Abstract
Purpose
Water reservoirs around the world suffer from accelerated sediment loads and, consequently, contamination. Notably, in water-scarce regions such as Jordan, this poses a threat to an important water source, and identifying the sediment sources is an important task. Thus, a sediment fingerprinting study in the Wadi Al-Arab catchment of northern Jordan was implemented with special attention directed to the development of suitable correction factors necessary to improve the comparability of source and sink sediments. The selection of seven conservative elements for the sediment fingerprinting was made, with specific attention directed to the chemical environment of the reservoir.
Materials and methods
Thirty-six samples from six different surface and subsurface sources and 38 sink samples from the Wadi Al-Arab reservoir were collected. In total, 27 organic and inorganic elements as well as radionuclides were analysed. Two vertical physicochemical water profiles provided information on the pH and Eh conditions and common element concentrations. The stepwise multiple regression analysis model (SMRAM) was developed to explore parameters that influence the element concentrations and their interrelations, and to calculate an element-specific correction factor. The standard selection procedure was expanded by the comparison of water and sink sediment element concentrations, a literature review concerning the pH and Eh conditions and, in selected cases, a correlation analysis.
Results and discussion
The combination of Al, Cr, Mn, Fe, 232Th, 228Th and 137Cs provided the best source discrimination, and based on Monte Carlo simulations, the mixing model revealed the existence of three major sediment source areas. These are as follows: (i) olive orchards on slopes, which delivered 59 ± 8 % of the sediments in the sink; (ii) cultivated fields on plateau and saddle positions contributed 11 ± 9 %; and (iii) slopes with natural vegetation used for grazing contributed 29 ± 15 % of the deposited sediment. With a mean residual error of 1.04 %, the sum of the source concentrations differs only slightly from sink concentrations and proves that the model is reliable.
Conclusions
The SMRAM model revealed that the different inorganic (total inorganic carbon, TIC) and organic (total organic carbon, TOC) carbon contents and the clay/sand content influence the element concentrations of the sediment samples. Due to the carbonatic environment, it was mainly necessary to correct for TIC. Applying an expanded literature review regarding the chemical environment under investigation, in addition to the standard mass conservation and Kruskal-Wallis test, prevented possible non-conservative elements from entering the discriminant analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In semi-arid regions, where water is scarce, soil erosion on slopes decreases the water infiltration potential, causes accelerated surface water runoff and thus reduces the recharge of aquifers. Eroded sediments are flushed into surface water reservoirs and often deteriorate the water quality and the reservoirs’ capacity (Palmieri et al. 2001; Slimane et al. 2013; Vanmaercke et al. 2011). In the extremely water-scarce nation of Jordan, surface reservoirs play a tremendous role in freshwater supply (Nortcliff et al. 2008). Increased sediment deposition due to erosion processes was reported by several authors in water reservoirs along the Lower Jordan Valley as they are the final sink for surface runoff and sediments (Ghrefat and Yusuf 2006; El-Radaideh 2010; Al-Ansari and Shatnawi 2011; Al-Ansari et al. 2012). To mitigate this problem, it is of great importance to define relevant sediment sources and their respective contribution to the sink.
Sediment fingerprinting has been proved to be a valuable tool for this task (Davis and Fox 2009; Owens and Xu 2011; Mukundan et al. 2012; Koiter et al. 2013a). Each potential source is characterized by discriminating properties or elements. By applying a multivariate mixing model, the source composition is iteratively determined by minimizing the sum of squared weighted errors of the element concentrations between the estimated source mixture and sink (Yu and Oldfield 1989; Walling et al. 1993; Collins et al. 2010; Wilkinson et al. 2012).
A variety of discriminating properties are used, for example, grain size, sediment colour, plant pollen, mineral magnetism, organic and inorganic elements, radionuclides, stable isotopes, rare earth elements and biogenic properties (Foster and Lees 2000; Collins and Walling 2002; Walling 2005, 2013; Davis and Fox 2009; Mukundan et al. 2012). The basic assumption of the method is that suitable properties are comparable between source and sink, and that they stay conservative during transport (Davis and Fox 2009; Koiter et al. 2013a). However, particle selection during transport can lead to an accumulation of smaller particles and organic matter with a higher specific surface area (SSA) in the sink (Koiter et al. 2013a; Walling 2013). Moreover, the larger the SSA of a particle, the more the elements are adsorbed to it and thus have the potential to hinder a direct elemental comparison between source and sink (Horowitz 1991; Collins et al. 1997; Motha et al. 2002; Koiter et al. 2013a; Walling 2013). In contrast, high carbonate concentrations in a sample dilute the elemental content compared to those with lower carbonate concentrations (Horowitz 1991).
To make a successful comparison between source and sink, one may restrict the element analysis to specific grain size fractions, for example, <2 to <63 μm (Motha et al. 2004; Collins et al. 2012; Wilkinson et al. 2012). This requires profound knowledge of the transport and the accompanied change in grain size composition (Koiter et al. 2013a, b; Walling 2013). Another option is to use correction factors (Collins et al. 1997, 2010, 2012; Walling et al. 1999; Russell et al. 2001; Krause et al. 2003; Foster et al. 2007; Koiter et al. 2013a; Smith and Blake 2014). Some authors additionally remove organic carbon as a second correction (Motha et al. 2004; Wilkinson et al. 2012).
Since the study presented here was preliminary, no previous knowledge existed concerning grain size fractions, particle selection, organic/inorganic carbon contents and their effects on the element concentrations. Consequently, we decided to apply correction factors to account for the comparability of the sediments, since selective grain size sampling or the removal of organic matter would have implied the possibility of overlooking important information within the data.
Correction factors were introduced by Collins et al. (1997) and have become a widely used procedure in fingerprinting studies (Walling et al. 1999; Krause et al. 2003; Foster et al. 2007; Collins et al. 2010, 2012). Correction factors have to be determined for those parameters that influence the element concentration, such as grain size and/or total organic carbon (TOC). To derive grain size correction factors, dividing the SSA in sink samples by the average SSA of the source samples has become a widely accepted approach (Walling et al. 1999; Krause et al. 2003; Foster et al. 2007; Collins et al. 2010, 2012). To correct the effects of TOC, the factor-determination is similar and the TOC content of the sink is divided by the TOC content of the source (Collins et al. 1997). In the mixing model, both correction factors are sometimes used as multipliers for each mean source element concentration, assuming that both influence each element independently but in the same positive linear way.
However, Collins et al. (1997) and Mukundan et al. (2012) report that the variances in element concentrations resulting from the influences of particle size and TOC often overlap. Thus, interrelations between the influential parameters particle size and TOC exist, and correcting for both would make an overcorrection likely. Russell et al. (2001) found that elements were positively correlated with logarithmic SSA. Furthermore, they argued that the TOC influence is negligible compared to the particle size and is due to the interrelations already covered by the particle size correction. Smith and Blake (2014) report that SSA and TOC may vary strongly due to temporal changes in and between the sources and not exclusively due to particle selectivity. Furthermore, they found inconsistencies of direction and strength in their linear correlation analysis between geochemical elements and SSA and TOC. They concluded that establishing specific relationships between elements and grain size fractions and correcting only for SSA should be done solely in cases where correction for particle selectivity seems appropriate (Smith and Blake 2014).
Further difficulties appear if changing conditions overturn the conservative behaviour of selected properties. This is the case in the study area, where sediments get flushed from an oxic environment into the Wadi Al-Arab Dam, where they settle into an anoxic environment. There, pH changes and important bio-geochemical reactions can occur, particularly on sediment–water interfaces (Stumm and Morgan 1996; Koiter et al. 2013a), possibly rendering fingerprinting elements non-conservative (Owens and Xu 2011; Koiter et al. 2013a). However, although well known, the influence of changing environments on, for example, dissolution or complexation of elements has rarely been discussed in fingerprinting studies (Salomons and Mook 1987; Horowitz 1991; Kelley and Nater 2000; Miller et al. 2005; Beutel et al. 2007; Foster et al. 2007; Kouhpeima et al. 2010; Slimane et al. 2013).
The objectives of the present study were as follows: (1) to introduce an alternative estimation of element-specific correction factors based on linear and nonlinear multiple regression analysis; (2) to include physicochemical water parameters in the selection process; (3) to evaluate the conservative nature of the fingerprinting elements; and (4) to calculate the relative contribution of each sediment source to the Wadi Al-Arab reservoir.
2 Study area
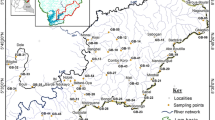
The Wadi Al-Arab catchment extends over 263.5 km2 in the north west of Jordan. The Wadi itself cuts its way from the Jordan Valley Escarpment (500 m a.s.l.) down to the Jordan Valley (-164 m a.s.l.) and is a tributary to the Jordan River. The annual precipitation ranges from ∼530 mm in the east to ∼380 mm in the west, resulting in a Mediterranean to semi-arid climatic regime. The catchment’s bedrock mainly consists of limestone and marl with terrestrial input (Al-Sharhan and Nairn 1997; Moh’d 2000). Figure 1 displays the extension of the three major lithological units of the Upper Cretaceous and Paleocene with the oldest unit (Al-Hisa Phosphorite/Amman Silicified Limestone) in the east and the youngest (Umm Rijam Chert) in the north and northwest.
Typical soil types for the region are Calcaric Cambi- or Lithosols on the slopes and Chromic Vertisols or Vertic Cambisols on top or saddle positions (MoA 1994). A plateau used for agriculture characterizes the east of the catchment. From there, an increase in relief is observed to the west and south. Bare rock/soil (34 %), olive orchards (27 %), fields (14 %) and natural shrubs on steep slopes (11 %) are the major land cover units in the catchment. At the western outlet, the Wadi Al-Arab reservoir collects the overland flow for agricultural projects in the Jordan Valley as well as, during periods of water shortage, for households in the capital, Amman. From our own bathymetric investigations in October 2011 and from GIS analysis, a yearly sedimentation volume of 67,000 m3 ± 6 % was calculated for the Wadi Al-Arab lake, posing an acute threat to the reservoir due to the position of the dam’s outlet (personal communication, Hussein Al-Shurieki 2011, dam director; own unpublished data 2011).
3 Definition of sediment sources
The erosive potential of several land units was the subject of previous work. Under olive trees on slopes, splash, rill and gully erosion occur and follow the downhill furrows, channelizing the runoff. Stone pedestals and siltation features in the rills and gullies are also visible and tillage erosion is likely (Kraushaar et al. 2014). On agricultural fields, ephemeral gullies as well as piping were occasionally observed. Naturally vegetated slopes experience intensive grazing and thus reduced resistance to water erosion (Yassoglou and Kosmas 1997; own unpublished results). In areas with sparse or no vegetation cover, such as south-exposed slopes or accumulations of new sediments from road construction, the accretion of rills, stone pedestals, gullies as well as the occurrence of badlands are common. The study aims to differentiate between these main sources: olive orchards (Source 1 = S1; Fig. 2a); fields for agriculture (Source 2 = S2; Fig. 2b); naturally vegetated slopes (Source 3 = S3; Fig. 2c) and the unconsolidated rock and recent sediments of the three main geological units Umm Rijam Chert (Source 4 = S4; Fig. 2d), Muwaqqar Chalk Marl (Source 5 = S5; Fig. 2e) and Al-Hisa Phosphorites/Amman Silicified Limestone (Source 6 = S6; Fig. 2f).
It was known from the literature that the heavy metal concentrations of the three different geological units were significantly different (Al-Sharhan and Nairn 1997; Moh’d 2000; Moh’d and Powell 2010). Furthermore, observations showed that the soil development on slopes (geomorphologically active positions) compared to the top and saddle positions (geomorphologically stable positions) is marginal. This shows in the enrichment of conservative elements due to solution weathering of the carbonatic rock and pedogenesis (Lucke 2007). The use of fertilizers and other agricultural additives is reflected in certain geochemical elements, for example, N, P, K and Cd (Ghrefat and Yusuf 2006). Finally, regular tillage practice is known to homogenize the concentration of fallout radionuclides, such as 137Cs, to the depth of the plough layer. Based on these findings, a differentiation between the mentioned sources seemed possible. Sources S1–S3 represent surface erosion from the mentioned land use units. Sources S4–S6 represent subsurface erosion from linear features, as well as unconsolidated rock and new sediments, differentiated by the three main geological units (Table 1, Fig. 2).
4 Methods
4.1 Source sampling
For sources S1–S3, six composite soil samples were obtained from different locations throughout the catchment (Fig. 1). Each composite sample consisted of 10–20 subsamples depending on the size of the orchard, field or slope and covered areas of 0.5–1 ha. Subsamples were collected along transects of 15–20 m in width, at a depth between 0 and 5 cm, and sieved to <2 mm. About 1 kg of soil was collected in this way per site, focusing on areas and material that are likely to erode, such as aggregates in downhill furrows or loose soil on bare rock on a slope.
Sources S4–S6 represent subsurface samples and thus included consolidated and unconsolidated geology. Consolidated geology samples were collected with a hammer and chisel from exposed rocks. Care was taken to ensure that the weathering crust and no material that had direct surface exposure entered the sample. In case of unconsolidated material, such as the Muwaqqar Chalk Marl (Fig. 1), 10 subsamples were collected with a plastic shovel as a composite sample from a depth of >30 cm.
Additionally, three samples of alluvial Wadi deposits (WD) along the principal valley of Wadi Al-Arab were collected. Sampling sites included two alluvial terraces from which material was derived from the cleaned profiles at a depth of >50 cm and one recent alluvial deposit, from which a surface sample of 0–5 cm depth was taken. The aim of these samples was to gather information on the composition of grain size fraction during transport, and included the element concentration in the modified element correction approach.
4.2 Sink sampling
4.2.1 Wadi Al-Arab Dam reservoir sediments
Five reservoir sediment cores with an average length of 40 cm were collected with a gravity corer of UWITEC (Umwelt und Wissenschaftstechnik; Mondsee, Austria) different locations of the reservoir (Fig. 1). Each sediment core was sliced into 5 cm intervals resulting in a total of 38 samples. This sampling strategy was chosen to represent the spatial and vertical variability of potential fingerprinting elements in the lake sediment.
4.2.2 Wadi Al-Arab Dam reservoir water profiles
In addition, two profiles over the whole water column (18 m) were investigated to compare water element concentrations with those of the reservoir sediments. A peristaltic pump was used to sample water in 2 m steps from the air–water surface down to the bottom sediment (n = 17). Physiochemical parameters (Eh, T, pH, EC) were analysed directly with a calibrated WTW Multiline P4 (Weilheim, Germany). The measurement of the redox potential was done to avoid any significant influence of air oxygen on the measured value. Each sample was filtered (0.45 μm CA-filter) and filled into two HDPE bottles (60 ml) for cation and anion analysis. Cation samples were acidified with 0.2 ml 10 % HCl to prevent adsorption on the sample bottle. The samples were kept cool (<5 °C) and dark until analysis.
4.3 Laboratory analysis
For sample preparation, the reservoir sediment samples were oven dried at 60 °C until constant weight. The resulting aggregates were carefully ground with a mortar and pestle for further analysis. The consolidated rock samples were crushed to a particle size of 2 mm with a jaw crusher (Retsch type B00; Haan, Germany). Table 2 gives an overview of the different analyses and instruments used on the sediment samples.
The grain size fractions were determined in the geochemical laboratory of the Technical University of Dresden following the Koehn method according to E DIN ISO 11277:06.94. Due to the generally low TOC content (mean, 2 %) compared to the higher inorganic carbon content (mean, 6.5 %), an exemption of the DIN was made concerning the destruction of organic carbon with hydrogen peroxide following Blume (2004). The elimination of organic carbon using H2O2 on heat plates would lead to the destruction of the carbonatic sand particles into silt and clay and cause a high error in these fractions (Döpke 2004). Thus, the TOC was not removed before the grain size analysis and therefore appears as an average error of 2 %, mainly in the sand fraction. Total inorganic content (TIC) and TOC of the samples were analysed with a LECO RC 412 Multiphase Carbon Determinator (St. Joseph, USA) in accordance with DIN EN 15936.
An energy dispersive X-ray fluorescence analyser (EDXRF—SPECTRO X-LAB 2000 , Kleve, Germany) was used to determine the elements As, Ba, Br, Cr, Cu, Fe, Mn, Ni, Pb, Rb, Sr, Th, Ti, U, V, Zn and Zr. For sample preparation, the homogenized sample mixture (4 g) and wax (0.9 g) were pressed to a pellet. Elements such as Al, Ca, K, Mg, Na, P, S and Si of a sample were analysed with a sequential wavelength dispersive X-ray fluorescence analyser (WDXRF—Bruker AXS S4 PIONEER, Billerica, USA) following the DIN EN ISO 12677 for sample preparation. A calibration function was generated for both XRF instruments with standards of a known sample with similar matrix.
Additionally, gamma ray-emitting radionuclides of the 238U- and the 232Th decay chain as well as 40K and 137Cs were measured with two GAMMA-X, N-type coaxial high purity Geranium detectors (type GMX90-S; ORTEC, Atlanta, USA). IAEA standards of similar matrix to the samples were used for the calibration of the detector. The sample material was filled in cylindrical plastic containers with a volume of 108 or 32 cm3, weighed and air-sealed to avoid the escape of the 222Rn gas and keep the activity equilibrium between the radionuclides of the 238U decay. The measurements were undertaken 3 weeks after filling to guarantee the activity equilibrium between long-life and short-life radionuclides. Measurements were performed for 12 h per sample; further processing was done using the Gamma-W software (Dr. Westmeier GmbH, Ebsdorfergrund, Germany). 210Pbex was calculated by subtracting the specific activity of 226Ra from the specific activity of 210Pb.
Major ions in the water samples were analysed with either inductively coupled plasma mass spectrometry (ICP-MS, Elan DRCe, Perkin-Elmer, Waltham, USA) (As, Cd, Cs, Fe, Mn, Rb, U—NIST Standard Reference Material 1643e) or inductively coupled plasma atomic emission spectroscopy (ICP-AES) (B, Ba, Ca, Fe, K, Li, Mg, Na, P, Si, Sr—CIROS-Spectro Analytical Instruments, Kleve, Germany) calibrated with matrix-adjusted standard solutions. NH4 (DIN 38406—E5), PO4 (DIN 38405—D11-1), NO3 (DIN 38405—D10) and NO2 (DIN 38405—D10) were photometrically determined using the EPOS Analyser 5060 (Eppendorf, Hamburg, Germany). Ion chromatography (ICS2000, Thermo Scientific (Dionex) Waltham, USA) following EN ISO 10304–2 and DIN 38405 was used for Br, Cl, NO3, and SO4.
4.4 Data correction—revised data correction factor
In the carbonatic catchment of Wadi Al-Arab, we considered the sand, silt and clay content; the TOC as well as the TIC percentage to potentially influence element concentrations, as described by Horowitz (1991). These parameters that affect the element content of a sample will be labelled as influential parameters. A stepwise multiple regression analysis model (SMRAM) was implemented to investigate linear and nonlinear relationships, according to the best fit, between grain size fractions, TOC and TIC, and the geochemical elements and radionuclides, respectively. Results were incorporated into an element correction factor while taking account of possible interrelations between all the influencing parameters.
The basic assumption of the method is that a part of the concentration variance of an element (element i ) can be explained by the concentration variances of different influential parameters. The common variances need to be minimized to reliably relate source to sink samples. Applying a regression model, the resulting R 2 indicates the impact, and the slope of the regression represents the intensity of the influential parameter on the element concentration. However, several influential parameters can impact the variance of the element i while at the same time also impacting each other (inter-correlation). Establishing correction factors independently for the parameters could lead to overcorrection. Therefore:
-
1.
The SMRAM is driven on semi-partial coefficients and Eq. (1) was chosen to simulate the natural concentration variance of each element (\( \overset{\wedge }{Y} \) i ).
-
2.
An element- and source-specific correction factor (CF is ) is produced from the ratio between simulated sink (\( {\overset{\wedge }{Y}}_{i_{\mathrm{sink}}} \)) and mean simulated source concentration of element i (\( \overset{\wedge }{Y} \) is ) (Eq.(2)).
-
3.
Finally, a corrected element data set Yc is is generated for the mixing model with Eq. (3).
The example given in Fig. 3 starts with bi-plots of the raw data of each element (Y raw; n = 21) in relation to the influencing parameters. Here, the two with the highest R 2 values at p level = 0.05 (*) are TIC and clay (Fig. 3a, b). The residuals of the regression with the highest R 2 (Fig. 3a) are then taken to a semi-partial regression analysis (Fig. 3c) to explore the interrelations between TIC and clay. In the case of the example, the best fit was given by a linear relationship. However, for each element, different positive and negative linear and nonlinear models were appropriate and therefore used. The semi-partial regression analysis is repeated until no more significant R 2 values between the residuals and the influencing parameters occur. In the example of Fig. 3, Si only needs to be corrected for TIC.
The SMRAM model implementation in two steps: (1) Regression analyses between Si and a influential parameter 1 (= TIC); b influential parameter 2 (= Clay). (2) Regression (a) shows a higher R 2 and therefore the residuals of Si (TIC) are then plotted against influential parameters 2 (= Clay) in a semi-partial regression analyses. The low R 2 of graph c indicates that the variance of TIC also explained the variance of clay
Once all significant influential parameters are identified for each element and their influences quantified in regression equations, they are summarized in one element-specific multivariate equation to merge the effects:
Where\( \overset{\wedge }{Y} \) i = simulated concentration of element i based on the related influential parameters; Y raw(IP1) = regression equation of Y raw and influential parameter1 with highest R 2; \( {Y_{\mathrm{res}}}_{{\mathrm{IP}}_1}\left({\mathrm{IP}}_2\right) \) = semi-partial regression equation with the residual of Y raw(IP1) and influential parameter2 with highest R 2; and n = number of influential parameters.
Using Eq. (1), the mean element concentration of the various sources and the sink is simulated based on the quantified relation of the relevant influential parameters content. This allows one to relate the simulated concentrations of sink (\( {\overset{\wedge }{Y}}_{i_{\mathrm{sink}}} \)) and source (\( \overset{\wedge }{Y} \) is ) and defines the source and element-specific correction factors (CF is ):
Where CF is = correction factor of elementi and sources; \( {\overset{\wedge }{Y}}_{i_{\mathrm{sink}}} \) = simulated mean sink concentration of elementi; \( \overset{\wedge }{Y} \) is = simulated mean concentration of elementi in sources.
The correction factors are then used to correct the raw element concentrations of the sources:
Where
Yc is = corrected concentration of element i in sources; r i = adjusted R 2 value of the multivariate regression equation; CF is = corresponding correction factor of element i in sources; and Yraw is = raw concentration of element i in sources.
The adjusted R 2 of the multivariate regression equations are included in Eq. (3) as a weighting. This ensures that raw element concentrations have more weight in the correction of “less influenced” elements compared to “strongly influenced” elements.
The element data was divided into two data sets: surface sources S1–S3 with the three Wadi deposit samples (n = 21) and subsurface sources S4–S6 (n = 18). Since the latter consisted of crushed stones, a grain size analysis was not applied and only TOC and TIC were considered as influential parameters. However, Fig. 6 displays the mean TIC contents of sources S4–S6, which are located above the mean sink content. A data correction of TIC effects based on the regression Eqs. (1–3) would therefore imply an extrapolation of the data set. This extrapolation is associated with increasing inaccuracies in the prediction of value simulations and could therefore lead to overcorrections (Freund et al. 2006). We decided to determine correction factors for sources S4–S6 by using the Collins et al. (1997) method for TIC together with the determined R 2 values of the regression analysis in Eq. (3) for data correction.
4.5 Element selection and mixing model
From the literature, we knew that all of the possible elements analysed have been identified in previous studies as potential fingerprinting properties and successfully implemented. Many rely on the conservativeness of P, 238U, 226Ra, 210Pb and 210Pbex (van Wijngaarden et al. 2002; Motha et al. 2002; Yeager et al. 2005; Collins and Walling 2007; Davis and Fox 2009; Evrard et al. 2011; Schuller et al. 2013). However, for Wadi Al-Arab, these elements were excluded prior to element selection because the geological unit Al-Hisa Phosphorites crops out in the Wadi bed (Fig. 1) and contains easily mobilized uranium and phosphorus (Moh’d and Powell 2010).
The procedure used in this study is based on commonly used selection tools in sediment fingerprinting (Collins et al. 2010; Davis and Fox 2009; Wilkinson et al. 2012) and was expanded by the inclusion of the water analysis results, a literature review and statistical analysis (shaded block; Fig. 4). This additional information ensured that the elements passing the mass conservation test and the Kruskal-Wallis test are surveyed a second time in regard to the corresponding element concentration in the water profile and characteristics mentioned in the literature concerning their solubility under the given pH and Eh. If an element does not pass one of the tests, it is excluded from further procedures.
The mass conservation test (Fig. 4a) ensured that the element concentrations of the sink are located in between the sources with the highest and lowest median concentration plus/minus the corresponding median absolute deviation (MAD). A violation hints at the element’s non-conservative behaviour and/or the absence of important sediment sources (Foster and Lees 2000; Haley 2010). The non-parametric Kruskal-Wallis test (Fig. 4b) identifies elements that can discriminate between at least two sources using the source median concentrations of an element (Collins and Walling 2002). The water analysis results (Fig. 4c) serve as an additional indicator to eliminate the obvious soluble elements and reach a quick reduction of elements for the following literature review. Therefore, the element concentration of the sediments (mg kg−1) and the element concentration of the water samples (mg l−1) were converted to parts per million. Following the simple assumption, if an element is soluble, then the content (ppm) in the water will be enriched by >1 % compared to the sediment content (ppm). Elements that did not show any peculiarities or were not analysed in the water samples were evaluated in regard to the prevailing pH and Eh conditions, following the literature. All elements that were described as soluble were excluded (Fig. 4d). All elements that are compound-dependent soluble were correlated with the respective elements and were rejected in cases of a two-tailed significant relationship.
The remaining elements entered a stepwise linear discrimination function analysis (Fig. 4e) to find the best source discriminating composite fingerprint based on the raw data. The element combination with the highest reclassification accuracy is used as the composite fingerprint in the mixing model proposed by Collins and Walling (2002) (Fig. 4f and Eq (4)). Instead of using a forward selection of elements driven by the minimization of Wilks’ lambda, as suggested by Collins et al. (1998), we agree with Haley (2010) that Wilks’ lambda may not necessarily provide the best element combination in view of the reclassification result due to variance inhomogeneity, non-normal distribution and co-linearity of the element data. Hence, the reclassification accuracy for all combinations of the remaining elements was tested.
Collins et al. (2012) advocate the use of normality tests of the data before element selection to decide if parametric or non-parametric scale and local estimators of selected elements are used in the mixing model. Due to the small sample number within each source (n = 6), median concentrations of the sources as local parameters and the corresponding MAD as scale parameters were used in this study. This guarantees a reduced influence of data outliers.
The mixing model according to Collins et al. (2010) and modified by Wilkinson et al. (2012; Eq. (4)) is solved iteratively by inserting random values for the unknown source composition parts P 1 to P m until the sum of squares of the weighted relative errors (ε) is minimized. This process is implemented under the following constrains:
Where ε = sum of squares of the weighted relative errors; n = number of elements used in the composite fingerprint; m = number of sediment sources; S i = sink concentration of element i ; P s = unknown composition part of sediment sources; V is = within-source-variability weighting of elementi and sources; Yc is = corrected concentration of elementi in sources and W i = discriminatory weighting of elementi.
To account for the heterogeneous nature of sediment sources, the model was not only solved for the mean element source concentrations, but also framed by a Monte Carlo sampling procedure giving information about the uncertainty of the source contributions based on the variability in element concentrations of the sources (Rowan et al. 2000; Small et al. 2002; Krause et al. 2003; Collins et al. 2010; Wilkinson et al. 2012). Student’s t distributions were computed for each element in each source by using the source medians as local estimators and the corresponding MAD as the range of the corrected element data (Yc is ). The mean element concentrations of the core samples reflect the sink concentrations in the mixing model. The mixing model was then solved 2000 times, ensuring a homogenous distribution, with randomly generated element fractions of Yc is within the two-tailed 95 % confidence interval of the Student’s t distributions. The mean of the 2000 mixing model results defines the source composition of the reservoir sediment and the standard deviation reflects the source uncertainties.
For providing information about the reliability of the estimated source composition, the mean relative error (MRE; Wilkinson et al. 2012) was used:
Where r = number of iterations; n = number of sources and ε = sum of squares of the relative element equation errors (Eq. (4)).
The data correction and sediment fingerprinting was mainly applied using the open source statistic program R (R Development Core Team 2011). Table S1 in the Electronic Supplementary Material provides an overview of the R-packages and functions used.
5 Results
5.1 Sink chemistry, sediments and influencing parameters
The physiochemistry of the Wadi Al-Arab reservoir showed an abrupt decrease from an alkaline (pH 8.2) to a neutral pH (pH 7.3) as well as a drop of redox potential to <300 mV below 8 m water depth, indicating anaerobic conditions in the lower water body of the meromictic lake (Beutel et al. 2007). The reservoir sediment cores showed neither vertically nor spatially significant variability in the element and isotope concentrations. Thus, the mean tracer concentrations of all core sediment samples were used in the subsequent analysis.
The grain size analysis from the sources and the sink showed a clear trend in grain size selectivity (Fig. 5). Sand accumulates mostly in the Wadi deposits. Clay and silt fractions are mainly transported through to the reservoir as suspended sediment yield. The TOC and TIC contents of the sources differ from the mean contents of the reservoir sediment (Fig. 6). Hence, grain size selectivity and substantial differences in organic matter and carbonate content are influencing parameters prohibiting the direct comparison between sink and source samples.
5.2 Data correction for sources one to three and Wadi deposits
A total of 27 elements were analysed with SMRAM for the influence of the influential parameters (i.e. sand, silt, clay, TOC, TIC). Table S2 in the Electronic Supplementary Material summarizes the results performed on the pedogenetic sources S1–S3 and the Wadi deposit samples. Significant relationships during the initial regression analysis are highlighted and show that clay (2), TOC (7) and TIC (14) each have an influence on 23 of 27 elements. Ni is not influenced by any of the tested parameters, whereas Ba, Br, Cr, Cu, Na, P, Sr, Zn, 210Pb and 137Cs are only influenced by one of the aforementioned influential parameters. Furthermore, it is noticeable that TOC-related elements are not influenced by sand, silt, clay and/or TIC, and vice versa. Apart from Ca and Sr, the direction of dependency between TIC and the related elements is negative, as is the dependency between sand and the related elements. Clay (with the exception of P) and TOC are always positively related with the elements. This study showed that the silt content of a sample has no significant influence on the element concentrations.
In the first-order semi-partial regression analysis, no further significant relationships were observed between the residuals from the initial regression analysis and the remaining influential parameters. Hence, the equations of the initial regression analysis serve as element correction functions (Eq. (1)) and the corresponding R 2 values serve as goodness of fit for the corrected data set development (Eq. (3)).
Table 4 shows the raw and corrected element concentrations following the SMARM in comparison to the method by Collins et al. (1997) using the SSA. Furthermore, we added the within-source variability weighting used in the mixing model. The SMARM-corrected values have a higher similarity to the original concentrations and are therefore more environmentally sensitive compared to the SSA method.
5.3 Data correction for sources 4 to 6
No significant relationships could be identified between TOC and the elements using the SMRAM, while TIC is positive linear related with Ca (R 2 = 0.97) and negative linear related with Cr (R 2 = 0.39), Ni (R 2 = 0.72), P (R 2 = 0.54), Si (R 2 = 0.82) and Zn (R 2 = 0.54) at p values <0.01.
5.4 Element selection
Table 3 summarizes the selection procedure and shows that Zn failed the mass conservation test. Ba, Br and Pb failed the Kruskal-Wallis test. The comparison of water and sink element content identified Na as non-conservative since the water samples were enriched by 10.93 % compared to the sediment concentration. The concentrations of Ca, Fe, K, Mg, Mn, Si and Sr were <1 % and together with the radionuclides they entered the literature review stage (Fig. 4d) concerning pH and Eh. Horowitz (1991) and Scheffer and Schachtschabel (2010) state that for an alkaline to neutral pH and a relatively high mean content of TIC—as found in the reservoir sediments—Al, Cr, Cu and Ni behave conservatively. Fe and Mn-oxihydroxides are, besides clay, important absorption partners for cations (Horowitz 1991; Slattery et al. 1999), which may become recycled under anaerobic conditions. However, in the Al-Arab reservoir, the pH never falls below 7.3 and Eh always exceeds +127 mV (own unpublished data; Saadoun et al. 2010), resulting in stable conditions (Scheffer and Schachtschabel 2010) and, hence, Mn and Fe can be considered as conservative. The oxides and associated minerals of Ti, Zr and Th (232Th, 228Th) are highly insoluble (Adriano 1986; Scheffer and Schachtschabel 2010) and consequently conservative, as is 137Cs (Motha et al. 2002).
Due to high phytoplankton production during winter (Saadoun et al. 2008), and subsequent in situ sedimentation of organic matter, TOC is excluded from the element selection. Furthermore, Adriano (1986), Horowitz (1991) and Scheffer and Schachtschabel (2010) reported that the nutrients K (including 40K) and Mg are possibly subject to leaching. Thus, they were excluded from the selection procedure as a precautionary measure. Overall, 12 components (Al, Cr, Cu, Fe, Mn, Ni, Si, Ti, Zr, 137Cs, 232Th and 228Th) proved to be conservative and were used for the stepwise linear discriminant analysis (Table 4).
The combination Al, Cr, Fe, Mn, 137Cs, 232Th and 228Th provided the best source discrimination with a cross-validated accuracy rate of 94.4 %. One sample of source S6 and one sample of source S5 were misclassified to source S4. All surface sources S1–S3 were correctly classified during cross-validation (Table 5).
5.5 Mixing model and Monte Carlo uncertainty estimation
The results of the mixing model indicate that 59 ± 8 % of the sampled reservoir sediments are delivered from sloped cultivated olive orchards (S1). Fields for agriculture on plateau and saddle positions (S2) are associated with 11 ± 9 %, with 29 ± 15 % derived from slopes used for grazing (S3) and 1 ± 1.4 % derived from the geological unit Al-Hisa Phosphorites/Amman Silicified Limestone (S6). However, with reference to the error, no sediment originated from the geological sources (S4–S6). It was possible to reconstruct the mean element concentrations of the sink with a mean residual error of 1.04 %.
6 Discussion
6.1 Data correction and element selection
Results showed that the grain size composition of the <63 μm fraction of source and sink sediments was substantially different (Fig. 5). Although Walling (2013) maintains that most sediments transported are <63 μm, the change in fraction volume would have prohibited a direct comparison of source and sink sediments, rendering the additional implementation of correction factors necessary. Sampling smaller grain sizes (<10 μm) as suggested by Wilkinson et al. (2012) implies that the source affiliation is only limited to this fraction and demands proof that the chosen fraction represents the major component of the sink sediment (Walling 2013). This was not the case for the Al-Arab reservoir sediments since the major component was silt (<63 μm).
Koiter et al. (2013a, b) shows that in catchments where disintegration and/or attrition of sediments during transport is likely, the general assumption that finer-grained material is more chemically reactive can be an incorrect conclusion and thus selective sampling of smaller grain size fractions is inappropriate. This might be of special relevance to carbonatic catchments, such as the Wadi Al-Arab, where the disintegration of carbonatic sand particles needs to be considered. However, we recognize that in certain cases, and with the necessary prior knowledge about the composition of the grain size fractions before and during transport as well as after deposition, the selective sampling approach has the potential to give better accuracy to the results than any numerical correction. Nevertheless, as in most cases, the work implemented in north Jordan was a pilot study and no prior knowledge existed as to grain size fractions, particle selectivity and organic or inorganic carbon content. Selective grain size sampling or the removal of organic material would have implied the risk of neglecting important information beforehand and, with regards to the results, would have followed incorrect assumptions.
The SMRAM confirms that sand and TIC generally dilute (negatively correlate) a sample, whereas TOC and clay enrich the concentration, corresponding to known literature (Adriano 1986; Horowitz 1991; Scheffer and Schachtschabel 2010). The positive relation of TIC with Ca (R 2 = 0.99) and Sr (R 2 = 0.42) leads to the suggestion that the TIC content, and thus the bedrock in the catchment, mainly consists of calcium carbonate and partly of strontium carbonate. The method further revealed that TIC, from all tested influential parameters, showed the highest R 2 values and best explains the variance of the 14 out of 27 related elements. Thus, the inclusion of TIC as an influencing parameter is judged as essential for carbonatic areas, as was recommended by Horowitz (1991). More importantly, the SMRAM revealed the absence of any further relationships after the first regression analysis, explained by the interrelations of the influential parameters (Table 6). Clay and TIC show a significant negative relationship, while sand and TIC have a significant positive one. This indicates that the sand fraction is made of carbonatic particles. TOC is free of interrelations with any other influential parameter. Collins et al. (1997), Russell et al. (2001) and Mukundan et al. (2012) report a general influence by TOC, which is most probably closely related to grain size influence, so one has to be careful of over correction when addressing both.
In north Jordan, existing uranium and phosphorus deposits in the bedrock prevent the use of P, 238U, 226Ra, 210Pb and 210Pbex as fingerprinting elements because they can enter the system in additional, undetermined amounts. Additionally, we tested the elements using the element selection procedure for reassurance. Results affirmed the decision for exclusion. 238U, 226Ra and 210Pb failed the mass conservation test, due to unknown enrichment. The 210Pbex core values were mainly negative because the unknown accumulation in 226Ra disturbs the comparability with 210Pb; both form the basis of the atmospheric 210Pbex calculation. Phosphorus was excluded because of its possible solubility (Scheffer and Schachtschabel 2010). The results generally suggest that a profound knowledge of the geological background of the study area is of advantage for the choice of suitable fingerprinting elements.
The water analysis results provided important information on the pH and Eh conditions in the water profile, thus allowing evaluation of the changing environmental conditions that the sediments go through while settling. Including the water element concentrations, the literature review and the correlation analysis into the expanded selection procedure helped to quickly eliminate elements whose conservative behaviour in water could be doubted. Results showed that the mass conservation and Kruskal-Wallis test did not suffice in rejecting the elements Ca, Na and Sr, which are undoubtedly soluble in water, but have been used as conservative fingerprinting properties in other studies analysing suspended sediment sources (e.g. Russell et al. 2001; Collins and Walling 2002; Carter et al. 2003; De Miguel et al. 2005). Furthermore, the problem of phytoplankton production in lakes is quite common and prohibits the use of organic properties, such as TOC. Additionally, K and Mg are potentially soluble and were therefore removed by the expanded procedure, although they are, together with TOC, considered as conservative fingerprinting properties in other studies using lake sediments (Kelley and Nater 2000; Kouhpeima et al. 2010; Slimane et al. 2013).
6.2 Results of the sediment fingerprinting
Despite the uncertainties associated with the source composition, the results clearly show that steep slopes with shallow, yellow, Mediterranean soils underlying cultivated olive orchards (S1 = 59 ± 8 %) and grazing land (S3 = 29 ± 15 %, Table 1), when combined, contributed ∼90 ± 17 % of the reservoir sediments. The marly substrate of the yellow Mediterranean soils have a high Na content, leading to unstable aggregates and surface crusting, concurrently delivering good conditions for runoff generation, favoured by the fragmented vegetation cover (Yassoglou and Kosmas 1997; Thornes 2005; García-Ruiz 2010; unpublished field observations). These developments are of special relevance within the catchment on the south-facing slopes as soil temperature and possible rain shadow visibly enhance this development strongly. These unfavourable characteristics of the present soils, together with the steep slope gradient and agricultural use, trigger the high soil erosion in these land units.
Our erosion measurements in seven olive orchards on slopes >10 % showed very high potential erosion rates (95 ± 8 t ha−1 year−1) mainly attributed to the downhill ploughing, the canalisation of runoff water and, consequently, the triggering of excessive water erosion (Fig. 2a, Kraushaar et al. 2014). These findings are supported by other studies from the Mediterranean on conventionally tilled olive orchards using different methods of investigation (Gómez et al. 2003; Milgroom et al. 2005; Bruggemann et al. 2005; Francia Martínez et al. 2006; Ramos et al. 2008; Barnevald et al. 2009; Vanwalleghem et al. 2010, 2011). Slopes that are not ploughed but undergo grazing are likely to suffer from different processes including thinning of vegetative coverage, compaction of soil surface, reduced infiltration and therefore increased runoff (Yassoglou and Kosmas 1997). Furthermore, as with Wadi Al-Arab, substantial evidence of mechanical translocation of the stony mulch layer that protects the surface is highly evident. A typical pattern of sheep and goat tracks exists on the majority of the slopes, leaving a reduced resistance to water erosion (Fig. 2c).
Fields used for agriculture on plateau and saddle positions that contain red Mediterranean soils (S2) show a much lower contribution to the sink at only 11 ± 9 %. The existence of red Mediterranean soils with their various pedogentic features (Yaalon 1997) and their advanced soil development can be interpreted as proof of stable geomorphic conditions (Verheye and de la Rosa 2005; Federoff and Courty 2013). However, recent changes in management caused shallow gullies in the red soils throughout the catchment. Broken road boundaries or the clearance of vegetation strips channelize and accelerate surface water runoff in upstream positions, increase transportation capacity and cause gullying, even in almost levelled locations. Typically, soils in grain fields are unprotected during the rainy season (October–February) and are, therefore, highly vulnerable to extreme rainfall events causing rills and shallow ephemeral gully erosion (García-Ruiz 2010). These results are supported by Khresat and Taimeh (1998) who report water erosion and improper farming practices to be the major causes of physical soil degradation in the study area.
Sources S4–S6 were, with regard to the error, not detected in the sink as these sediments do not contain 137Cs that was present in the lake sediments. These sources were chosen to represent subsurface samples as C-horizons from gully erosion, in addition to new sediments derived from construction work on roads where enormous masses of unconsolidated bedrock sediments are produced and dumped onto the slopes. However, with regards to the fingerprinting data, either they did not deliver enough material to the sink for the model to detect, and/or the bedrock samples do not represent these sources well enough. Future research should focus on sampling the aforementioned sources directly and consider the vertical sampling of profiles to differentiate topsoil from gully erosion for their relative contribution to the sink.
6.3 Implications for the Wadi Al-Arab reservoir
The reservoir experienced ∼9 % loss of total water storage volume in 26 years. The clayey and silty sediments accumulate at the wall of the dam near the water outlet. Here, the sediment line is around 1–2 m below the outlet, posing an acute threat to the reservoir as there is no available removal equipment (personal communication, Hussein Al-Shurieki 2011, dam director; own unpublished data 2011).
Particle selectivity leads to an enrichment of the heavy metals, such as chromium (228 mg kg−1), nickel (99.5 mg kg−1) and cadmium (4 mg kg−1), in the reservoir sediments, exceeding the probable effect level for an ecosystem (USEPA 2014: Cr = 90 mg kg−1; Ni = 36 mg kg−1; Cd = 3.53 mg kg−1). Due to the amount of clay, the high concentration of iron, and the carbonatic background (mean TIC = 7.4 %), and thus a pH >7, the heavy metals are considered as immobile in the reservoir sediments (Blume 2004). However, sediments should not be hauled to the surface and used as agricultural amendments since heavy metals might become mobile under changing conditions, such as agricultural use. Thus, the only effective way of managing the reservoir is to prevent sediments from entering it, with counter erosion measurements on the slopes, predominantly in the most affected source areas, such as olive orchards and slopes used for grazing.
7 Conclusions
A sediment fingerprinting procedure was implemented for a Mediterranean to semi-arid catchment in north Jordan with six potential sediment sources. On the basis of this pilot study, an adapted correction factor approach is suggested to guarantee comparability of sink and source samples. This approach includes the exploration of possible linear, as well as nonlinear, positive and negative relationships between individual elements, radionuclides and the identified influential parameters of clay, silt, sand, TOC and TIC. Correction factors were developed that incorporate the concept of interrelations in the calculation of element-specific correction factors. Results highlight the influential impacts of TIC, particularly in the carbonatic Wadi Al-Arab catchment, and the majority of the elements needed to be corrected for differences in TIC content. TOC did not show interrelations to other influential parameters, such as clay, silt or sand. However, when more than one influential parameter existed (usually clay, sand and TIC), the correction of TIC was sufficient to erase the influence of the other parameters due to interrelations between them. Finally, Ni did not need any correction. The strong influence of the TIC content is assumed to exist in regions of carbonatic bedrock as well as in areas of carbonatic dust input. Carbonate-free areas should be free on any TIC influence on the element concentration.
The correction factors are calculated element—but not source-specific since the regression analyses are sensitive to outliers in small data sets. The general trend (positive or negative) between the influential parameters and the element are assumed to be similar for each source. Nevertheless, it would be of great advantage to further explore source-specific relations with an expanded data set.
The element selection procedure should not rely solely on a cross check of the element’s application in other, similar research studies nor the conventional element selection procedure comprising a mass conservation test and the Kruskal-Wallis test. Both approaches are not enough to reliably eliminate non-conservative elements, especially when considering reservoir or lake sediments. Additional physiochemical information on the sink environment gained through water samples, a literature review and correlation analysis helped to reject further elements. However, the study also showed that an additional comprehensive and conservative literature review with consideration of prevailing pH and Eh conditions alone would have eliminated these respective elements. When using lake or reservoir sediments in sediment fingerprinting, organic elements need to be carefully evaluated for their conservativeness due to possible phytoplankton production, and thus an unspecified additional organic source.
We conclude from the analysis of the lake sediments that the element concentrations did not vary significantly in the core samples and throughout the lake, and thus indicate similar source contributions throughout the 26 years of the reservoir’s existence. Furthermore, no exhaustion of the identified surface sources, and the related human impact on the system, could be detected. The observed sediment delivery to the reservoir, and associated sedimentation, has resulted in an acute threat to the reservoir’s life span.
References
Adriano DC (1986) Trace elements in the terrestrial environment. Springer, New York
Al-Ansari N, Shatnawi A (2011) Siltation of three small reservoirs in Jordan. Publications by the Institute of Earth and Environmental Sciences, Al al-Bayt University Jordan
Al-Ansari N, Alroubai A, Knutsson S (2012) Bathymetry and sediment survey for two old water harvesting schemes, Jordan. J Earth Sci Geotech Eng 2:13–23
Al-Sharhan AS, Nairn AEM (1997) Sedimentary basins and petroleum geology of the Middle East. Elsevier, Amsterdam
Barnevald RJ, Bruggeman A, Sterk G, Turkelboom F (2009) Comparison of two methods for quantification of tillage erosion rates in olive orchards of north-west Syria. Soil Till Res 103:105–112
Beutel MW, Leonarda TM, Denta SR, Mooreb BC (2007) Effects of aerobic and anaerobic conditions on P, N, Fe, Mn, and Hg accumulation in waters overlaying profundal sediments of an oligo-mesotrophic lake. Water Res 42:1953–1962
Blume HP (ed) (2004) Handbuch des Bodenschutzes. Bodenökologie und –belastung - Vorbeugende und abwehrende Schutzmaßnahmen, 3rd edition. ecomed, Landsberg am Lech, pp 723–769
Bruggemann A, Masri Z, Turkelboom F, Zöbisch M, El-Naeb H (2005) Strategies to sustain productivity of olive groves on steep slopes in the northwest of the Syrian Arab Republic. In: Benites J, Pisante M, Stagnari F (eds) Integrated soil and water management for orchard development; role and importance, vol 10, FAO land and water bulletin. FAO, Rome, pp 75–87
Carter J, Owens PN, Walling DE, Graham JLL (2003) Fingerprinting suspended sediment sources in a large urban river system. Sci Total Environ 314–316:513–534
Collins AL, Walling DE (2002) Selecting fingerprint properties for discriminating potential suspended sediment sources in river basins. J Hydrol 261:218–244
Collins AL, Walling DE (2007) Sources of fine sediment recovered from the channel bed of lowland groundwater-fed catchments in the UK. Geomorphology 88:120–138
Collins AL, Walling DE, Leeks GJL (1997) Source type ascription for fluvial suspended sediment based on a quantitative composite fingerprinting technique. Catena 29:1–27
Collins AL, Walling DE, Leeks GJL (1998) Use of composite fingerprints to determine the provenance of the contemporary suspend sediment load transported by rivers. Earth Surf Process Landf 23:31–52
Collins AL, Walling DE, Webb L, King P (2010) Apportioning catchment scale sediment sources using a modified composite fingerprinting technique incorporating property weightings and prior information. Geoderma 155:249–261
Collins AL, Zhang Y, McChesney D, Walling DE, Haley SM, Smith P (2012) Sediment source tracing in a lowland agricultural catchment in southern England using a modified procedure combining statistical analysis and numerical modelling. Sci Total Environ 414:301–317
Davis CM, Fox JF (2009) Sediment fingerprinting: review of the method and future improvements for allocating nonpoint source pollution. J Environ Eng 135:490–504
De Miguel E, Charlesworth S, Ordonez A, Seijas E (2005) Geochemical fingerprints and controls in the sediments of an urban river: River Manzanares, Madrid (Spain). Sci Total Environ 340:137–148
Döpke G (2004) Regionale und substratabhängige verteilung von schwermetallen in oberflächennahen sedimenten der inner Kingston basin, Ontariosee. University of Osnabrück, Austria
El-Radaideh NM (2010) Using bottom reservoir sediments as a source of agricultural soil: Wadi El-Arab Reservoir as a case study, NW Jordan. ABHATH AL-YARMOUK: Basic Sci Eng 19:75–91
Evrard O, Navratil O, Ayrault S, Ahmadi M, Némery J, Legout C, Lefèvre I, Poirel A, Bonté P, Esteves M (2011) Combining suspended sediment monitoring and fingerprinting to determine the spatial origin of fine sediment in a mountainous river catchment. Earth Surf Process Landf 36:1072–1089
Federoff N, Courty M-A (2013) Revisiting the genesis of red Mediterranean soils. Turkish J Earth Sci 22:359–375
Foster IDL, Lees JA (2000) Tracers in geomorphology—theory and applications in tracing fine particulate sediments. In: Foster IDL (ed) Tracers in geomorphology. Wiley, Chichester, pp 3–21
Foster IDL, Boardman J, Keay-Bright J (2007) Sediment tracing and environmental history for two small catchments, Karoo uplands, South Africa. Geomorphology 90:126–143
Francia Martínez JR, Durán Zuazo VH, Martínez Raya A (2006) Environmental impact from mountainous olive orchards under different soil-management systems (SE Spain). Sci Total Environ 358:46–60
Freund RJ, Wilson WJ, Sa P (2006) Regression analysis—statistical modeling of a response variable, 2nd edn. Elsevier, Oxford
García-Ruiz JM (2010) The effects of land uses on soil erosion in Spain: a review. Catena 81:1–11
Ghrefat H, Yusuf N (2006) Assessing Mn, Fe, Cu, Zn, and Cd pollution in bottom sediments of Wadi Al-Arab Dam, Jordan. Chemosphere 65:2114–2121
Gómez JA, Battany B, Renschler CS, Fereres E (2003) Evaluating the impact of soil management on soil loss in olive orchards. Soil Use Manag 19:127–134
Haley S (2010) The application of sediment source fingerprinting techniques to river floodplain cores, to examine recent changes in sediment sources in selected UK river basins. University of Exeter, Exeter, Unpublished PhD thesis
Horowitz AJ (1991) A primer on sediment-trace element chemistry, 2nd edn. Lewis Publishers, Chelsea
Kelley DW, Nater EA (2000) Source apportionment of lake bed sediments to watersheds in an Upper Mississippi basin using a chemical mass balance method. Catena 41:277–292
Khresat SA, Taimeh AY (1998) Properties and characterization of Vertisols developed on limestone in a semi-arid environment. J Arid Environ 40:235–244
Koiter AJ, Owens PN, Petticrew EL, Lobb DA (2013a) The behavioural characteristics of sediment properties and their implications for sediment fingerprinting as an approach for identifying sediment sources in river basins. Earth-Sci Rev 125:24–42
Koiter AJ, Lobb DA, Owens PN, Petticrew EL, Tiessen KHD, Li S (2013b) Investigating the role of connectivity and scale in assessing the sources of sediment in an agricultural watershed in the Canadian prairies using sediment source fingerprinting. J Soils Sediments 13:1676–1691
Kouhpeima A, Hashemi SAA, Feiznia S, Ahmadi H (2010) Using sediment deposited in small reservoirs to quantify sediment yield in two small catchments of Iran. J Sustain Dev 3(3):133–139
Krause AK, Franks SW, Kalma JD, Loughran RJ, Rowan JS (2003) Multi-parameter fingerprinting of sediment deposition in a small gullied catchment in SE Australia. Catena 53:327–348
Kraushaar S, Herrmann N, Ollesch G, Vogel HJ, Siebert C (2014) Mound measurements—quantifying medium term soil erosion under olive trees in Northern Jordan. Geomorphology 213:1–12
Lucke B (2007) Demise of the decapolis. Past and present desertification in the context of soil development, land use and climate. Brandenburg Technical University of Cottbus, Germany, Unpublished PhD thesis
Milgroom J, Auxiliadora Soriano M, Garrido JM, Gómez JA, Fereres E (2005) The influence of a shift from conventional to organic olive farming on soil management and erosion risk in southern Spain. Renewable Agric Food Syst 22:1–10
Miller JR, Lord M, Yurkovich S, Mackin G, Kolenbrander L (2005) Historical trends in sedimentation rates and sediment provenance, Fairfield Lake, Eastern north Carolina. J Am Water Resour Assoc 41.5:1053–1075
MoA (Ministry of Agriculture) (1994) National soil map and land use project—the soils of Jordan. Volume 3. Hunting technical services LTD, Jordan (Level 2), Amman, Jordan
Moh’d BK (2000) The geology of Irbid and Ash Shuna Ash Shamaliyya (WAQQAS), Map Sheets No. 3154-II and 3154-III.Geological Directorate. Geological Mapping Division, Bulletin 46, Amman, Jordan
Moh’d BK, Powell JH (2010) Uranium distribution in the Upper Cretaceous-Tertiary Belqa Group, Yarmouk Valley, northwest Jordan. Jordan J Earth Environ Sci 3:49–62
Motha JA, Wallbrink PJ, Hairsine PB, Grayson RB (2002) Tracer properties of eroded sediment and source material. Hydrol Process 16:1983–2000
Motha JA, Wallbrink PJ, Hairsine PB, Grayson RB (2004) Unsealed roads as suspended sediment sources in an agricultural catchment in south-eastern Australia. J Hydrol 286:1–18
Mukundan R, Walling DE, Gellis AC, Slattery MC, Radcliffe DE (2012) Sediment source fingerprinting: transforming from a research tool to a management tool. J Am Water Resour Assoc 48:1241–1257
Nortcliff S, Carr G, Potter RB, Darmame K (2008) Jordan’s water resources: challenges for the future. The University of Reading, Reading, Geographical Paper No. 185
Owens PN, Xu Z (2011) Recent advances and future directions in soils and sediments. J Soils Sediments 11:875–888
Palmieri A, Shah F, Dinar A (2001) Economics of reservoir sedimentation and sustainable management of dams. J Environ Manag 61:149–163
R Development Core Team (2011) R: a language and environment for statistical computing, R Foundation for statistical computing, Vienna, Austria. Available at: http://www.R-project.org (last access: 13 October 2013)
Ramos MI, Feito FR, Gil AJ, Cubillas JJ (2008) A study of spatial variability of soil loss with high resolution DEMs: a case study of a sloping olive grove in southern Spain. Geoderma 148:1–12
Rowan JS, Goodwill P, Franks SW (2000) Uncertainty estimation in fingerprinting suspended sediment sources. In: Foster IDL (ed) Tracers in geomorphology. Wiley, Chichester, pp 279–290
Russell MA, Walling DE, Hodkinson RA (2001) Suspended sediment sources in two small agricultural lowland catchments in the UK. J Hydrol 252:1–24
Saadoun I, Bataineh E, Al-Handal AY (2008) The primary production conditions of Wadi Al-Arab Dam (reservoir), Jordan. Jordan J Biol Sci 1:67–72
Saadoun I, Batayneh E, Alhandal A, Hindieh M (2010) Physicochemical features of the Wadi Al-Arab Dam (reservoir), Jordan. Intern J Ocean Hydrobiol 39:191–205
Salomons W, Mook WG (1987) Natural tracers for sediment transport studies. Cont Shelf Res 7:1333–1343
Scheffer F, Schachtschabel P (2010) Lehrbuch der bodenkunde, 16th edn. Spektrum Akademischer Verlag, Heidelberg
Schuller P, Walling DE, Irouméc A, Quilodránd C, Castilloa A, Navase A (2013) Using 137Cs and 210Pbex and other sediment source fingerprints to document suspended sediment sources in small forested catchments in south-central Chile. J Environ Radioact 124:147–159
Slattery WJ, Conyers MK, Aitken RL (1999) Soil pH, aluminium, manganese and lime requirement. In: Peverill KI, Sparrow LA, Reuter DJ (eds) Soil analysis—an interpretation manual. CSIRO, Collingwood, pp 103–128
Slimane AB, Raclot D, Evrard O, Sanaa M, Lefèvre I, Ahmadi M, Tounsi M, Rumpel C, Mammou AB, Le Bissonnais Y (2013) Fingerprinting sediment sources in the outlet reservoir of a hilly cultivated catchment in Tunisia. J Soils Sediments 13:801–815
Small IF, Rowan JS, Franks SW (2002) Quantitative sediment fingerprinting using a Bayesian uncertainty estimation framework. In: Dyer FJ, Thoms MC, Olley JM (eds) The structure, function and management implications of fluvial sedimentary systems. IAHS Press, Wallingford, pp 443–450, IAHS Publ 276
Smith HG, Blake WH (2014) Sediment fingerprinting in agricultural catchments: a critical re-examination of source discrimination and data corrections. Geomorphology 204:177–191
Stumm W, Morgan JJ (1996) Aquatic chemistry: chemical equilibria and rates in natural waters, 3rd edn. Wiley, New York
Thornes JB (2005) Coupling erosion, vegetation and grazing. Land Degrad Dev 16:127–138
United States Environment Protection Agency - USEPA (2014) Water quality standards handbook—Chapter 3: Water quality criteria (40 CFR 131.11) http://water.epa.gov/scitech/swguidance/standards/handbook/chapter03.cfm (last checked 12.06.2014)
van Wijngaarden M, Venema LB, De Meijer RJ, Zwolsman JJG, Van Os B, Gieske JMJ (2002) Radiometric sand–mud characterisation in the Rhine–Meuse estuary part A. Fingerprinting Geomorphology 43:87–101
Vanmaercke M, Poesen J, Verstraeten G, de Vente J, Ocakoglu F (2011) Sediment yield in Europe: spatial patterns and scale dependency. Geomorphology 130:142–161
Vanwalleghem T, Laguna A, Giráldez JV, Jiménez-Hornero FJ (2010) Applying a simple methodology to assess historical soil erosion in olive orchards. Geomorphology 114:294–302
Vanwalleghem T, InfanteAmte J, González de Molina M, Soto Fernández D, Gómez JA (2011) Quantifying the effect of historical soil management on soil erosion rates in Mediterranean olive orchards. Agric Ecosyst Environ 142:341–351
Verheye W, de la Rosa D (2005) Mediterranean soils. In: Encyclopedia of Life Support Systems (EOLSS) developed under the Auspices of the UNESCO (ed) Land use and land cover. Eolss Publishers, Oxford, http://digital.csic.es/bitstream/10261/38282/1/Mediterranean%20soils.pdf . Accessed 3 May 2015
Walling DE (2005) Tracing suspended sediment sources in catchments and river systems. Sci Total Environ 344:159–184
Walling DE (2013) The evolution of sediment source fingerprinting investigations in fluvial systems. J Soils Sediments 13:1658–1675
Walling DE, Woodward JC, Nicholas AP (1993) A multiparameter approach to fingerprinting suspended sediment sources. In: Peters NE, Hoehn E, Liebundgut C, Tase N, Walling DE (eds) Tracers in hydrology. IAHS Press, Wallingford, pp 329–338, IAHS Publ 215
Walling DE, Owens PN, Leeks GJL (1999) Fingerprinting suspended sediment sources in the catchment of the River Ouse, Yorkshire, UK. Hydrol Process 13:955–975
Wilkinson SN, Hancock GJ, Bartley R, Hawdon AA, Keen RJ (2012) Using sediment tracing to assess processes and spatial patterns of erosion in grazed rangelands, Burdekin River basin, Australia. Agric Ecosyst Environ 180:90–102
Yaalon DH (1997) Soils in the Mediterranean region: what makes them different? Catena 28:157–169
Yassoglou NJ, Kosmas C (1997) Desertification in the Mediterranean Europe. A case in Greece. In: RALA (ed) Rangeland desertification report, Report No. 200. RALA Ed, Reykjavik, Iceland, pp 27–33
Yeager KM, Santschi PH, Phillips JD, Herbert BE (2005) Suspended sediment sources and tributary effects in the lower reaches of a coastal plain stream as indicated by radionuclides, Loco Bayou, Texas. Environ Geol 47:382–395
Yu L, Oldfield F (1989) A multivariate mixing model for identifying sediment source from magnetic measurements. Quat Res 32:168–181
Acknowledgments
The authors would like to thank A. Rämmler, J. Fröhlich, D. Sonntag, D. Bednorz, I. Volkmann, H-J. Stärk, C. Bönisch, M. Wunderlich and S. Mothes (UFZ), M. Weber (MLU Halle) and B. Winkler (TU Dresden) for analytics and interpretation advice. We are grateful to H. Al-Shurieki (Al-Arab reservoir) and M. Raggad and E. Salameh (Jordan Univ.) for their steady support on site. We also wish to thank the German tax payer for funding the SMART II project (FKZ 02-WM1080) through the Federal Ministry of Education and Research, the IPSWat Program and Helmholtz Interdisciplinary Graduate School for Environmental Research (HIGRADE).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hugh Smith
Rights and permissions
About this article
Cite this article
Kraushaar, S., Schumann, T., Ollesch, G. et al. Sediment fingerprinting in northern Jordan: element-specific correction factors in a carbonatic setting. J Soils Sediments 15, 2155–2173 (2015). https://doi.org/10.1007/s11368-015-1179-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1179-2