite of the high total concentrations of the potentially hazardous elements (aluminium, arsenic, cadmium, copper, chromium, lead and zinc), their concentrations in the available fraction were small representing less than 4 % of the total concentrations (Table 2). The values for these element concentrations in the available fraction indicate that the soils may not represent a source of hazardous elements spread and even a potential hazard for biodiversity. The concentrations of the elements, considered as nutrients (calcium, iron, magnesium, manganese, nickel and potassium), in the soil available fraction were a relatively small percentage of their total concentrations (ranging from 0.4 to 6 %), with exception of calcium and magnesium (10–20 %), and sodium that was mostly available (58–70 %). It is important not to forget that these soils are in permanent contact with salt water from Tagus estuary containing high concentrations of sodium, magnesium and calcium.
Correlations were found between the concentrations of arsenic, chromium, lead, magnesium, manganese, sodium and zinc in the soil available fraction and their total concentrations in the soils (0.91 < r < 1.00, depending on the element). Trace element availability can also be a consequence of the soil chemical and mineralogical composition (e.g. presence of sulfides, iron and manganese oxides), as well as other characteristics (e.g. pH, Eh and organic matter) (Otero and Macías 2002; Weis and Weis 2004; Reboreda and Caçador 2007b). In the present study, no significant correlations were established between the available concentrations of the elements and the pH and iron oxide values. However, organic matter seems to decrease the availability of copper, iron and nickel, as well as the manganese oxides for aluminium, chromium, lead and zinc (−0.90 < r < −1.00, depending on the element). These results are in agreement with the data obtained in the sediments from Tagus estuary which showed that copper was mainly associated to organic matter, whereas only less than 10 % of the total concentrations of lead and zinc were bound to manganese oxides (Reboreda and Caçador 2007b).
3.2 Spontaneous halophyte plant behaviour
The concentrations of the elements in the roots and shoots of the halophyte plants collected in the field are given in Table 3. For the majority of the elements, similar concentrations in the roots were obtained for the three species being those concentrations always higher than the available concentrations in the soils (Table 2). Nevertheless, the concentrations of sodium in the roots varied with the species reaching the highest concentrations in A. tripolium. A wide range of elemental concentration in the roots of the three studied species is reported by several authors (Caçador et al. 2000, 2009; Fitzgerald et al. 2003; Reboreda and Caçador 2007a; Sousa et al. 2008; Vinagre et al. 2008; Caetano et al. 2008; Duarte et al. 2010; Santos-Echeandía et al. 2010; Milić et al. 2012; Andrades-Moreno et al. 2013), which are different from those obtained in the present study. This fact can be related to the variations in the total and/or the available concentrations of the elements in the soils where the plants grew.
The uptake of the elements by the roots was positively correlated with their concentrations in the soil available fraction, whose values depend on the element and the species (A. tripolium, r Ca = 0.98, r Cd = 0.91; H. portulacoides, r As = 0.93, r Pb = 0.96; Sarcocornia sp., r Al = 0.99, r Cd = 0.93, r Cr = 1.00, r Zn = 0.90). Nonetheless, roots can also restrict the uptake of some elements from the soil available fraction or even exclude some of them (Kabata-Pendias 2011): A. tripolium, Fe (r = −1.00); H. portulacoides Ca, Fe, Mn and Ni (−0.93 < r < −0.99) and Sarcocornia sp. Cu, Fe and Ni (−0.95 < r < −1.00).
According to the biological absorption coefficients (BAC) (Table 4), the uptake of the elements depended on the species, the element and, in some cases, even the plant sample. The values for the biological absorption coefficients for arsenic, cadmium, chromium, copper and lead indicate, according to Perelman (1966), an intensive absorption capacity by the plant roots independently of the species, while for calcium and sodium, the absorption capacity was strong. The three species presented a similar strong to intensive absorption capacity for iron and manganese. Regarding the BAC for aluminium and nickel, the H. portulacoides showed higher uptake of these elements than the other two species. The uptake of zinc varied with the species, being intensive for H. portulacoides, strong for A. tripolium, and Sarcocornia sp. showed both behaviours. The coefficients ([element]root / [element]sediment) calculated by Caçador et al. (2000), Caetano et al. (2008) and Sousa et al. (2008) for arsenic, cadmium, chromium, copper, iron, lead, manganese, nickel and/or zinc, for S. fruticosa and H. portulacoides growing in sediments from River Tagus, by Fitzgerald et al. (2003) for A. tripolium from Suir Estuary, and by Milić et al. (2012) for H. portulacoides growing in the Mediterranean coast of Montenegro were much lower than the values obtained in the present study. This fact can be associated with the different extraction methodology of elements from the sediments, which represent either the total (or pseudo-total) concentrations of the elements or their available fraction concentrations. The uptake and accumulation of the elements by salt marsh plants also depend on other factors such as the age and growth stage of the plants, seasonal variations and soils/sediments characteristics (Caçador et al. 2000; Weis and Weis 2004; Kabata-Pendias 2011; Milić et al. 2012).
After absorption by the roots, the transport of the elements within the plant is important because it can guarantee the existence of adequate concentrations of nutrients to the various physiological processes. Weis and Weis (2004) stated that the element translocation is dependent on the species, element and environmental conditions; however, no differences were observed among the studied species. Similar behaviours of the elements’ translocation were observed for the three species. The elements (except sodium and in some cases calcium) were stored in the roots (TranslC < 1). These results agreed with previously published data on the same species growing in different contaminated and non-contaminated areas (Caçador et al. 2000, 2009; Fitzgerald et al. 2003; Reboreda and Caçador 2007a; Sousa et al. 2008; Caetano et al. 2008; Duarte et al. 2010; Milić et al. 2012; Andrades-Moreno et al. 2013). According to Sousa et al. (2008), H. portulacoides mostly retains metals in the cell wall especially in the roots.
The concentrations of the elements in the shoots were similar in all species, except for copper and zinc. The shoots of A. tripolium had the highest concentration of copper and zinc, which are significantly different from H. portulacoides and Sarcocornia sp., respectively. The analysis of the published data concerning the three species growing in different contaminated and non-contaminated areas showed a wide range for the concentrations of the elements in the shoots (Caçador et al. 2000, 2009; Fitzgerald et al. 2003; Reboreda and Caçador 2007a; Caetano et al. 2008; Sousa et al. 2008; Duarte et al. 2010; Milić et al. 2012; Sousa 2012; Andrades-Moreno et al. 2013), being the obtained data within those range.
The low translocation of potentially hazardous elements from roots to shoots of all the studied species is an efficient tolerance mechanism to avoid the phytotoxic concentrations in the aboveground part of the plants. In fact, concentrations of arsenic, cadmium, chromium, copper, lead, manganese, nickel and zinc in the plant shoots are in the range considered normal/sufficient and below the toxic levels for plants in general (Kabata-Pendias 2011). However, some shoot samples had concentrations of copper and manganese considered deficient (A. tripolium: Mn; H. portulacoides: Cu and Mn; Sarcocornia sp.: Mn). Nutrient deficiencies due to the competition with sodium were reported by Hu and Schmidhalter (2005); however, this competition, evaluated by statistical analysis, was not observed for the studied species. Nonetheless, antagonism interactions involving other elements can also contribute to nutrient imbalances (Kabata-Pendias 2011). In this way, concentrations of copper and nickel in Sarcocornia sp. shoots seem to affect negatively the manganese concentrations in the same organ (r = −0.98 and −0.94, respectively). However, for the other species, no influence was observed for the studied elements.
According to the soil-to-plant transfer coefficients (always lower than one), the three species are accumulators of sodium but non-accumulators of the analysed potentially hazardous elements and nutrients. The low translocation of the potentially hazardous elements, high concentrations of elements in roots, elemental concentrations in shoots below the phytotoxic level and the non-accumulator behaviour of the species are important factors in the selection of these plant species for phytostabilisation purposes of salt marsh sediments and soils.
3.3 Microcosm assay
3.3.1 Irrigation water characteristics
The chemical characteristics of the soil (Section 3.1) and the lagoon water, both used in the microcosm assay, are summarised in Tables 1, 2 and 5. The two water samples presented similar physical and chemical characteristics (Table 5). The waters are slightly alkaline, strongly saline and had high concentrations of calcium, magnesium, potassium and sodium, cations that are naturally abundant in marine waters as well as chlorides. The concentrations of the potentially hazardous elements were low. However, according to the Canadian Water Quality Guidelines for the Protection of Aquatic Life–marine water, both samples can be considered contaminated with cadmium (>0.12 μg/L; CCME 2014).
3.3.2 Chemical and ecotoxicological characterisation of the soils
The chemical characteristics of the soil and the concentrations of the elements in the pore water are given in Table 6. During the assay, the plant growth did not affect the pH of the soil; however, the EC varied significantly. The EC increased along the assay, especially in the control CG1 (sediments without plants) where the concentrations of the elements in the pore water were also higher than those in the sediments with T. africana (TG1).
Independently of the plant growth, the concentrations of iron and manganese in the soils pore waters, at the end of the experiments, were reduced when compared to those found in the pore water of the soil SPLS4 used to fill the pots (Table 6). This decrease can be explained by the observed formation of iron and manganese oxides. The notorious increase of the concentrations of zinc at the end of the experiment can be explained by the interaction of the soil with the walls of the pots, which, in spite of being enamelled, could be damaged exposing the underlying metal (zinc enriched) to the wet sediment. Although vegetated soils can concentrate more elements in the pore water than non-vegetated soils due to the alteration of chemical conditions of the soils (Otero and Macías 2002), this fact was not observed in the present study. The opposite was observed for calcium, magnesium and potassium whose concentrations were higher in the control (CG1) than in the experiments with plants (TG1).
The increase of the elements’ concentrations in the pore water can be associated to the enrichment through the lagoon water used for irrigation, which had higher concentrations of some elements (Table 5) than the pore water of the soil before the assay.
The concentrations of the elements in the peat used to grow the T. africana (CG2-negative control) are shown in Table 7. In general, the total concentrations of the studied elements in the soil (Table 2), especially the potentially hazardous elements, were higher than those in the peat, but the opposite was observed to calcium, chromium, magnesium, nickel and potassium. The concentrations of the elements in the available fraction (extracted with RHIZO solution) of the peat are much lower than in the SPS4 soil used to grow the T. africana, except for aluminium and chromium (Tables 2 and 7).
The aquatic toxicity tests, done in the lagoon waters and sediments’ elutriates, were valid according to the criteria established in the guidelines for both bioassays (MicroBioTests 2003, 2012). No toxic effects higher than 10 % were observed in the lagoon waters as well as in the sediments’ elutriates from microcosm assay (with and without plants), at the end of the assay. The growth of T. africana did not influence the ecotoxicity of elutriates.
3.3.3 Plants behaviour
Approximately 8 days after transplantation to the contaminated soil, S. salviifolia and F. tinctoria died, while T. africana presented 90 % of survival. Although S. salviifolia and F. tinctoria occur naturally in areas more distant from estuaries (Gómez-Mercado et al. 2012), these species, growing in peat substrata, tolerated the irrigation with saline water from the lagoon and did not show visual signs of saline stress. However, after transplantation, a cumulative effect of salinity and the contamination or the textural characteristics of the soil could be occurred leading to the death of the plants.
The concentrations of the elements in the roots and shoots of T. africana are presented in Table 8. Regardless the substrata where the plants grew, the concentrations of the elements in the roots (except for aluminium in control CG2) were higher than those either in the pore water or in the RHIZO solution (Tables 6, 7 and 8). This fact can be explained by the low selectivity of this species in the elements uptake (Storey and Tomson 1994; Manousaki et al. 2008).
Significant variations were obtained between elements concentrations in the roots of T. africana from TG1 and from CG2. The concentrations of aluminium, arsenic, chromium, copper, iron, lead, sodium and zinc in the roots of the plants growing in the soil (TG1) were higher than in the plant roots from the control (CG2), while the opposite was observed for calcium (CG2 > TG1). Variations in the concentrations of the elements from the pore water of the soil (TG1) and the available fraction from the control (CG2) (Tables 6 and 7) did not explain the differences in the concentrations of the elements in the roots. Nonetheless, the salinity of the substrata can stimulate the uptake of the elements and their accumulation in the roots (Kadukova and Kalogerakis 2007). Several authors (Kadukova and Kalogerakis 2007; Manousaki et al. 2008, 2009; Moreno-Jiménez et al. 2009) reported much higher concentrations of arsenic, cadmium and lead, in the roots of Tamarix sp. (growing in hydroponic conditions or organic substrate mixture enriched with these elements) than those found in the plant roots growing in both substrata in the present study. Concentrations of calcium, copper, chromium, manganese and sodium in the roots of Tamarix nilotica (Ehrenb.) Bunge from banks of the River Nile (Fawzy et al. 2006) were higher than those found in the roots of T. africana collected from both substrata used in this study. Nonetheless, the opposite was observed for cadmium, nickel and zinc (Fawzy et al. 2006).
The uptake capacity, evaluated by biological absorption coefficient (Table 9), varied especially with the substrata, but in the plants from control (CG2), the uptake capacity was dependent on the element. According to the Perelman classification, the uptake of all the elements in the plants from soil (TG1) was intensive. However, in plants from control (CG2), the uptake of cadmium, chromium, copper, manganese, nickel and zinc was intensive, while for arsenic, iron and sodium was strong and for aluminium was intermediate.
After the absorption of the elements, T. africana growing in both soil and peat stored mostly aluminium, arsenic, cadmium, chromium and nickel in the roots (TranslC < 1), while the nutrients and sodium were translocated from the roots to shoots (Table 9). The same translocation behaviour was observed in T. nilotica for copper, magnesium, manganese, potassium, sodium and zinc, but not for calcium, chromium and nickel (Fawzy et al. 2006). Both translocation behaviours were observed for arsenic in plants from both substrata as well as for cadmium in plants growing in the soil TG1. This fact can be due to a variation on the substrata salinity and its effect on the uptake and translocation of the elements. Manousaki et al. (2008, 2009) reported that the salinity leads to the increase of the uptake and translocation of cadmium from the roots to the aerial part in Tamarix smyrnensis Bunge growing in soils and hydroponic experiments.
The translocation behaviour of iron and lead was dependent on the substrata. The plants growing in the soil TG1 also stored iron and lead in the roots, while the plants growing in the peat (CG2) translocated lead to the shoots and had both behaviours for iron. Fawzy et al. (2006) observed, in T. nilotica growing in a non-contaminated area, the storage of lead in the roots and translocation of iron from roots to leaves. These observations did not agree with the results obtained in the present study for plants from CG2. Differences between lead concentrations in the roots and shoots were also reported for T. smyrnensis suggesting an important translocation restriction of this cation, which is stored in the roots in order to avoid toxicity (Kadukova and Kalogerakis 2007; Kadukova et al. 2008). However, Manousaki et al. (2009) reported that the increase of salinity decrease the lead uptake and accumulation in roots, whereas Kadukova et al. (2008) found that there was no clear relationship between lead uptake by the plant and the increase of the salinity.
The highest concentrations of arsenic, cadmium, sodium and zinc were obtained in the shoots of the plants growing in the contaminated soil, while for calcium and manganese, the highest concentrations were reached in the control (CG2). Although the element concentrations in the shoots depend on several biological processes of the species (e.g. uptake, accumulation in roots, translocation from roots to shoots and tolerance capacity; Kabata-Pendias 2011), the concentrations of calcium and manganese in the pore water (TG1) and in the soil available fraction (GG2) seem to be related to the concentrations of the same elements in the shoots.
In the shoots of the plants from both substrata, the concentrations of the studied elements (except manganese) were within the range considered sufficient and below the phytotoxic level for various plant species (Kabata-Pendias 2011). The concentrations of manganese were very low and considered deficient (Kabata-Pendias 2011) as was also observed in the halophytic plants collected in the field. Although the deficiency of manganese can be associated to antagonism interactions, no negative effect with the studied elements was found. A wide range of elemental concentrations in Tamarix sp. shoots, from plants growing in mining areas, non-contaminated areas and microcosm assays enriched with potentially hazardous elements, are reported by several authors (Del Río et al. 2002; Boularbah et al. 2006; Fawzy et al. 2006; Kadukova and Kalogerakis 2007; Manousaki et al. 2008; Moreno-Jiménez et al. 2009). These concentrations are, in general, higher than those measured in the plants of the present study. This variation can be related not only to the species but also to the elements’ concentrations in soil/sediments (total and/or available fractions).
According to the calculated soil-to-plant transfer coefficients (always lower than one), plants from both substrata were non-accumulators of potentially hazardous elements and nutrients. For sodium and zinc, the accumulation behaviour varied with substrata; plants from soil TG1 were non-accumulators of zinc but accumulators of sodium, while the opposite was observed in plants from control (CG2). For calcium, the plants from soils presented both accumulator behaviours (TransferC 0.8–1). On the contrary, T. nilotica was accumulator of all the studied elements except calcium, cadmium and copper (Fawzy et al. 2006).
3.3.4 Salts formed at the surface of the Tamarix africana leaves
Tamarix species have leaf salt glands, which are responsible for the salt exclusion and, consequently, the tolerance to the salinity (Manousaki et al. 2008; Abou-Jaoudé 2011). Nonetheless, salt glands also provide a mechanism for the exclusion of toxic ions (Weis and Weis 2004; Flowers et al. 2010).
The secretory product of T. africana comprised a large variety of ions (Table 10) related to the chemical composition of the aqueous solutions in the rhizosphere, which is in accordance with the results reported by several authors for different species of the Tamarix genus (Storey and Thompson 1994; Manousaki et al. 2005, 2009; Fawzy et al. 2006). In fact, the most abundant elements in the salts (aluminium, calcium, iron, potassium, magnesium, manganese, sodium and zinc) corresponded to elements with higher availability from the soil (Table 6). Leaves of T. nilotica from banks of river Nile also secreted high amounts of calcium, iron, potassium, magnesium, manganese, sodium and zinc, and in less quantity cadmium, chromium, copper, lead and nickel, which were determined in the salts of the plant leaves (Fawzy et al. 2006). Studies done with T. smyrnensis also showed excretion of cadmium and lead on the surface of their leaves (Manousaki et al. 2005, 2008; Kadukova and Kalogerakis 2007; Kadukova et al. 2008).
The low translocations of aluminium, cadmium, iron, nickel and lead (Table 9) found in T. africana can be explained by the great amounts of these elements, which were exuded through the salts ([element]salts/[element]shoots 1.0–8.5, depending on the element), corresponding to a metal detoxification mechanism. The existence of great amounts of sodium in the salts also contributes to the plant detoxification ([element]salts/[element]shoots 8.0) balancing the translocation of this element to shoots. Arsenic concentration in the salts was lower than in the shoots ([element]salts/[element]shoots 0.7) suggesting that the small concentrations of this element in the shoots are only due to its low translocation. Copper and zinc, due to their physiological function in shoots, were strongly translocated from roots to the shoots, not being highly excreted ([element]salts/[element]shoots 0.6 and 0.3, respectively). On the contrary, calcium was excreted ([element]salts/[element]shoots 1.6) in spite of the values (1.8–3.0, Table 9) obtained for the translocation coefficient of this element being, probably a consequence of its high concentration in the lagoon water. The deficiency in manganese observed in the plant shoots (Table 8) can be related to the great amounts of this element exuded by the leaves ([element]salts/[element]shoots 1.9) (Table 10).
The different behaviour observed for nutrients and potentially hazardous elements in the T. africana plants growing in the contaminated soil suggests the existence of an efficient mechanism of tolerance associated to chemical elements exclusion through the salt formation at the surface of the leaves.
4 Conclusions
The soils developed on sediments of the lagoon, on which the former steel plant deposited a considerable quantity of hazardous wastes, can be considered contaminated by arsenic, cadmium, chromium, copper, lead and zinc. However, the availability of these potentially toxic elements in the soils was very small. Although the lagoon water is only contaminated with cadmium and presented very high salinity, no ecotoxicological effect of soil elutriates was observed for both aquatic species (A. franciscana and B. plicatillis).
The halophytic plants that grew spontaneously in the salt marsh soils (A. tripolium, H. portulacoides and Sarcocornia sp.) are tolerant to the contaminants present in the soils and play an important role in their stabilisation. The low translocation of the potentially hazardous elements from roots to shoots, the small concentrations of these elements in the shoots and the non-accumulator behaviour of these plant species indicate their potential for phytostabilisation of the contaminated salt marsh soils.
Tamarix africana was well adapted to the contaminated saline soils; however, the same did not occur with S. salviifolia and F. tinctoria that died after 8 days from transplantation into the contaminated soil. Tamarix africana was able to decrease the contaminants’ concentrations in the pore water at the end of the experiment. At ecotoxicological level, no differences were observed in the elutriates from the soil with and without plant growth. The low concentrations of the potentially hazardous elements in the T. africana shoots can be associated with their low translocation from roots to shoots, and/or active excretion mechanism by the salt glands. The great absorption of chemical elements, storage of potentially hazardous elements in the roots, their concentrations below phytotoxic level in the shoots, low elements’ translocation and high tolerance to the high salinity with the presence of potentially hazardous elements in the substrata indicate T. africana as a promising phytostabilisation species.
References
Abou-Jaoudé R (2011) Harnessing the biodiversity of Italian Tamarix species: populations, plants and leaf responses to extreme environmental constraint. Dissertation, University of Tuscia. http://dspace.unitus.it/bitstream/2067/2367/1/raboujaoude_tesid.pdf Accessed March 2014
Abreu MM, Godinho B, Magalhães MCF (2014) Risk assessment of Arbutus unedo L. fruits from plants growing on contaminated soils in the Panasqueira mine area, Portugal. J Soil Sediment 14:744–757
Activation Laboratories (2014a) 1H - total digestion - ICP/INAA. http://www.actlabs.com/page.aspx?page=506&app=226&cat1=549&tp=12&lk=no&menu=64&print=yes. Accessed in March 2014
Activation Laboratories (2014b) 6 – Hidrogeochemistry – ICP/MS. http://www.actlabs.com/page.aspx?page=544&app=226&cat1=549&tp=12&lk=no&menu=64&print=yes. Accessed in March 2014
Activation Laboratories (2014c) 2D – Vegetation Ash – ICP/MS. http://www.actlabs.com/page.aspx?page=538&app=226&cat1=549&tp=12&lk=no&menu=64&print=yes. Accessed in March 2014
Allan IJ, Vrana B, Greenwood R, Mills GA, Roig B, Gonzalez C (2006) A “toolbox” for biological and chemical monitoring requirements for the European Union’s Water Framework Directive. Talanta 69:302–322
Almeida CMR, Mucha AP, Vasconcelos MT (2011) Role of different salt marsh plants on metal retention in an urban estuary (Lima estuary, NW Portugal). Estuar Coast Shelf Sci 91:243–249
Andrades-Moreno L, Cambrollé J, Figueroa ME, Mateos-Naranjo E (2013) Growth and survival of Halimione portulacoides stem cuttings in heavy metal contaminated soils. Mar Pollut Bull 75:28–32
APA (2008) REA 2007 Portugal – Relatório do Estado do Ambiente. Agência Portuguesa do Ambiente, Lisboa
Baran A, Tarnawski M (2013) Phytotoxkit/Phytotestkit and Microtox® as tools for toxicity assessment of sediments. Ecotox Environ Safe 98:19–27
Bianchi TS (2007) Biogeochemistry of estuaries. Oxford University Press, New York
Boularbah A, Schwartz C, Bitton G, Aboudrar W, Ouhammou A, Morel JL (2006) Heavy metal contamination from mining sites in South Morocco: 2. Assessment of metal accumulation and toxicity in plants. Chemosphere 63:811–817
Caçador I, Vale C, Catarino F (1996) Accumulation of Zn, Pb, Cu and Ni in sediments between roots of the Tagus estuary salt marshes, Portugal. Estuar Coast Shelf Sci 42:393–403
Caçador I, Vale C, Catarino F (2000) Seasonal variation of Zn, Pb, Cu and Cd concentrations in the root-sediment system of Spartina maritima and Halimione portulacoides from Tagus estuary salt marshes. Mar Environ Res 49:279–290
Caçador I, Caetano M, Duarte B, Vale C (2009) Stock and losses of trace metals from salt marsh plants. Mar Environ Res 67:75–82
Caetano M, Vale C, Cesário C, Fonseca F (2008) Evidence for preferential depths of metal retention in roots of salt marsh plants. Sci Total Environ 390:466–474
CCME (2001) Sediment quality guidelines for the protection of aquatic life. Canadian Council of Ministers of the Environment, Winnipeg
CCME (2014) Water quality guidelines for the protection of aquatic life. Canadian Council of Ministers of the Environment, Winnipeg
Chao TT (1972) Selective dissolution of manganese oxides from soils and sediments with acidified hydroxylamine hydrochloride. Soil Sci Soc Am 36:762–768
De Endredy AS (1963) Estimation of free iron oxides in soils and clays by photolytic methods. Clay Miner Bull 9:209–217
Del Río M, Font R, Almela C, Vélez D, Montoro R, Bailo ADH (2002) Heavy metals and arsenic uptake by wild vegetation in the Guadiamar river area after the toxic spill of the Aznalcóllar mine. J Biotech 98:125–137
Duarte B, Caetano M, Almeida PR, Vale C, Caçador I (2010) Accumulation and biological cycling of heavy metal in four salt marsh species, from Tagus estuary (Portugal). Environ Pollut 158:1661–1668
Égner H, Riehm H, Domingo WR (1960) Untersuchhungen uber die chemicheboden: analyse als grundlage für die beurteilung der nahrstonffzustandes der boden. II. Chemiche extraktions, methoden zur phosphor, und kalium bestimmung. Kungl Lantbrukshoegst 26:199–215
Fawzy EM, Soltan ME, Sirry SM (2006) Mobilization of different metals between Tamarix parts and their crystal salts – soil system at the banks of river Nile, Aswan, Egypt. Toxicol Environ Chem 88(4):603–618
Feng M, Shan X, Zhang S, Wen B (2005) A comparison of the rhizosphere-based method with DTPA, EDTA, CaCl2 and NaNO3 extraction methods for prediction of bioavailability of metals in soil to barley. Environ Pollut 137:231–240
Fitzgerald EJ, Caffrey JM, Nesaratnam ST, McLoughlin P (2003) Copper and lead concentrations in salt marsh plants on the Suir Estuary, Ireland. Environ Pollut 123:67–74
Flowers TJ, Galal HK, Bromham L (2010) Evolution of halophytes: multiple origins of salt tolerance in land plants. Funct Plant Biol 37:604–612
García-Lorenzo ML, Martínez-Sánchez MJ, Pérez-Sirvent C (2014) Application of a plant bioassay for the evaluation of ecotoxicological risks of heavy metals in sediments affected by mining activities. J Soils Sediments 14:1753–1765
Gómez-Mercado F, Torres FDM, Luque EG, Lozano SDH (2012) Salinity tolerance of the hygrophilous plant species in the wetlands of the south of the Iberian Peninsula. Not Bot Horti Agrobot 40:18–28
Hagemeyer J, Waisel Y (1988) Excretion of ions (Cd2+, Li2+, Na+ and Cl−) by Tamarix aphylla. Physiol Plant 73:541–546
Hu Y, Schmidhalter U (2005) Drought and salinity: a comparison of their effects on mineral nutrition of plants. J Plant Nutr Soil Sci 168:541–549
INIA-LQARS (2000) Manual de fertilização das culturas. Laboratório Químico Agrícola Rebelo da Silva, Lisboa
Kabata-Pendias A (2011) Trace elements in soils and plants, 4th edn. CRC, Boca Raton
Kadukova J, Kalogerakis N (2007) Lead accumulation from non-saline and saline environment by Tamarix smyrnensis Bunge. Eur J Soil Biol 43:216–223
Kadukova J, Manousaki E, Kalogerakis N (2008) Pb and Cd accumulation and phyto-excretion by salt cedar (Tamarix Smyrnensis Bunge). Int J Phytoremediat 10:31–46
Loureiro S, Ferreira AL, Soares AM, Nogueira AJ (2005) Evaluation of the toxicity of two soils from Jales Mine (Portugal) using aquatic bioassays. Chemosphere 61:168–177
Manousaki E, Kadunova J, Naxakis G, Kalogerakis N (2005) Release of Pb and Cd by the leaves of the mediterranean plant Tamarix smyrnensis. Proc 9th Int Conf Environ Sci Technol A921-A927
Manousaki E, Kadunova J, Papadantonakis N, Kalogerakis N (2008) Phytoextraction and phytoexcretion of Cd by the leaves of Tamarix smyrnensis growing on contaminated non-saline and saline soils. Environ Res 106:326–332
Manousaki E, Kokali F, Kalogerakis N (2009) Influence of salinity of Pb and Cd accumulation by the salt ceader tamarix (T. semyrnesis Bu). J Chem Tech Biotech 84:877–883
MicroBioTests (2003) Artoxkit MTM – Artemia toxicity screening test for estuarine and marine waters. MicroBioTests Inc, Mariakerke (Gent)
MicroBioTests (2012) Rotoxkit MTM – Rotifer toxicity screening test for estuarine and marine waters. Standard operational procedure. MicroBioTests Inc, Mariakerke (Gent)
Mil-Homens M, Branco V, Lopes C, Vale C, Abrantes F, Boer W, Vicente M (2009) Using factor analysis to characterize historical trends of trace metal contamination in a sediment core from the Tagus prodelta, Portugal. Water Air Soil Pollut 197:277–287
Milić D, Luković J, Ninkov J, Zeremski-Škorić T, Zorić L, Vasin J, Milić S (2012) Heavy metal content in halophytic plants from inland and maritime saline areas. Cent Eur J Biol 7:307–317
Moreno-Jiménez E, Esteban E, Carpena-Ruiz RO, Peñalosa JM (2009) Arsenic- and mercury- induced phytotoxicity in the Mediterranean shrubs Pistacia lentiscus and Tamarix gallica grown in hydroponic culture. Ecotoxicol Environ Saf 72:1781–1789
Otero XL, Macías F (2002) Spatial and seasonal variation in heavy metals in interstitial water of salt marsh soils. Environ Pollut 120:183–190
Perelman AI (1966) The geochemistry of land areas in Russian. Izd Vish Shk, Moscow. In: Nagaraju A, Karimulla S (2002) Accumulation of elements in plants and soils in and around Nellore Mica Belt, Andhra Pradesh, India - a biogeochemical study. Environ Geol 41:852–860
Póvoas I, Barral MF (1992) Métodos de análise de solos. Comunicações do Instituto de Investigação Científica Tropical. Serie Ciências Agrárias 10, Lisboa
Reboreda R, Caçador I (2007a) Halophyte vegetation influences in salt marsh retention capacity for heavy metals. Environ Pollut 146:147–154
Reboreda R, Caçador I (2007b) Copper, zinc and lead speciation in salt marsh sediments colonised by Halimione portulacoides and Spartina maritima. Chemosphere 69:1655–1661
Rollo MF (2005) Memórias da Siderurgia: contribuições para a história da indústria siderúrgica em Portugal. História, Lisboa
Santos JLG (1965) Ensaios com o minério de ferro de Moncorvo na Lurgi Gesellschaft fur chemie und huttenwesen m.b.H. (Frankfurt-Main). Bol Minas 2(1):3–17
Santos-Echeandía J, Vale C, Caetano M, Pereira P, Prego R (2010) Effect of tidal flooding on metal distribution in pore waters of marsh sediments and its transport to water column (Tagus estuary, Portugal). Marine Environ Res 70:358–367
Schwertmann U (1964) Differenzierung der eisenoxidedes bodensdurch extraktionmit ammonium oxalat-lösung. Z Pflanzenernähr Düng Bodenk 105:194–202
Serafim A, Company R, Lopes B, Pereira C, Cravo A, Fonseca VF, França S, Bebianno MJ, Cabral HN (2013) Evaluation of sediment toxicity in different Portuguese estuaries: ecological impact of metals and polycyclic aromatic hydrocarbons. Estuar Coast Shelf Sci 130:30–41
Sousa ARB (2012) Explorar o potencial da Sarcocornia perennis para saladas frescas ou em pó (Sal Verde) como alternativa ao sal. University of Algarve, Dissertation
Sousa AI, Caçador I, Lillebø AI, Pardal MA (2008) Heavy metal accumulation in Halimione portulacoides: intra- and extra-cellular metal binding sites. Chemosphere 70:850–857
Storey R, Thomson WW (1994) An X-ray microanalysis study of the salt glands and intracellular calcium crystals of Tamarix. Ann Bot 73:307–313
Vassilev A, Schwitzguébel JP, Thewys T, van der Lelie D, Vangronsveld J (2004) The use of plants for remediation of metal-contaminated soils. Sci World J 4:9–34
Vinagre C, Cabral HN, Caçador I (2008) Influence of halophytes and metal contamination on salt marsh macro-benthic communities. Estuar Coast Shelf Sci 76:715–722
Weis J, Weis P (2004) Metal uptake, transport and release by wetland plants: implications for phytoremediation and restoration. Environ Int 30:685–700
Acknowledgments
The authors would like to thank to José Correia (ISA, University of Lisbon) and Dr. M. Teresa Caldeira (University of Aveiro) for their technical assistance, and the Portuguese Foundation for Science and Technology (FCT) for financial research support for LEAF- Linking Landscape, Environment, Agriculture and Food (FCT-UID/AGR/04129/2013), CICECO-Aveiro Institute of Materials (FCT-UID/CTM /50011/2013), financed by national funds through the FCT/MEC and when appropriate co-financed by FEDER under the PT2020 partnership agreement, and the PhD grant (SFRH/BD/80198/2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jaume Bech
Rights and permissions
About this article
Cite this article
Santos, E.S., Abreu, M.M., Peres, S. et al. Potential of Tamarix africana and other halophyte species for phytostabilisation of contaminated salt marsh soils. J Soils Sediments 17, 1459–1473 (2017). https://doi.org/10.1007/s11368-015-1333-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1333-x