Abstract
Purpose
Heavy metals are natural soil constituents; however, the intense use of agrochemicals can increase total contents above background levels as well as the available fractions of the more toxic elements. In this study, the occurrence of some metals was investigated in agricultural soils from an intensely farmed rural area of Greece (Argolida) aiming to evaluate the various available pools and examine their relationship with the reactive and pseudototal soil fraction.
Materials and methods
Thirty soil samples were selected from a large database of a previous geochemical survey in the study area. The pseudototal (aqua regia), reactive (0.43 M HNO3), potentially phytoavailable (0.05 M EDTA), mobilizable (0.43 M HAc), and mobile (0.01 M CaCl2) pools of Cu, Zn, Cd, and Mn were determined. Soil properties were also examined including pH, total organic carbon, calcium carbonate content, and amorphous Al, Mn, and Fe oxides. In order to combine all geochemical information and elucidate the association between available metal pools and soil chemical characteristics, multiple linear regression was employed. The various proportions of available metal pools (%) were expressed as a function of the pseudototal or reactive metal content and general soil properties.
Results and discussion
The mobile pool of trace elements in the studied soils was quite low, while notable amounts of Cu were released by EDTA indicating a greater tendency for complexation. The acetic acid extraction yielded increased percentages of Cd and Mn and for these elements the reactive pools were quite representative of the pseudototal content. Extractable amounts of Zn by all the applied reagents were very low indicating that a considerable part of this metal is of geogenic origin. The empirical regression models showed that prediction of the available pools of Cu, Zn, and Cd from the pseudototal and reactive content is feasible. Although pH is known to be a key factor of at least exchangeable metal pools, its narrow range blurs its influence in the present study.
Conclusions
The relatively low extraction yields of the studied elements show that the potential hazard of metal transfer to plants in the area is minimal despite some elevated pseudototal concentrations. The geochemical reactive and various available pools of Cu, Zn, and Cd in Mediterranean calcareous citrus soils can be determined by a single soil extraction test using dilute nitric acid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heavy metals are natural constituents in soil, originating from the weathered parent rocks. However, their extensive use in various anthropogenic activities and applications sometimes leads to significant increase in the soil content (Lv et al. 2015; Rodríguez Martín et al. 2015; Sungur et al. 2015). Agricultural soils are threatened by metal contamination either due to rapidly expanding urban and industrial areas or because of the extensive use of agrochemicals for crop protection, e.g., Cu-based fungicides, and the enhancement of growth, e.g., to counteract Zn deficiency (Behera et al. 2011; Rodríguez Martín et al. 2013; Shan et al. 2013; Jiao et al. 2014).
Many trace metals such as Cu, Mn, and Zn are essential for plant and animal growth and participate in metabolic functions in certain concentrations while others, e.g., Cd, have no known positive effects and are undeniably toxic in concentrations above tolerance limits. Essential metals can also cause toxic effects above certain limits and lead to crop yield decrease. The accumulation of heavy metals in plant tissues and subsequent transfer through the food chain can pose a risk to the health of animals and eventually of humans (Dudka and Miller 1999; Rodrigues et al. 2010).
The common practice in risk assessment is to compare the total or pseudototal (aqua regia) content of heavy metals in soil with regulatory concentration limits. Although the European legislation limits refer to total concentrations (e.g., Directive 86/278/EEC), it is widely accepted that the total content of heavy metals is not necessarily related to potential risks. Thus, an approach that includes the total (inert plus reactive), the reactive, and the available metal pools has been suggested in assessing the potential risk by exposure to contaminated soil (Gupta et al. 1996; Peijnenburg et al. 2007; Römkens et al. 2009). The reactive fraction represents precipitates of metals or metal ions reversibly sorbed on binding sites located on surfaces of soil organic matter, amorphous metal oxides, and clay. Metals in the inert pool are strongly bound within the crystalline matrix of the solid phase and are virtually unavailable for transport or plant uptake since they are released only in aerobic soils by slow weathering processes in a time frame of years. The available pool can be related to the uptake of metals by soil organisms and plants and their potential leaching to groundwater (Römkens et al. 2009; Rodrigues et al. 2010). The relationship between the available pools and the reactive metal pools in agricultural soil has not received much attention, except for the paddy fields in Taiwan (Römkens et al. 2009) and some low-polluted calcareous soils from a periurban area in Madrid, Spain (de Santiago-Martín et al. 2015).
In the last decades, several chemical extraction methods have been developed and modified with the aim to understand the interaction of heavy metals with the soil. Commonly applied extractants include neutral salts, mild acids, and complexing agents trying to simulate the chemical reactions that are responsible for metal mobilization to the solution phase, such as changes in soil pH, temperature, redox potential, and organic matter decomposition (Peijnenburg et al. 2007). Although the application of mild extracting solutions usually yields low values of metals, the usefulness of single, selective extractions is recognized for providing rapid indication on remobilization processes and potential risks for the environment (Sahuquillo et al. 2002).
Copper-based fungicides and chemical fertilizers have been traditionally used in vineyards and orchard soils throughout the world for preventing downy mildew and promoting crop growth. Their long-term application has resulted into extensive accumulation of Cu and other heavy metals in the receiving soils (Komárek et al. 2008; Kelepertzis 2014). There are concerns about the risks that the accumulation of metals in these soils may pose not only to human health but also to the long-term sustainability of agricultural land. It has been suggested that metals introduced in the soil by anthropogenic activities have increased potential availability and reactivity compared to naturally occurring metals (Fernández-Calviño et al. 2008). Furthermore, most studies concerning metal contamination in agricultural fields where Cu-based fungicides have been used are principally based on the analysis of total and available content only of Cu. In this sense, the various available pools of other anthropogenic metals associated with the application of agrochemicals have not received the appropriate attention.
In this work, we have studied the occurrence and distribution of Cu, Zn, Cd, and Mn in various operationally defined fractions in agricultural soil samples from the rural area of Argolida, Greece. In this area, we observed an accumulation of Cu, Zn, and Cd in the citrus soils due to diverse management practices including applications of fungicides, pesticides, and fertilizers. However, no general pattern in the use of specific amendments could be recognized based on information acquired by the local farmers. Manganese was evaluated to exhibit a mixed source from both the local parent materials and the applied agrochemicals (Kelepertzis 2014). For a detailed discussion on the contribution of agrochemicals on soil geochemistry in Argolida, supported by maps and statistical analysis, the reader is referred to previously published work (Kelepertzis 2014). Our present assessment is based on several widely used single extraction procedures for evaluating the heavy metal availability; their yield is compared with the reactive and pseudototal (aqua regia) content. Furthermore, soil properties were also examined including pH, total organic carbon, calcium carbonate content, and amorphous Al, Mn, and Fe oxides. In order to combine all geochemical information and produce a better understanding of the soil factors that influence metal availability, we follow the empirical approach suggested recently (Luo et al. 2012; Rodrigues et al. 2013; Kelepertzis and Argyraki 2015). Specifically, the various proportions of available metal (%) are expressed as a function of the pseudototal and reactive metal content and general soil properties by applying multiple linear regression on the analytical data.
The aims of our study were (a) to evaluate the various available pools of Cu, Zn, Cd, and Mn in the rural agricultural soil of a typical Mediterranean environment (Argolida, Greece) and (b) to ascertain the relationship between operationally defined available pools of metals and their respective reactive or pseudototal content. Since the aqua regia is a standard method for assessing heavy metals in soil, exploring the relationship between this extraction and metal availability by milder chemical reagents is of great interest for metal assessment in agricultural soil.
2 Materials and methods
2.1 Sample selection
Thirty soil samples (0–20 cm depth) were selected from the sample database of a previous detailed soil geochemical survey (Kelepertzis 2014). The samples were collected from a rural area surrounding Argos town (∼22,000 inhabitants) in Peloponnese, Greece. This area has been subjected to intense cultivation of oranges and mandarins during the last five decades. The site is typical of a Mediterranean climate with rainy winters and warm, dry summers. The selection of the investigated samples was based on Cu concentrations because this metal represents the typical marker of anthropogenic inputs in citrus cultivated soils (Kabata-Pendias and Pendias 2001; Fernández-Calviño et al. 2008). Samples with pseudototal (aqua regia-extractable) Cu content higher than 80 mg kg−1 were included in the present study in order to ensure the inclusion of soils that have been influenced by fertilizer use and Cu-based fungicides. The soil samples had been air-dried, disaggregated, and sieved to <2 mm fraction. Representative portions of each soil sample had been further sieved through a nylon 100-μm sieve and stored in room temperature.
2.2 Methods
2.2.1 Selection of extracting agents for the evaluation of heavy metal availability
Among the numerous extraction schemes, we chose in this study to use the 0.01 M CaCl2, 0.43 M CH3COOH (HAc), and the 0.05 M EDTA extracting agents for assessing the availability of Cu, Zn, Mn, and Cd in the studied agricultural soils. These chemical extractions are not selective in terms of providing specific chemical forms of metals having an operationally defined character but have been widely applied with the aim to evaluate the availability of metals in agricultural soils (Schramel et al. 2000; Mirlean et al. 2007; Duplay et al. 2014; Qasim et al. 2015). The neutral salt extractant provides the most useful indication of metal plant uptake exhibiting an ionic strength similar to that of typical soil solutions (Menzies et al. 2007). The acetic acid chemical reagent represents one of the most abundant organic acids present in the rhizosphere of many plants (Meers et al. 2007). Furthermore, EDTA is believed to simulate complexing behavior of root exudates extracting organically bound metal fractions (Sahuquillo et al. 2002). The buffering at pH 7.0 provides the exclusion of effects involving carbonate dissolution (Meers et al. 2007). Additionally, the above three chemical procedures have been validated at the laboratory level by two certified reference materials (BCR 483 and BCR 484) aiding to monitor pitfalls that may occur during the performance of the soil extraction protocols (Quevauviller et al. 1997). The metal fractions obtained by the CaCl2, HAc, and EDTA chemical extractions were operationally defined as mobile (Gupta et al. 1996), mobilizable (Gupta et al. 1996; Sahuquillo et al. 2003), and potentially phytoavailable (Komárek et al. 2008), respectively.
2.2.2 Laboratory procedures
The pH of the samples was determined on the <2 mm soil fraction using a Jenway bench top pH meter with a glass electrode in a 1:5 (v/v) suspension of soils in ultrapure Milli-Q water according to the ISO 10390 1994 procedure. Experimental work described below was performed on the <100 μm soil fraction. Total organic carbon (TOC) was determined using a modified method based on Walkley and Black (1934) and Gaudette et al. (1974). Between 0.25 and 0.5 g of soil sample is treated for 2 h with potassium dichromate (1 N) and concentrated sulfuric acid at room temperature. This treatment oxidizes all of the organic material and the excess potassium dichromate is subsequently titrated using ammonium iron (II) sulfate. The result is expressed as percent organic carbon. Each sample was analyzed in duplicate. The carbonate content of the soils was estimated using a modification of the procedure described in Loring and Rantala (1992). Approximately 1 g of soil was treated with HCl 6 M for 1 min and the percent carbonate content was calculated by the mass difference before and after the treatment. Amorphous Fe oxides (Feox), Mn oxides (Mnox), and Al oxides (Alox) were determined by the acid ammonium oxalate extraction in the dark (Schwertmann 1964). Iron, Mn, and Al concentrations in the filtrates were analyzed by flame atomic absorption spectrometry (FAAS) at the Laboratory of Environmental Chemistry, University of Athens. The pseudototal content of the investigated metals was determined after the aqua regia digestion protocol at the Acme Analytical Laboratories Ltd of Canada using inductively coupled plasma-mass spectrometry (ICP-MS). Replicates, in-house reference materials, and reagent blanks were used for quality control.
The chemical extractions described below were performed at the Laboratories of Environmental Chemistry and Economic Geology and Geochemistry, University of Athens. Pro Analysis-grade chemicals and ultrapure Milli-Q water were used. The following cleaning procedures were adopted in order to avoid contamination. All glass laboratory utensils for metal analysis were washed with detergent, then soaked for at least 24 h in 10 % HNO3 acid solution and rinsed repeatedly with ultrapure Milli-Q water. Single-use plastic ware (centrifuge tubes and bottles for the extracts) were also soaked for at least 24 h in 10 % HNO3 acid solution and rinsed repeatedly with ultrapure Milli-Q water. The potentially phytoavailable fraction was obtained from subsamples of 2.5 g of soil leached with 25 ml of 0.05 M EDTA solution (adjusted to pH 7) for 1 h at room temperature (Ure et al. 1993). The mobilizable pool was determined by treating 1 g of soil sample with 40 ml of 0.43 M HAc solution for 16 h at room temperature (Quevauviller et al. 1997). The mobile amounts (CaCl2 extraction) were obtained following the methodology described by Novozamsky et al. (1993). The reactive forms of metals were extracted by mixing 1 g of soil material with 40 ml of a 0.43 M HNO3 solution and shaken for 2 h at room temperature. We modified the proposed 1:10 (w/v) ratio of Rodrigues et al. (2010) because of the calcareous nature of the investigated soils ensuring that pH values of the final extraction fluids were within the range 0.8–1.0. All the mixtures from these extractions were shaken in a mechanical and end-over-end shaker at 25 rpm. The extracts were separated from the solid residue by centrifugation at 3500 rpm for 10 min. A portion of the supernatant was subsequently removed by using a disposable syringe and filtered (Millipore 0.45 μm filters using a small filtration device). Concentrations of Cu, Zn, Cd, and Mn were measured by flame (Varian SpectrAA 200) and graphite furnace (Varian SpectrAA 640Z with Zeeman background correction) atomic absorption spectrometers at the facilities of Laboratory of Environmental Chemistry, University of Athens.
Procedural blanks and three analytical duplicates were included in each analytical batch (batches were based on chemical extraction) for quality control purposes. The relative percent difference (RPD) was calculated for each pair of duplicates as an indication of method precision revealing mean RPD values lower than 20 % for all the considered elements. To assess the accuracy of the EDTA, HAc, and CaCl2 extractions, two certified reference materials (BCR 483 and BCR 484) for extractable trace element concentrations (Quevauviller et al. 1997) were included in duplicate in each batch. Average recovery percentages of the different elements were 96 % (Cu), 105 % (Zn), and 89 % (Cd) for the EDTA extraction; 83 % (Cu), 88 % (Zn), and 89 % (Cd) for the HAc extraction; and 123 % (Cu), 128 % (Zn), and 85 % (Cd) for the CaCl2 extraction.
2.3 Statistical analysis
The data were summarized using mean, range, median, and standard deviation values. Available metal concentrations in relation to their respective reactive and pseudototal content were plotted on box-and-whisker diagrams. The length of the box indicates the interquartile range and the horizontal line inside the box indicates the median. The whiskers extend to the maximum and minimum data point within 1.5 box heights from the top or the bottom of the box. The star characters are the maximum and minimum values and the square sign represents the mean value.
Multiple linear regression analysis was performed to examine the relationship between available metal pools and their respective pseudototal or reactive content and basic soil properties. To overcome the influence of extreme values on the regression analysis and the non-normality of the chemical data, the logarithms of the original chemical data were used (Reimann et al. 2008). The first approach consisted of selecting the most significant parameters to describe the metal availability. The Pearson correlation coefficients between each of the explanatory soil chemistry variables, i.e., pseudototal and reactive concentrations and soil properties (pH, TOC, total CaCO3, Feox, Mnox, Alox), and the dependent variable (available metal concentration) were tested to select the soil parameters with the strongest linear relationship with the available metal pools. Empirical regression models were derived by stepwise selection of explanatory variables. In this way, the most salient parameters that influence the metal availability remained in the equation. The least significant parameters, defined by their largest p value, were discarded until all the remaining variables (including the intercepts) contributed significantly to the regression model at the 95th percentile confidence level (p < 0.05).
3 Results and discussion
3.1 Physicochemical characteristics of the studied soils
The descriptive statistics of physicochemical properties and aqua regia-extractable concentrations of heavy metals for the studied soil samples are presented in Table 1. All soil samples are slightly alkaline (median 7.6) with relatively low organic carbon content ranging from 1.0 to 2.7 %. Notable variations were observed in Fe, Mn, and Al amounts extracted by the ammonium oxalate dissolution (1030–3640, 252–2970, and 795–3680 mg/kg, respectively). The citrus soils yielded significant differences in their CaCO3 content with percentages fluctuating between 2.2 and 17.5 % probably indicating the variable contribution of the surrounding carbonate rocks to soil mineralogy.
The pseudototal content of Cu, Zn, Mn, and Cd exhibited the following concentration ranges: 83–275, 75.2–289, 557–3495, and 0.41–6.10 mg/kg, respectively. The content of Cu falls within the range of values reported in the literature for agricultural fields affected by application of Cu-based fungicides (Fernández-Calviño et al. 2008; Komárek et al. 2008; Fan et al. 2011). The Zn and Cd concentrations from our study are also comparable with data obtained for similarly affected soils from other countries (Mirlean et al. 2007; Duplay et al. 2014). In most cases, the investigated soils displayed concentrations lower than the maximum values of 140 mg/kg for Cu, 300 mg/kg for Zn, and 3 mg/kg for Cd allowed by the European Union for sewage sludge application on agricultural soils of pH between 6 and 7 (CEC 1986). The only deviation was the Cu and Cd contents for three and two soil samples, respectively. Manganese, a metal that has been rarely investigated in similar soil surveys, demonstrated a significant enrichment (median 1494 mg/kg) that has been previously ascribed to both the application of agrochemicals and the surrounding geological formations comprising serpentinized ultramafic rocks (Kelepertzis 2014).
3.2 Evaluation of heavy metal extractability
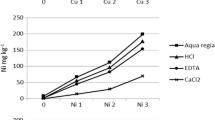
The chemical results of single extractions for Cu, Zn, Mn, and Cd are shown in Table 2. Results were also expressed as percentages of amounts dissolved by the CaCl2, EDTA, HAc, and HNO3 chemical procedures over the pseudototal content (Fig. 1) and as ratios of the various available pools to the reactive content (Fig. 2). The median extractability ratios in relation to the pseudototal pools in decreasing order are Zn (0.35 %) > Cd (0.31 %) > Cu (0.24 %) > Mn (0.07 %) for the mobile fraction, Cd (27 %) > Cu (24 %) > Mn (10 %) > Zn (6 %) for the potentially phytoavailable fraction, Cd (40 %) > Mn (21 %) > Zn (5 %) > Cu (4 %) for the mobilizable fraction, and Mn (72 %) > Cd (55 %) > Cu (39 %) > Zn (18 %) for the reactive fraction. The size of the available metal pools relative to the reactive content decreases in the order Zn (2.01 %) > Cu (0.63 %) > Cd (0.53 %) > Mn (0.09 %) for the mobile fraction, Cu (62 %) > Cd (49 %) > Zn (32 %) > Mn (13) for the potentially phytoavailable fraction, and Cd (69 %) > Mn (29 %) > Zn (28 %) > Cu (9 %) for the mobilizable fraction.
In general, the extractable concentrations presented various patterns depending on the element and the specific reagent used. The only exception is the CaCl2 soil test for which extractable concentrations were low for all four studied elements representing <0.5 % of the pseudototal metal content in the soil. Similarly, the mobile pools represent only a minor fraction of the reactive content in the soil (Fig. 2). Such low leachate amounts are consistent with Sahuquillo et al (2003) and Li et al (2014) who highlighted the low extraction yields of CaCl2 extraction, especially in the pH range characterizing the studied soils (Fan et al. 2011).
The EDTA yielded notable proportions of the pseudototal content for Cu and Cd (Fig. 1), indicating that these two elements are prone to complexation. Indeed, roots can also release soluble organic compounds into the rhizosphere which are capable of complexing Cu and, hence, to increase its potential uptake by plants (Rajkumar et al. 2012). Thus, in contaminated soils, extraction with organic complexing agents such as EDTA are reported to be better adapted for estimating Cu bioavailability (Schramel et al. 2000; Brun et al. 2001), especially in slightly alkaline soils (Komárek et al. 2008). For Zn and Mn, a minor fraction is released by the EDTA extraction demonstrating their incorporation to more stable mineral phases and highlighting that these elements are not sensitive to complexation processes. The EDTA-extracted amounts of Cu can be as high as 84 % of the reactive fraction, reflecting the fact that a significant portion of the reactive fraction of this metal is in a potentially phytoavailable form for the studied agricultural soils.
The mildly acidic leaching by acetic acid is notably less effective for Cu and Zn than for the other two studied elements (Figs. 1 and 2). A considerable increase in the ratios of the mobilizable amounts of Zn, Mn, and Cd relative to the reactive content is observed compared to the pseudototal fraction. The physical significance of this observation is that the mobilizable patterns that are sensitive to changes in the pH of the soil system are modulated by the action of the reactive metal phases. The low HAc-extractable Cu compared to the EDTA is attributed to the fact that acetic acid is hardly able to attack the strong copper-humic acid complexes (Schramel et al. 2000). Manganese and Cd seem to be prone to soil acidification, a problem linked to prolonged use of ammonium N fertilizer (Bouman et al. 1995; Fan et al. 2011) or a result of acid rain. The most striking feature of the mobilizable fraction is the high liberated Cd amounts (up to 63 % and up to 100 % of the pseudototal content and reactive content, respectively) attributed to its association with labile ion-exchangeable soil components and easily soluble hydrous oxides (Meers et al. 2007; Rodrigues et al. 2013). The high mobilizable pools of Cd signify the necessity of maintaining the alkaline nature of the studied soils by controlling the type of applied fertilizers (e.g., ammonium fertilizers produce more acidity than nitrate fertilizers). Considering that metal mobilization with acetic acid is basically attributed to carbonate dissolution (Sahuquillo et al. 2003) that is not taking place during the pH conditions of the EDTA extraction (pH 7.0), it may also be inferred that some Cd and Mn reside in the carbonate fraction of the studied soils.
The dilute nitric acid extracted significant proportions of the pseudototal content of elements with the exception of Zn which is mostly present in chemically inert mineral phases (Fig. 1). This probably reflects Zn that is included in minerals (e.g., oxides and silicates), highlighting that despite its accumulation in Argolida soil (Kelepertzis 2014), a considerable part of this metal is of geogenic origin. Higher chemical extractabilities of HNO3 compared to other extractants are due to its capacity to dissolve Fe and Mn oxides (Römkens et al. 2009). Especially for Mn and to a lesser extent for Cd, the reactive pools are quite representative of the pseudototal content (Fig. 1) indicating that the geochemically active fraction for these metals is almost equal to the pseudototal content in the soil.
3.3 Soil geochemical characteristics controlling metal availability
The influence of pseudototal content on metal availability is shown in Fig. 3 and Table 3. With respect to the concentrations extracted by CaCl2, only the pseudototal Cu content was found to exert some control on predicting the respective mobile amounts as evidenced by their strong correlation (r = 0.61, p < 0.001; Fig. 3). In particular, 35.6 % of the total variability in the mobile Cu concentrations is explained by a linear regression model with pseudototal Cu content as predictor variable (logCuCaCl2 = −2.51 + 0.950 log CuAR). Regression equations (Table 3) demonstrated that the variations in the potentially phytoavailable and mobilizable pools of metals were explained by the pseudototal content in the soil (48.7 % for CuHAc up to 91.4 % for CdEDTA). Indeed, the influence of total metal contents on corresponding extractabilities has been expressed in the literature (Wu et al. 2010; Luo et al. 2012; Li and Zhang 2013). The influence of pseudototal content is significantly more pronounced for Cd available pools explaining 88.1 % of variance for CdHAc and 91.4 % for CdEDTA.
In Table 4, results of multiple regression analysis for the investigated metals are presented using the reactive content as the independent variable. Relationships between the reactive content and the available pools are also graphically shown in Fig. 4. The use of HNO3 results to significantly higher R 2 adj values for the potentially phytoavailable and mobilizable amounts of both Cu and Zn indicating that the reactive pools provided better predictions of the available contents of these elements. The same pattern was also seen for the mobile Cu amounts for which the explained variance reached 43 % of total based on the reactive Cu fraction (logCuCaCl2 = −1.59 + 0.625 log CuHNO3). This can be explained by the fact that aqua regia extracts the inert fraction of the soil components that is not related to the available chemical forms (Römkens et al. 2009), for instance the fraction of Cu and Zn that is occluded in the crystal matrix or is bound to crystalline metal oxides. Another explanation could be driven by the chemical forms that are mobilized by these soil extractions. All EDTA, HAc, and dilute HNO3 are known to extract soluble organic metal complexes that are weakly adsorbed to soil surfaces through weak forces of attraction; the chemical nature of the aqua regia extraction that is able to destroy the strongest chemical bonds of metals with organic matter may blur the influence of pseudototal fraction on the availability of Cu and Zn. Interestingly, the available pools of Cd were described more accurately by the pseudototal content in the soil. This observation indicates that pseudototal fraction of Cd includes a geochemical phase that is in equilibrium with its available pools (Römkens et al. 2009). Another reason for this pattern could be spectral interferences deriving from the sample matrix composition in the determination of Cd that affect the measurements and blur the statistical relationship between the reactive and available fractions.
Inspecting Figs. 3 and 4, it is evident that the pseudototal and reactive contents of Cu, Zn, and Cd appear rather concentrated, especially because the highest isolated values concern the same samples. In general, extreme values take on a special importance in regression analysis because their presence away from the apparent trend in the data can strongly influence the final regression results (Reimann et al. 2008). Nevertheless, for the present data set, the removal of the two outliers from the regression analysis does not influence the output results. This may be ascribed to the usage of the log-transformed values that reduces the influence of extreme values. Only the influence of pseudototal and reactive fraction of Cd on its availability is lowered, but the close association of soil pseudototal content to Cd available pools still remains.
The contribution of some soil properties only slightly improved the performance of models for individual available metal pools (Table 4). A dubious effect of Alox on the potential phytoavailable pools of Cd and the mobilizable pools of Cu was observed since the partial correlations are both positive and negative. Especially, the negative correlation coefficient is difficult to explain considering the expected adsorption of Cu to active forms of metal oxides (Rodrigues et al. 2010) highlighting the difficulties of statistical models to overcome the complex interactions of metals with the soil components. For the studied soils, the influence of pH and TOC on the available metals pools is not so crucial as in other studies (Wu et al. 2010; Behera et al. 2011; Kelepertzis and Stathopoulou 2013; Sungur et al. 2014). This may be due to the limited range of pH and TOC values characterizing the citrus soils from Argolida that does not allow the importance of these principal soil properties in governing metal availability to be expressed.
In particular, soil pH is one of the most important factors controlling the availability of heavy metals to plants. The studied soils have a calcareous character and this is probably a limiting factor of metal uptake. This is particularly important considering the relatively high pseudototal concentration of Cu in the soil. Although Cu mobility and solubility is expected to be remarkably high in acidic soils (Chaignon et al. 2003), Cu phytotoxicity has also been documented for agricultural plants grown on calcareous soils (Michaud et al. 2007). This is possible as the rhizosphere pH is often more acidic than that of the bulk soil, and thus larger amounts of heavy metals are dissolved and possibly taken up in the vicinity of the root. However, in view of the relatively low extraction yields of the studied elements, it is suggested that the potential hazard of plant toxicity in the area is not significant and that despite their elevated pseudototal concentrations, heavy metals are effectively retained in soil phases. Further experimentation is needed to clarify the role of additional soil parameters such as crystalline Fe and Mn oxides and cation exchange capacity in controlling the heavy metal availability for the studied agricultural system.
4 Conclusions
Various single extraction procedures were used to evaluate the heavy metal (Cu, Zn, Mn, Cd) extractability in citrus agricultural soils from Argolida, an area carrying a long history of application of Cu-based fungicides and fertilizers. The pseudototal, reactive, potentially phytoavailable, mobilizable, and mobile fractions of these metals were determined. Among the protocols used for the determination of available metal pools, the highest Cu extractability was observed by EDTA. Greater amounts of both Cd and Mn were solubilized by the HAc soil test compared to the EDTA, whereas the extraction efficiencies for Zn were low in all the applied chemical reagents. Although the application of agrochemicals is an important source of accumulated heavy metals in the studied soils, their available concentrations are not always high as a result of their presence in less mobile solid fractions and the alkaline nature of the studied soil. Differences in the available pools of Cu, Zn, and Cd could be mostly explained by their respective pseudototal or reactive content in the soil while available Mn is not correlated to either chemical methods. We conclude that a single soil extraction test by dilute nitric acid can be applied for the estimation of both geochemical reactive and various available pools of Cu, Zn, and Cd in Mediterranean calcareous citrus soils.
References
Behera SK, Singh MV, Singh KN, Todwal S (2011) Distribution variability of total and extractable zinc in cultivated acid soils of India and their relationship with some selected soil properties. Geoderma 162:242–250
Bouman OT, Curtin D, Campbell CA, Biederbeck VO, Ukrainetz H (1995) Soil acidification from long-term use of anhydrous ammonia and urea. Soil Sci Soc Am J 59:1488–1494
Brun LA, Maillet J, Hinsinger P, Pépin M (2001) Evaluation of copper availability to plants in copper-contaminated vineyard soils. Environ Pollut 111:293–302
CEC (1986) Council Directive 12 June 1986 on the protection of the environment, and in particular the soil when sewage sludge is used in agriculture. Official Journal of the European Community. No L.181 (86/278/CEC). Brussels: Commission of the European Communities; pp 6–12
Chaignon V, Sanchez-Neira I, Herrmann P, Jaillard B, Hinsinger P (2003) Copper bioavailability and extractability as related to chemical properties of contaminated soils from a vine-growing area. Environ Pollut 123:229–238
de Santiago-Martín A, Van Oort F, González C, Quintana J, Lafuente A, Lamy I (2015) Improving the relationship between soil characteristics and metal bioavailability by using reactive fractions of soil parameters in calcareous soils. Environ Toxicol Chem 34:37–44
Dudka S, Miller WP (1999) Accumulation of potentially toxic elements in plants and their transfer to human food chain. J Environ Sci Health B 34:681–708
Duplay J, Semhi K, Errais E, Imfeld G, Babcsanyi I, Perrone T (2014) Copper, zinc, lead and cadmium bioavailability and retention in vineyard soils (Rouffach, France): the impact of cultural practices. Geoderma 230–231:318–328
Fan J, He Z, Ma LQ, Stoffella PJ (2011) Accumulation and availability of copper in citrus grove soils as affected by fungicide application. J Soils Sediments 11:639–648
Fernández-Calviño D, Rodríguez-Suárez JA, López-Periago E, Arias-Estévez M, Simal-Gándara J (2008) Copper content of soils and river sediments in a winegrowing area, and its distribution among soil or sediment components. Geoderma 145:91–97
Gaudette HE, Flight WR, Toner L, Folger DW (1974) An inexpensive titration method for the determination of organic carbon in recent sediments. J Sediment Res 44:249–253
Gupta SK, Vollmer MK, Krebs R (1996) The importance of mobile, mobilisable and pseudo total heavy metal fractions in soil for three-level risk assessment and risk management. Sci Total Environ 178:11–20
ISO 10390 (1994) Soil quality—determination of pH
Jiao W, Ouyang W, Hao F, Wang F, Liu B (2014) Long-term cultivation impact on the heavy metals behavior in a reclaimed wetland, Northeast China. J Soils Sediments 14:567–576
Kabata-Pendias A, Pendias H (2001) Trace elements in soils and plants, 3rd edn. CRC Press LLC, Boca Raton
Kelepertzis E (2014) Accumulation of heavy metals in agricultural soils of Mediterranean: insights from Argolida basin, Peloponnese, Greece. Geoderma 221–222:82–90
Kelepertzis E, Argyraki A (2015) Geochemical associations for evaluating the availability of potentially harmful elements in urban soils: lessons learnt from Athens, Greece. Appl Geochem 59:63–73
Kelepertzis E, Stathopoulou E (2013) Availability of geogenic heavy metals in soils of Thiva town (central Greece). Environ Monit Assess 185:9603–9618
Komárek M, Száková J, Rohošková M, Javorská H, Chrastný V, Balík J (2008) Copper contamination of vineyard soils from small wine producers: a case study from the Czech Republic. Geoderma 147:16–22
Li Y, Zhang M-K (2013) A comparison of physiologically based extraction test (PBET) and single-extraction methods for release of Cu, Zn, and Pb from mildly acidic and alkali soils. Environ Sci Pollut Res 20:3140–3148
Li L, Wu H, van Gestel CAM, Peijnenburg WJGM, Allen HE (2014) Soil acidification increases metal extractability and bioavailability in old orchard soils of Northeast Jiaodong Peninsula in China. Environ Pollut 188:144–152
Loring DH, Rantala RTT (1992) Manual for the geochemical analyses of marine sediments and suspended particulate matter. Earth Sci Rev 32:235–283
Luo X-S, Yu S, Li X-D (2012) The mobility, bioavailability, and human bioaccessibility of trace metals in urban soils of Hong Kong. Appl Geochem 27:995–1004
Lv J, Liu Y, Zhang Z, Dai J, Dai B, Zhu Y (2015) Identifying the origins and spatial distributions of heavy metals in soils of Ju country (Eastern China) using multivariate and geostatistical approach. J Soils Sediments 15:163–178
Meers E, Laing GD, Unamuno V, Ruttens A, Vangronsveld J, Tack FMG, Verloo MG (2007) Comparison of cadmium extractability from soils by commonly used single extraction protocols. Geoderma 141:247–259
Menzies NW, Donn MJ, Kopittke PM (2007) Evaluation of extractants for estimation of the phytoavailable trace metals in soils. Environ Pollut 145:121–130
Michaud AM, Bravin MN, Galleguillos M, Hinsinger P (2007) Copper uptake and phytotoxicity as assessed in situ for durum wheat (Triticum turgidum durum L.) cultivated in Cu-contaminated, former vineyard soils. Plant Soil 298:99–111
Mirlean N, Roisenberg A, Chies JO (2007) Metal contamination of vineyard soils in wet subtropics (southern Brazil). Environ Pollut 149:10–17
Novozamsky I, Lexmond TM, Houba VJG (1993) A single extraction procedure of soil for evaluation of uptake of some metals by plants. Int J Environ An Ch 51:47–58
Peijnenburg WJGM, Zablotskaja M, Vijver MG (2007) Monitoring metals in terrestrial environments within a bioavailability framework and a focus on soil extraction. Ecotoxicol Environ Saf 67:163–179
Qasim B, Motelica-Heino M, Joussein E, Soubrand M, Gauthier A (2015) Potentially toxic element phytoavailability assessment in Technosols from former smelting and mining areas. Environ Sci Pollut Res 22:5961–5974
Quevauviller P, Rauret G, Rubio R, López-Sánchez J-F, Ure A, Bacon J, Muntau H (1997) Certified reference materials for the quality control of EDTA- and acetic acid-extractable contents of trace elements in sewage sludge amended soils (CRMs 483 and 484). Fresen J Anal Chem 357:611–618
Rajkumar M, Sandhya S, Prasad MNV, Freitas H (2012) Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol Adv 30:1562–1574
Reimann C, Filzmoser P, Garrett R, Dutter R (2008) Statistical data analysis explained: applied environmental statistics with R. Wiley-Blackwell, Chichester
Rodrigues SM, Henriques B, Ferreira da Silva E, Pereira ME, Duarte AC, Römkens PFAM (2010) Evaluation of an approach for the characterization of reactive and available pools of twenty potentially toxic elements in soils: part I—the role of key soil properties in the variation of contaminants’ reactivity. Chemosphere 81:1549–1559
Rodrigues SM, Cruz N, Coelho C, Henriques B, Carvalho L, Duarte AC, Pereira E, Römkens PFAM (2013) Risk assessment for Cd, Cu, Pb, and Zn in urban soils: chemical availability as the central concept. Environ Pollut 183:234–242
Rodríguez Martín JA, Ramos-Miras JJ, Boluda R, Gil C (2013) Spatial relations of heavy metals in arable and greenhouse soils of a Mediterranean environment region (Spain). Geoderma 200–201:180–188
Rodríguez Martín JA, De Arana E, Ramos-Miras JJ, Gil C, Boluda R (2015) Impact of 70 years urban growth associated with heavy metal pollution. Environ Pollut 196:156–163
Römkens P, Guo H-Y, Chu C-L, Liu T-S, Chiang C-F, Koopmans G (2009) Characterization of soil heavy metal pools in paddy fields in Taiwan: chemical extraction and solid-solution partitioning. J Soils Sediments 9:216–228
Sahuquillo A, Rigol A, Rauret G (2002) Comparison of leaching tests for the study of trace metals remobilisation in soils and sediments. J Environ Monit 4:1003–1009
Sahuquillo A, Rigol A, Rauret G (2003) Overview of the use of leaching/extraction tests for risk assessment of trace metals in contaminated soils and sediments. Trends Anal Chem 22:152–159
Schramel O, Michalke B, Kettrup A (2000) Study of the copper distribution in contaminated soils of hop fields by single and sequential extraction procedures. Sci Total Environ 263:11–22
Schwertmann U (1964) Differenzierung der Eisenoxide des Bodens durch photochemische Extraktion mit saurer Ammoniumoxalat-Lösung. Z Pflanzenernaehr Bodenkd 105:194–202
Shan Y, Tysklind M, Hao F, Ouyang W, Chen S, Lin C (2013) Identification of sources of heavy metals in agricultural soils using multivariate analysis and GIS. J Soils Sediments 13:720–729
Sungur A, Soylak M, Ozcan H (2014) Investigation of heavy metal mobility and availability by the BCR sequential extraction procedure: relationship between soil properties and heavy metals availability. Chem Speciat Bioavailab 26:219–230
Sungur A, Soylak M, Yilmaz E, Yilmaz S, Ozcan H (2015) Characterization of heavy metal fractions in agricultural soils by sequential extraction procedures: the relationship between soil properties and heavy metal fractions. Soil Sediment Contam 24:1–15
Ure AM, Quevauviller P, Muntau H, Griepink B (1993) Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission of the European Communities. Int J Environ An Ch 51:135–151
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 37:29–37
Wu C, Luo Y, Zhang L (2010) Variability of copper availability in paddy fields in relation to selected soil properties in Southeast China. Geoderma 156:200–206
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Rongliang Qiu
Rights and permissions
About this article
Cite this article
Kelepertzis, E., Paraskevopoulou, V., Argyraki, A. et al. Evaluation of single extraction procedures for the assessment of heavy metal extractability in citrus agricultural soil of a typical Mediterranean environment (Argolida, Greece). J Soils Sediments 15, 2265–2275 (2015). https://doi.org/10.1007/s11368-015-1163-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1163-x