Abstract
Purpose
This study assessed soils from 36 parks and gardens (Vigo City, NW of Spain) where there are different degrees of traffic intensity and activity.
Materials and methods
The soils were characterised, and the content of Ba, Ca, Cr, Cu, Fe, Mg, Mn, Na, Ni, Pb, Si, Sr and Zn was analysed. Further assessment determined the geoaccumulation index, enrichment factor and the contamination degree by metals with adverse effects on human health and environmental quality.
Results and discussion
The results reveal the existence of a moderate degree of contamination by Ba, Pb and Cu, which contribute the most to soil contamination due to the influence of industrial areas and main transport routes. Correlation and cluster analyses suggest that the metals included in the study have three possible origins: “natural” (Na and Si), “mixed” (two groups with different source intensity: Ca and Sr and Cr, Fe, Mg, Mn and Ni) and two possible “urban” sources: traffic (Cu, Pb, Zn) and mixed (Ba).
Conclusions
None of the soils can be classified as strongly contaminated but more than 61 % of the moderate contamination degree determined in the studied soils is explained by the Ba, Cu and Pb contents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Urban soils are increasingly attracting the attention of numerous investigators, who are focusing on soils, vegetation and demographic and economic factors, mainly due to the fact that almost 49 % of the world population lives in urban areas, and it is estimated that this figure will be increased to 84 % in 2050. In Europe, 75 % of the population lives in urban or peri-urban areas covering only 9 % of the total land surface, and in 2020 this value will rise to 80 % (Ajmone-Marsan and Biasioli 2010; Picket et al. 2011). These soils are being subjected to severe anthropogenic pressure because of urban development. There are different “urban factors” which directly or indirectly affect their physical, chemical and biological properties (Picket et al. 2011). The urban soils are created by the processes of urbanisation, and they are materials that have been manipulated, disturbed or transported by man’s activities in the urban environment. Their physical, chemical and biological properties are generally unfavourable as rooting medium (Craul 1992). Therefore, urban soils are different from agricultural or natural ones as these are soils that were transformed by supply of amendments and fertilisation, ploughing, sowing, etc., which makes them fertiles, suitables for life development, particularly plants for animal and human consumption, and for livestock production. Hence, the conditions of the urban soils vary from one city to another depending on the urban structure, the distribution of vehicles and the types of fuels used (Kabata-Pendias 2010; Minguillón et al. 2014).
Soils play an important role as they act not only as sources but also as sinks of various contaminants. Unlike in the atmosphere or water masses, contaminants are accumulated in soils for long periods due to their interaction with colloidal particles (Metreveli and Frimmel 2007). Furthermore, green areas are essential components of urban ecosystems, not only because of their capacity to purify toxic substances but also because of their role in the microclimate (e.g. dampen temperature and water content variations), among other factors (De Kimpe and Morel 2000; Li et al. 2013).
The natural processes and anthropogenic activities, such as the atmospheric emissions of industries and transport, the construction waste disposal and landfills (Puskás and Farsang 2009) influence the metal content in urban soils. The proximity of humans and animals to contamination sources in urban areas (industries, transport infrastructures, etc) increases the risk of developing various diseases through the inhalation, ingestion or dermal exposure, enhancing mortality and morbidity rates (Rydin et al. 2012; Minguillón et al. 2014). Therefore, they are commonly used as indicators of environmental quality and pollution (Loska et al. 2004; Ajmone-Marsan and Biasioli 2010; Szolnoki et al. 2013).
After the ban on leaded petrol in most industrialised countries around the end of the 1990s, Pb is no longer the only indicator of pollution and heavy traffic in urban environments. As a result, in recent years, Ba, Cd, Cu, Ni, Pt, Pd or Zn have been introduced as other indicators of traffic-related contamination (Monaci and Bargagli 1997; Sutherland 2000; Zechmeister et al. 2005). For example, the origin of Ba is attributed to enrichment due to brake linings in cars and later emissions due to frictional processes (Fernández–Espinosa and Ternero-Rodríguez 2004).
To control risks and determine the degree of soil contamination with respect to natural or background values, different legislations include generic reference or intervention levels (GRL in air, water or soil). They allow to estimate the soil quality and to study any risk above accepted levels for human health or protection of ecosystems.
The objectives of this study were (1) to study the concentrations of several elements (Ba, Ca, Cr, Cu, Fe, Mg, Mn, Na, Ni, Pb, Si, Sr and Zn), focussing on those with implications for environmental implications in the urban soils from Vigo (NW, Spain); (2) to determine the degree of soil quality and (3) to identify the possible origin (natural and/or anthropogenic) of the metals in the soils for obtaining the spatial distribution of the possible pollutants.
2 Materials and methods
2.1 Study area
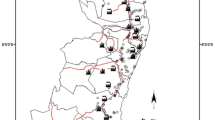
The city of Vigo (296,479 inhabitants in 2013) is located in the NW of Spain (datum ETRS89/UTM 29 N 42.2333 N, -8.7166 W) on the shores of the Ría de Vigo. It is the largest city in the region, and the 14th largest in Spain. Important commercial and industrial activities are operating in this area, in the SW of the city there is a car manufacturing plant whilst in the NW and NE the most noteworthy companies are involved in shipbuilding (dockyards). There is also an important commercial and fishing port as well as an airport situated 9 km E of the city (Fig. 1). The number of vehicles is around 170,000 (±50 % diesel), and the average daily traffic at the end of 2013 was approximately 500,000. The climate ranges from oceanic to Mediterranean, with mild temperatures (an average of 14.8 °C) and high precipitation (1450 mm year−1). The dominant winds are from the SW. The geology of the area is mainly granite rock in the W and gneiss in the E, the latter occupying more than 50 % of the surface.
2.2 Sampling and analytical method
A total of 36 soil samples were collected (0–20 cm depth) from green areas scattered around the city of Vigo. The main focus when sampling was paid to the city centre during a dry period (1–15 October 2013) (Fig. 1). In order to obtain a good perspective of the evolution of the city, samples were taken from at least 10-year-old areas that had not undergone important changes. They were also selected attending to the degree and type of vegetation coverage as this is an important factor that influences the soil properties and functions. The soil structure, organic matter content, water holding capacity, pH, etc. are directly related to the plant coverage and also influence the retention of the different pollutants. The selected soils were also sampled attending to the intensity of surrounding traffic and the distance to different areas of activities (residential, industrial, riverside walks, etc.) (Table 1). Most of these green areas are subjected to some kind of human intervention (irrigation, fertilisation and periodical changes in ornamental vegetation, trampling of the herbaceous vegetation by pedestrians, etc.).
After the elimination of the superficial layer of vegetation (2–3 cm depth), the samples were taken using an Eijkelkamp Mod.04.20.SA sampler (three subsamples per sampling point) and stored in polyethylene bags to be transported to the laboratory. They were air dried, sieved (<2 mm) and homogenised to make up a compound sample, which was then subdivided into three subsamples for further analysis.
After sieving, the coarse component of the soil was determined (Eriksson and Holmgren 1996). The fraction <2 mm was analysed for soil pH, total Kjeldahl-N and organic matter content (OM). They were determined, respectively, with a pH electrode in 2:1 water/soil extracts, according to Bremner and Mulvaney (1982) and following the Walkley and Black (1934) procedure. Available P was determined as described by Olsen and Sommers (1982). Exchangeable cations (Ca2+, K+, Mg2+, Na+ and Al3+) were extracted with 0.1 M BaCl2 (Hendershot and Duquette 1986). The concentrations of Al, Ca, K, Mg and Na and available P were analysed by ICP-OES (Perkin Elmer Optima 4300DV).
The total concentrations of Ba, Ca, Cr, Cu, Fe, Mg, Mn, Na, Ni, Pb, Si, Sr and Zn in the soils were determined by means of X-ray fluorescence (XRF) (Siemens; model SRS-3000, Hannover, Germany). For each sample, three 40-mm-diameter pellets were prepared by compacting 5 g of the ground sample under 25 Mg of pressure. The overall accuracy and precision of the analytical procedures for total contents (XFR method) were verified through the analysis of two standard reference materials (SRM 2710a Montana I Soil and 2711a Montana II Soil) from the National Institute of Standards and Technology (NIST). Spikes and duplicates were also used as a part of our quality control. The blank determinations were also performed in triplicate throughout all the experiments (data included as supplementary data, Table S1, see Electronic Supplementary Material).
2.3 Statistical and spatial analysis
All the analyses were carried out in triplicate, and each parameter is expressed as the average on a dry weight basis. Pearson correlation analyses (p < 0.05, p < 0.01) were applied in order to identify the relationships between the metal contents. Cluster analyses (CA) were performed in order to determine the different geochemical groups by means of the complete link method; the results are presented in a dendrogram with Euclidean distances. The statistical analyses were carried out with R 3.0.3 (R Development Core Team 2014). The distribution maps were performed with QGIS 2.0.2 (QGIS Development Team, 2014), using the BCN25-BTN25 (1:25,000) cartography from the Instituto Geográfico Nacional de España (2014) and the inverse distance weighting (IDW) method.
2.4 Assessment of pollution
Different environmental quality indicators were used, such as the geoaccumulation index (Igeo), the enrichment factor (EF), the contamination factor (ci f ) and the contamination degree (Cdeg) (Table 2), on the basis of the concentrations of the earth’s crust in accordance with several studies (Loska et al. 2004; Srinivasa Gowd et al. 2010). As there can be endogenous concentrations which are higher than in the terrestrial average (Loska et al. 2004), in this study, the different environmental quality indicators where calculated on the basis of the background values (Table 3) and GRL for the Galician soils (Macías and Calvo de Anta 2009).
2.4.1 Geoaccumulation index
The geoaccumulation index introduced by Müller (1979) was calculated on the basis of the background levels (Loska et al. 2004) by using the following equation:
Cn is the total concentration measured in each soil and for each metal, Bn is the background value for each metal and the factor of 1.5 is used to correct any possible lithological variations.
As it was previously indicated, Igeo was calculated on the basis of the regional background values (Table 3). The different Igeo values are classified into six degrees or classes (Table 2) where Igeo ≥ 5 indicates an enrichment of at least 100-fold with respect to the background value.
2.4.2 Enrichment factor
The enrichment factor (EF) proposed by Buat-Menard and Chesselet (1979) is based on the standardisation of the elements measured against one used as a reference (Al, Fe, Mn, Sc and Ti). Some authors (Covelli and Fontolan 1997; Loska et al. 2004) indicated that it is better to use than other environmental indices. In this work, Fe was used as a reference value for the standardisation following the criteria of Daskalakis and O’Connor (1995) and Acevedo-Figueroa et al. (2006). They indicated that (1) the geochemistry of Fe and many trace metals are similar both in oxic and anoxic conditions; (2) its geochemistry is similar to that of many trace metals and (3) its natural sediment concentration tends to be uniform. As a result, there are five categories defined for the different EF values (Table 2). The EF was calculated according to the following equation:
2.4.3 Contamination factor and contamination degree
The global assessment of the contamination in the soil was carried out by means of the contamination factor (c i f) and the degree of contamination (Cdeg). According to Hakanson (1980), c i f is the contamination factor for each element and Cdeg represents the sum of all the contamination factors. Each contamination factor was calculated following the equation:
c i 0-1 is the average metal content from at least five sampling points; c i n is the pre-industrial concentration of each metal (the regional background levels, Table 3). Table 2 shows the classes proposed by Hakanson (1980) into which both the contamination factor and the degrees of contamination values are included.
3 Results and discussion
3.1 Properties of urban soil samples
The values of the different soil characteristics are shown in Table 3. The high content of coarse component (18.2 ± 7.3 %; 5–50.4 %) is due to the materials used to refill the areas planned for gardens as there are different artefacts and rubble coming from those materials. The pHH2O is between 4.30 and 7.79 (average 5.87); the highest values are from soils close to beach areas (S2, S35) and the influence of CaCO3 from the mollusc shells probably influence the pH of the soils. In fact, most of the soils are moderately to slightly acid as a result of the influence of the geological parent material of the soil (granite rocks), which was attenuated by the construction waste materials among other factors (Li et al. 2013).
The average of the N Kjeldahl concentration is 1061 ± 650 mg kg−1 (60–3400 mg kg−1). The values are enough to support the growth of vegetation (Li et al. 2013) and similar to those measured in soils of other cities such as Anji, China (540–1310 mg kg−1; Yang et al. 2014), Seville (400–5200 mg kg−1; Ruiz-Cortés et al. 2005) and Denver-Boulder, Co, USA (2100 mg kg−1; Golubiewski 2006). Nevertheless, the OM values are, in general, low 29,600 ± 12,900 mg kg−1 (and lower than those found by other authors for urban soils in Torino (5200–82,700 mg kg−1; Biasioli et al. 2006) or Seville (8400–80,500 mg k−1; Ruiz-Cortés et al. 2005).
The average of the available P contents is 25.65 ± 24.75 mg kg−1 (4–146.90 mg kg−1), and they are congruent not only with the soil types but also with the undergoing processing and modifications on a periodic basis. However, in some soils (S29, S30, S31), the content of the available P is over 70 mg kg−1, and there are different reasons for that as an overuse of phosphate fertilisers, the OM content or because they are in more disturbed soils (transport, industry, etc.) (Pouyat et al. 2007; Li et al. 2013).
The average of the cation exchange capacity (CEC) of the soils is 10.56 cmol(+) kg−1 (it ranges from 4.10 to 31.36 cmol(+) kg−1). In most of the soils, this value is lower than 15 cmol(+) kg−1, but there are some exceptions (31.36 cmol(+) kg−1; S35) detected in areas near beaches where depositions of mollusc shells would influence the CEC as in the case of pH. In fact, the content of exchangeable Ca2+ is high in the soils from these areas, although in most of the soils it is also the principal exchangeable cation and the contents vary from 0.99 to 30.17 cmol(+) kg−1). In addition, the exchangeable complex saturation is generally high (average value 96.29 % and range 49.02–100 %). In any case, the CEC is similar to the urban soils from other cities like Seville (2.1–35.4 cmol(+) kg−1; Ruiz-Cortés et al. 2005), Torino (4.7 to 26.3 cmol(+) kg−1; Biasioli et al. 2006) or Beijing (4.23–20.29 cmol(+) kg−1; Xia et al. 2013).
As urban soils are subjected to garden-tending processes, they usually reveal higher values for pH, OM, N, available P, CEC and exchangeable complex saturation than rural or natural soils. The direct addition of fertilisers, pruning waste, fallen leaves and organic residues originating from vegetation and animals influence the soil urban properties, but they also receive indirect additions of N and P through construction waste materials and the emissions of vehicles (Pouyat et al. 2007; Lorenz and Lal 2008).
Attending to the previous results, the urban soils from Vigo have low pH and OM contents that may increase the mobility and availability of different contaminants. On the other hand, a high amount of available P may counteract this effect by reducing mobility (Kabata-Pendias 2010).
3.2 Metal concentrations
Tables 3 and 4 show the total concentrations of the analysed metals and the corresponding degree of enrichment with respect to the background level. The average values for enrichment factor are 2.64 (Ba), 2.43 (Cr), 1.91 (Cu), 0.68 (Mn), 0.52 (Ni), 2.07 (Pb) and 1.73 (Zn).
The total content of Ba varies between 237.82 and 1145.04 mg kg−1 (an average of 516.75 mg kg−1), and seven soils exceed the corresponding GRL for urban soils (600 mg kg−1) (Fig. 2). Nevertheless, the average Igeo value (0.59) indicates that, in general, the soils are not contaminated or that the contamination is moderate. The EF data (0.93–4.57) also indicates a moderate enrichment in Ba.
The total contents of Cr range between 33.48 and 195.30 mg kg−1(average 68.55 mg kg−1) and only two soils (S9 and S27) exceed the GRL for urban soils (100 mg kg−1) (Fig. 2). With the exception of S9 (0.70), all the soils reveal negative Igeo values (an average of −0.90) reflecting no contamination. However, the EF values show a moderate enrichment (they range from 1.18 to 3.63). The results are in agreement with Kabata-Pendias (2010) as Cr is an element with low mobility under oxidation conditions and with pH close to neutral.
According to the background levels, Cu contents are rather high, that vary from 22.56 to 208.22 mg kg−1, (average 66.12 mg kg−1) but only three soils exceed the GRL (100 mg kg−1) (S3, 104.11 mg kg−1; S4, 208.22 mg kg−1 and S14, 130.14 mg kg−1) (Fig. 3). The average Igeo value was −0.18 (−1.84 to 0.70) showing that in general, there is no contamination but according to EF values few soils were classified as with significant enrichment (EF = 6.61).
Lead contents are high according to the background levels. They range from 34.38 to 259.11 mg kg−1 (average 96.29 mg kg−1), and 12 soils exceed the GRL (100 mg kg−1) (Fig. 4). Although the average Igeo value is indicative of uncontaminated soils (−0.02), it is necessary to highlight the existence of 11 soils classified as Class 1 and five soils with moderate contamination (Class 2). In addition, the average EF values indicate moderate enrichment (0.48–7.88).
The levels of Mn (Table 3) are below the GRL (1500 mg kg−1) (Fig. 3). Furthermore, both the average Igeo values (−1.34) and the EF (0.68) reveal no contamination or minimum enrichment (Table 4). As with Mn, the levels of Ni are below the GRL (100 mg kg−1) (Fig. 4) but they are also lower than the regional background level (65 mg kg−1). They range between 11.50 and 60 mg kg−1 (average 32.01 mg kg−1) and accordingly the average Igeo (−1.71) values indicate there is no contamination and the EF values (0.51) that there is minimum enrichment or deficient.
Zinc contents are relatively high (ranging from 116 to 268 mg kg−1 and an average of 192.92 mg kg−1) (Fig. 5) compared to the background levels, but they do not exceed the GRL (500 mg kg−1). In fact, according to the average Igeo values, there is contamination but according to the EF values, there is Zn moderate enrichment in the 12 soils where EF > 2.
In general, the metal contents in the studied soils are below their corresponding GRL and although some soils have high contents suggesting contamination, none of them can be classified as strongly contaminated or significantly enriched. Comparing the results with those obtained in the urban soils from other Spanish cities, in general, the metal contents in Vigo are lower than in cities with more population like Seville (Biasioli et al. 2007) or Madrid (De Miguel et al. 2007), but also in smaller cities with potentially polluting industrial activity like Avilés (Gallego et al. 2002) where there is a Zn smelter or A Coruña (Cal-Prieto et al. 2001) or Huelva (Guillén et al 2012), both with a petrochemical complex.
3.3 Contribution of particular metals to the contamination of soils in Vigo
In order to assess the contamination of the soils, the contamination factor and the contamination degree were calculated. As it was previously indicated by the Igeo and EF values, the new results also reveal low contamination factor (c i f) for Cr, Mn and Ni, and moderate for Ba, Cu, Pb and Zn (Table 4). The degree of metal contamination (Cdeg) indicates moderate contamination (9.03) (Table 4). The contribution of each metal to this index is as follows: Ba (26 %), Pb (19.38 %), Cu (16.57 %), Zn (16.50 %), Cr (9.49 %), Mn (6.92 %) and Ni (5.45 %).
3.4 Correlation of metals and source identification
By means of GIS techniques, it is possible to represent in maps the spatial distribution of the elements in the soils. These techniques also allow to relate them to “hot-spots” (Rodríguez-Salazar et al. 2011) by means of using interpolation methods such as Kriging or IDW. Inverse distance weighting (IDW) uses easier parameters than Kriging, giving rise to results that are more easy to define and to understand. When there are outliers or very different values (Azpurua and Ramos 2010), IDW interpolation gives better results than the model Kriging or SPLINE. As urban soils are very heterogeneous environments, with very high spatial variability in physical, chemical and biological properties, IDW is a better choice than Kriging. In fact, different studies about metal distribution in urban soils (Zhang 2006; Lee et al. 2006; Carr et al. 2008; Slavik et al. 2012; Karim et al. 2014) indicated that using IDW is a good choice for identifying a contamination pattern, or determining the variation of soil metal concentration.
The sampling points, transport routes and industrial zones are indicated in Fig. 1, and the pollution hotspot can be identified with the distribution maps (Figs. 2, 3, 4 and 5). They suggest a strong influence of industries and traffic on soil metal accumulation as was also indicated for Shanghai urban soils by Shi et al. (2008). The distribution of Ba (Fig. 2), Cu (Fig. 3) and Pb (Fig. 4) was mainly associated to transport routes and to the city’s industrial areas. Chromium seems to be somewhat more localised (S6, S7 and S8, Fig. 2), and the garden-tending processes (protectors or wooden fences treated with chromate) is the most probable origin. On the other hand, the distribution of Mn, Ni and Zn is more diffuse (Figs. 3, 4 and 5, respectively) and the distribution maps also showed there are no hotspot in accordance with the previously environmental factors that revealed no contamination.
The correlation (Table 5) and cluster (Fig. 6) analyses were performed with the total metal content of the soils. Together with the Igeo, EF and Cdeg (Table 4), it is possible to explain the relationships between the metals and their probable origins. Is it possible also to distinguish between “natural”, “mixed” and “urban” origins. Two possible sources in both mixed and urban contents were identified:
-
i).
Natural: the cluster of natural origin is made up of Na and Si. Their origin in the soils is supposed to be lithogenic as to the parent material is mainly granite.
-
ii).
Mixed: the two sources identified are mainly natural and urban. There is a cluster made up of Ca and Sr. Both elements, but also Mg, were not included in the “natural” cluster, therefore, their origin was supposed to be mixed (anthropogenic and natural). They probably undergo different geochemical changes with respect to the original sources (De Miguel et al. 1997). They could be resuspended in the air and latter deposited in the soils since they are present in particles of cement from building materials and concrete manufacturing companies (Santacatalina et al. 2012).
The second cluster classified as mixed origin is made up of Mg, Cr, Fe, Mn and Ni (Fig. 6). Except for Cr, the other studied metals are always below the GRL for the studied soils. The concentration of each element that made up the mixed origin cluster is positive and significantly correlated with the concentration of the other elements that belong to the same cluster (Table 5). These facts suggest the existence of a common source (natural or anthropogenic). It is worth highlighting that, except for Cr, the enrichment factors are low, suggesting that a priori the origin is lithogenic (Massas et al. 2013). However, the negative correlation between their contents and the ones of the elements identified as natural origin (Na and Si), along with no correlation with the ones of Ca (Table 5), indicate a mixed origin.
-
iii).
Urban: the urban origin cluster is made up of Ba, Cu, Pb and Zn. The maps (Figs. 2, 3, 4 and 5, respectively) show the distribution of these elements in the study area and the hot spots for those that are higher than the GRL (7 soils of Ba, 3 soils of Cu and 12 soils of Pb). There are no hot spots for Zn as the contents did not exceed the GRL. Nevertheless, the Zn contents are relatively high and the EF values indicate there is moderate enrichment. As a result, the urban or anthropogenic origin is deduced for Ba, Cu, Pb and Zn as the Igeo, EF and c i f values indicated there is enrichment and moderate contamination of the soils by Ba, Cu, Pb and Zn.
Moreover, according to the cluster analysis (Fig. 6), it is possible to identify two urban sources (one for Ba and another one for Cu, Pb and Zn). In addition, the positive correlation between their concentrations (Table 5) also suggests the common origin. The distribution map of Pb (Fig. 4) shows that traffic is the main probable origin as Pb from petrol is the main source of Pb in soils (43.51 % average) (Rodríguez-Seijo et al. 2015). The Pb comes from the additives used in petrol, that were later banned, and their accumulation in the soil during decades, as well as from the combustion of fuel oil, the wear and corrosion of metal vehicle parts and urban fixtures, paints, etc. Furthermore, the main sources of Cu and Zn are lubricants, abrasion processes of tyres, brakes, etc. and corrosion of metal parts in contact with fuel. Other sources of Zn in urban environments are vulcanised tyres and galvanised components of vehicles, street furniture, roofs, etc. (De Miguel et al 1997; Sutherland 2000; Saeedi et al 2012).
The origin of Ba in the soils is also assigned to an urban origin. The total Ba contents are high as it was previously indicated. For decades, the importance of Ba in areas with intense road traffic was ignored, but different processes may give rise to an increase of Ba contents in soils. Barium is a constituent of alloys, paint, ceramic, glass or plastic cements but the friction processes of tyres and brakes also release Ba to the environment (Monaci and Bargagli 1997; Sutherland 2000; Fernández–Espinosa and Ternero-Rodríguez 2004; Minguillón et al. 2014). Even traffic is an important source of Ba; according to several authors, Ba can be forming part of very fine particles and it can be transported long distances. As a result, its origin in the soils is mixed, from natural and anthropogenic sources, as it was already stated in previous studies (De Miguel et al. 1997; Zechmeister et al. 2005; Chen et al. 2014).
Three groups were obtained; the first one named the “urban” group is influenced by transport. The second is composed of elements with industrial origin or mixed and the third one encompasses elements from natural origin. Nevertheless, the elements included in the second and third group can vary according to geology and industrial activity (De Miguel et al. 1997; Wei et al. 2010; Rodríguez-Salazar et al. 2011, Saeedi et al. 2012; Oliva and Fernández-Espinosa 2007).
4 Conclusions
In general, the metal contents in the studied soils are below their corresponding GRL, and although some soils have high metal concentrations suggesting contamination, none of them can be classified as strongly contaminated or significantly enriched.
Barium, Cu and Pb explain more than 61 % of the moderate contamination degree of the studied soils and they come from the same source.
There are three main different sources that determine the metal contents: “natural”, “mixed” and “urban” origins. The urban origin is mainly explained by traffic.
The obtained results will allow to help in the selection of “hot-spots” to carry out more in-depth studies into bioavailability.
References
Acevedo-Figueroa D, Jiménez BD, Rodríguez-Sierra CJ (2006) Trace metals in sediments of two estuarine lagoons from Puerto Rico. Environ Pollut 141:336–342
Ajmone-Marsan F, Biasioli M (2010) Trace elements in soils of urban areas. Water Air Soil Pollut 213:121–143
Azpurua MA, Ramos KD (2010) A comparison of spatial interpolation methods for estimation of average electromagnetic field magnitude. Prog Electromagn Res M 14:135–145
Biasioli M, Barberis R, Ajmone-Marsan A (2006) The influence of a large city on some soil properties and metals content. Sci Total Environ 356:154–164
Biasioli M, Grcman H, Kralj T, Madrid F, Díaz-Barrientos E, Ajmone-Marsan F (2007) Potentially toxic elements contamination in urban soils: a comparison of three European cities. J Environ Qual 36:70–79
Bremner JM, Mulvaney CS (1982) Nitrogen-total. In: Page AL, Miller RH, Keeney RS (eds) Method of soil analysis: part 2. Chemical and microbiological properties, vol Agronomy monographs no. 9, 2nd edn. American Society of Agronomy and Soil Science Society of America, Madison, pp 595–624)
Buat-Menard P, Chesselet R (1979) Variable influence of the atmospheric flux on the trace metal chemistry of oceanic suspended matter. Earth Planet Sci Lett 42:399–411
Cal-Prieto MJ, Carlosena A, Andrade JM, Martínez ML, Muniategui S, López-Mahía P, Prada D (2001) Antimony as a tracer of the anthropogenic influence on soils and estuarine sediments. Water, Air, and Soil Pollut 129:333–348
Carr R, Zhang C, Moles N, Harder M (2008) Identification and mapping of heavy metal pollution in soils of a sports ground in Galway City, Ireland, using a portable XRF analyser and GIS. Environ Geochem Health 30:45–52
Chen H, Lu X, Li LY, Gao T, Chang Y (2014) Metal contamination in campus dust of Xi’an, China: a study based on multivariate statistics and spatial distribution. Sci Total Environ 484:27–35
Craul PJ (1992) Urban soil in landscape design. Wiley, New York, 396 p
Covelli S, Fontolan G (1997) Application of a normalization procedure in determining regional geochemical baselines. Environ Geol 30:34–45
Golubiewski NE (2006) Urbanization increases grassland carbon pools: effects of landscaping in Colorado’s Front Range. Ecol Appl 16:555–71
Daskalakis KD, O’Connor TP (1995) Normalization and elemental sediment contamination in the coastal United States. Environ Sci Technol 29(2):470–477
De Kimpe CR, Morel JL (2000) Urban soil management: a growing concern. Soil Sci 165:31–40
De Miguel E, Llamas JF, Chacón E, Berg T, Larssen S, Røyset O, Vadset M (1997) Origin and patterns of distribution of trace elements in street dust: unleaded petrol and urban lead. Atmos Environ 31:2733–2740
De Miguel E, Iribarren I, Chacón E, Ordoñez A, Charlesworth S (2007) Risk-based evaluation of the exposure of children to trace elements in playgrounds in Madrid (Spain). Chemosphere 66:505–513
Eriksson CP, Holmgren P (1996) Estimating stone and boulder contents in forest soils—evaluating the potential of surface penetration methods. Catena 28:121–134
Fernández–Espinosa AJ, Ternero-Rodríguez M (2004) Study of traffic pollution by metals in Seville (Spain) by physical and chemical speciation methods. Anal Bioanal Chem 379:684–699
Gallego JLR, Ordóñez A, Loredo J (2002) Investigation of trace element sources from an industrialized area (Avilés, northern Spain) using multivariate statistical methods. Environ Int 27:589–596
Guillén MT, Delgado J, Albanese S, Nieto LA, De Vivo B (2012) Heavy metals fractionation and multivariate statistical techniques to evaluate the environmental risk in soils of Huelva Township (SW Iberian Peninsula). J Geochem Explor 119–120:32–43
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14:975–1001
Hendershot WH, Duquette M (1986) A simple barium chloride method for determining cation exchange capacity and exchangeable cations. Soil Sci Soc Am J 50:605–608
Instituto Geográfico Nacional de España. (2014). Base Cartográfica Numérica 1:25.000 (BCN25), Base Topográfica Nacional 1:25.000 (BTN25). Cedido por © Instituto Geográfico Nacional de España. https://www.cnig.es/ Accessed 04 April 2014
Kabata-Pendias A (2010) Trace elements in soils and plants, 4th edn. CRC, New York
Karim Z, Qureshi BA, Mumtaz M, Qureshi S (2014) Heavy metal content in urban soils as an indicator of anthropogenic and natural influences on landscape of Karachi—a multivariate spatio-temporal analysis. Ecol Indic 42:20–31
Lee CS-L, Li X, Shi W, Cheung SC-N, Thornton I (2006) Metal contamination in urban, suburban, and country park soils of Hong Kong: a study based on GIS and multivariate statistics. Sci Total Environ 356:45–61
Li Z-G, Zhang G-S, Liu Y, Wan K-Y, Zhang R-H, Chen F (2013) Soil nutrient assessment for urban ecosystems in Hubei, China. PLoS One 8(9):e75856
Lorenz K, Lal R (2008) Biogeochemical C and N cycles in urban soils. Environ Int 35:1–8
Loska K, Wiechulla D, Korus I (2004) Metal contamination of farming soils affected by industry. Environ Int 30:159–165
Macías F, Calvo de Anta R (2009) Niveles Genéricos de Referencia de Metales Pesados y otros elementos de traza en suelos de Galicia. Xunta de Galicia 2009. Santiago de Compostela, Spain (in Spanish)
Massas I, Kalivas D, Ehaliotis C, Gasparatos D (2013) Total and available heavy metal concentrations in soils of the Thriassio plain (Greece) and assessment of soil pollution indexes. Environ Monit Assess 185:6751–6766
Metreveli G, Frimmel FH (2007) Influence of Na-bentonite colloids on the transport of heavy metals in porous media. In: Frimmel FH, Frank F, Hans-Curt F (eds) Colloid transport in porous media. Springer, Berlin, pp 29–53
Minguillón MC, Cirach M, Hoek G, Brunekreef B, Tsai M, de Hoogh K et al (2014) Spatial variability of trace elements and sources for improved exposure assessment in Barcelona. Atmos Environ 89:268–281
Monaci F, Bargagli R (1997) Barium and other trace metals as indicators of vehicle emissions. Water Air Soil Pollut 100:89–98
Müller G (1979) Schwermetalle in den sedimenten des Rheins-Veränderungen seit 1971. Umschau 79(24):778–783 (in German)
Oliva SR, Fernández-Espinosa AJ (2007) Monitoring of heavy metals in topsoils, atmospheric particles and plant leaves to identify possible contamination sources. Microchem J 86:131–139
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL, Miller RH, Keeney RS (eds) Method of soil analysis: part 2. Chemical and microbiological properties. Agronomy monographs no. 9, 2nd edn. American Society of Agronomy and Soil Science Society of America, Madison, pp 403–430
Picket STA, Cadenasso ML, Grove JM, Nilon CH, Boone CG, Groffman PM et al (2011) Urban ecological systems: scientific foundations and a decade of progress. J Environ Manage 92:331–362
Pouyat RV, Yesilonis ID, Russell-Anelli J, Neerchal NK (2007) Soil chemical and physical properties that differentiate urban land-use and cover types. Soil Sci Soc Am J 71:1010–1019
Puskás I, Farsang A (2009) Diagnostic indicators for characterizing urban soils of Szeged, Hungary. Geoderma 148:267–281
QGIS Development Team (2014) QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org Accessed 22 January 2014
R Development Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org Accessed 21 January 2014
Rodríguez-Salazar MT, Morton-Bermea O, Hernández-Álvarez E, Lozano R, Tapia-Cruz V (2011) The study of metal contamination in urban topsoils of Mexico City using GIS. Environ Earth Sci 62:899–905
Rodríguez-Seijo A, Arenas-Lago AML, Vega FA (2015) Identifying sources of Pb pollution in urban soils by means of MC-ICP-MS and TOF-SIMS. Environ Sci Pollut Res 22:7859–7872
Ruiz-Cortés E, Reinoso R, Díaz-Barrientos E, Madrid L (2005) Concentrations of potentially toxic metals in urban soils of Seville: relationship with different land uses. Environ Geochem Health 27:465–474
Rydin Y, Bleahu A, Davies M, Dávila JD, Friel S, De Grandis G et al (2012) Shaping cities for health: complexity and the planning of urban environments in the 21st century. Lancet 379:2079–2108
Saeedi M, Li LY, Salmanzaeh M (2012) Heavy metals and polycyclic aromatic hydrocarbons: pollution and ecological risk assessment in street dust of Tehran. J Hazard Mater 227–228:9–17
Santacatalina M, Yubero M, Mantilla E, Carratalá A (2012) Relevance of the economic crisis in chemical PM10 changes in a semi-arid industrial environment. Environ Monit Assess 184:6827–6844
Shi G, Chen Z, Xu S, Zhang J, Wang L, Bi C, Teng J (2008) Potentially toxic metal contamination of urban soils and roadside dust in Shanghai, China. Environ Pollut 156(2):251–260
Slavik R, Julinová M, Labudíková M (2012) Screening of the spatial distribution of risk metals in topsoil from an industrial complex. Ecol Chem Eng S 19:259–272
Srinivasa Gowd S, Ramakrishna Reddy M, Govil PK (2010) Assessment of heavy metal contamination in soils at Jajmau (Kanpur) and Unnao industrial areas of the Ganga Plain, Uttar Pradesh, India. J Hazard Mater 174:113–121
Sutherland RA (2000) Bed sediment-associated trace metals in an urban stream, Oahu, Hawaii. Environ Geol 39:611–627
Szolnoki Z, Farsang A, Puskás I (2013) Cumulative impacts of human activities on urban garden soils: origin and accumulation of metals. Environ Pollut 177:106–115
Walkey A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 34:29–38
Xia X, Zhao X, Lai Y, Dong H (2013) Levels and distribution of total nitrogen and total phosphorous in urban soils of Beijing, China. Environ Earth Sci 69:1571–1577
Yang L, Li Y, Peng K, Wu S (2014) Nutrients and heavy metals in urban soils under different green space types in Anji, China. Catena 115:39–46
Wei B, Jiang F, Li X, Mu S (2010) Heavy metal induced ecological risk in the city of Urumqi, NW China. Environ Monit Assess 160:33–45
Zechmeister HG, Hohenwallner D, Riss A, Hanus-Illnar A (2005) Estimation of element deposition derived from road traffic sources by using mosses. Environ Pollut 138:238–249
Zhang C (2006) Using multivariate analyses and GIS to identify pollutants and their spatial patterns in urban soils in Galway, Ireland. Environ Pollut 142:501–511
Acknowledgments
We thank the Xunta de Galicia for funding project EM2013/018. F.A. Vega is hired under a Ramón y Cajal contract at the University of Vigo. A. Rodríguez-Seijo would like to thank the University of Vigo for his pre-doctoral fellowship (P.P. 00VI 131H 64102) and would like to thank the Concello de Vigo (Spain) for their help in collecting samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This research does not contain any studies with human or animal subjects. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Responsible editor: Maria Manuela Abreu
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 389 kb)
Rights and permissions
About this article
Cite this article
Rodríguez-Seijo, A., Andrade, M.L. & Vega, F.A. Origin and spatial distribution of metals in urban soils. J Soils Sediments 17, 1514–1526 (2017). https://doi.org/10.1007/s11368-015-1304-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1304-2